Review Article Open Access
Controlled Expansion of Mammalian Cell Populations by Reversible Immortalization
Hirofumi Noguchi1* and Naoya Kobayashi2*1Department of Gastroenterological Surgery, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama 700-8558, Japan
2Okayama Saidaiji Hospital, Okayama 704-8192, Japan
- Corresponding Authors:
- Hirofumi Noguchi
Department of Gastroenterological Surgery
Okayama University Graduate School of Medicine
Dentistry and Pharmaceutical Sciences 2-5-1 Shikata-cho
Okayama 700-8558, Japan
Tel: +81-86-235-7257
Fax: +81-86-221-8775
E-mail: noguch-h@cc.okayama-u.ac.jp, noguchih2006@yahoo.co.jp - Naoya Kobayashi
Okayama Saidaiji Hospital, Okayama 704-8192, Japan
E-mail: n-kobayashi@saidaiji-hp.or.jp
Received date: April 25, 2013; Accepted date: May 15, 2013; Published date: May 20, 2013
Citation: Noguchi H, Kobayashi N (2013) Controlled Expansion of Mammalian Cell Populations by Reversible Immortalization. J Biotechnol Biomater 3:158. doi:10.4172/2155-952X.1000158
Copyright: © 2013 Noguchi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
In 1996, reversible immortalization using SV40 large T antigens and the Cre/LoxP system was successfully achieved with primary human fibroblasts. The concept of reversible immortalization involves introducing an immortalizing agent, SV40 large T antigens, into primary cells, expanding the cells in the culture, and finally, efficiently removing the immortalizing agent using Cre/LoxP site-specific recombination. The resulting cell population is essentially identical to the initial primary cells, but greatly increased in number. Since this report, reversible immortalization has been realized with hepatocytes, pancreatic β-cells, hepatic stellate cells, endothelial cells, renal epithelial cells and myogenic cells. This method facilitates the study of cell transplantation as well as cell differentiation, the cell cycle and senescence, by allowing one to control cell proliferation.
Keywords
Reversible immortalization; myogenic cells; β-cells
Introduction
Various research strategies have been hampered by difficulties in obtaining populations of primary cells that actively divide, while maintaining their stage of differentiation. Transferring specific oncogenes can generate cell lines that propagate in an intermediate stage of differentiation, a process known as cell immortalization. The simian virus 40 gene encoding the large tumor antigen (SV40Tag) is widely used to obtain continuously growing cell lines. Unlike most other oncogene products, SV40Tag alone can immortalize cells in the absence of other oncoproteins, owing to its multiple effects on the cell cycle [1]. SV40Tag is able to induce transformation in cell culture and in vivo transgenic systems, a phenomenon effected through its interaction with at least five cellular targets: hsc70, the three Rb tumor suppressor proteins (pRb, p107, a nd p130), and the tumor suppressor p53 [2,3]. SV40Tag binds p53 through interactions with exposed amino acids on the surface of its ATPase domain [4]. Similarly, the three Rbproteins bind to an LXCXE motif located in the flexible linker between the J domain and the origin binding domain. Finally, the J domain governs recruitment and activation of hsc70, a cellular chaperone [5,6]. SV40Tag has been shown to interact with another three targets, and these interactions could contribute to transformation as well, but they have been less studied. Two of these factors, the checkpoint kinase Bub1 and the cullin Cul7, interact with SV40Tag via the flexible linker near the LXCXE motif [7-10]. Finally, the transcriptional adapter proteins CBP/p300 [11-13], bind SV40Tag through interactions with p53, and could also contribute to transformation.
Recently, the telomerase expression has also been used, either alone or in the company of other immortalizing genes, to create genetically stable, nontumorigenic cell lines capable of apparently indefinite proliferation. The introduction of SV40Tag sometimes does not induce immortalization, but rather extends the in vitro life span of cells. This event reflects the critical attrition of telomere length, and can be overcome by expressing the catalytic component of the enzyme telomerase (human telomerase reverse transcriptase: hTERT) [14]. However, established cell lines frequently exhibit different characteristics from the primary cells, especially in terms of cell growth.
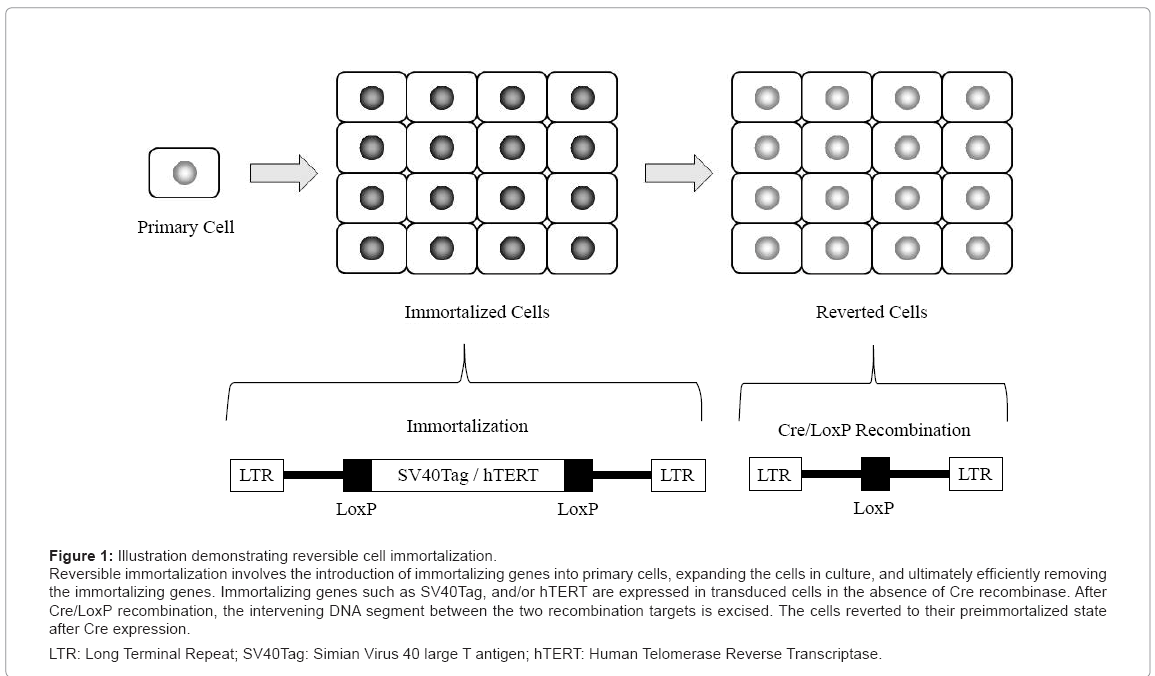
Recently, the Cre/LoxP system has been used to temporarily “immortalize” primary cells, in order to obtain populations of primary cells that actively divide in vitro without entering senescence [15]. Cre recombinase catalyzes site-specific recombination between two specific 34-base-pair direct repeats called LoxP. The binding of Cre to LoxP results in the formation of a synapse between both LoxP sites, in addition to Cre-mediated excision of the intervening sequences, which are permanently removed from the genome. Reversible immortalization involves the introduction of immortalizing genes, such as SV40Tag, and/or hTERT, into primary cells, expanding the cells in culture, and ultimately efficiently removing the immortalizing genes using the Cre/LoxP system (Figure 1). The cells reverted to their preimmortalized state after Cre expression, as indicated by changes in both the growth characteristics and p53 levels, and their fate conformed to the telomere hypothesis of replicative cell senescence [15]. Reversible immortalization has been realized with fibroblasts, hepatocytes, hepatic stellate cells, endothelial cells, renal epithelial cells, myogenic cells and pancreatic beta cells.
Figure 1: Illustration demonstrating reversible cell immortalization. Reversible immortalization involves the introduction of immortalizing genes into primary cells, expanding the cells in culture, and ultimately efficiently removing the immortalizing genes. Immortalizing genes such as SV40Tag, and/or hTERT are expressed in transduced cells in the absence of Cre recombinase. After Cre/LoxP recombination, the intervening DNA segment between the two recombination targets is excised. The cells reverted to their preimmortalized state after Cre expression.
LTR: Long Terminal Repeat; SV40Tag: Simian Virus 40 large T antigen; hTERT: Human Telomerase Reverse Transcriptase.
In this review, we focus on reversible cell immortalization using gene transfer and site-specific recombination.
Reversibly Immortalized Human Hepatocytes
The transplantation of hepatocytes, which has been proposed as temporary metabolic support in patients awaiting liver transplantation or spontaneous reversion of liver disease, is an attractive alternative to liver transplantation. Our group demonstrated the reversible immortalization of human hepatocytes [16]. A highly-differentiated cell line, NKNT-3, was established via retroviral transfer. We performed intrasplenic transplantation of NKNT-3 cells treated with Cre recombinase (reverted NKNT-3 cells) into a rat model of liver failure induced by 90% hepatectomy. The reverted NKNT-3 cells provided lifesaving metabolic support during acute liver failure.
Our group also showed the reversible immortalization of human hepatocytes using a retroviral vector expressing a catalytic subunit of hTERT flanked by a pair of LoxP recombination targets [17]. One of the 24 clones was further subjected to transfection with the plasmid pCAGMerCreMer/PuroR, which expresses a Cre recombinase protein fused to two mutant estrogen-receptor ligand-binding domains (MerCreMer), under the control of the CAG promoter. Cre/LoxP recombination was performed in the established cells using simple exposure to 500 nM of tamoxifen for one week. Following transfection with the pCAGMerCreMer/PuroR, a resultant clone, 16-T3, was isolated for further studies based on the Cre/LoxP recombination efficiency. 16-T3 cells grew steadily in the culture and doubled in number within approximately 48 hours. The 16-T3 cells that reverted after treatment with tamoxifen showed an increased expression of hepatic markers, in association with enhanced levels of transcription factors. Transplantation of the reverted 16-T3 cells significantly prolonged the survival of pigs with acute liver failure induced by d-galactosamine injection.
Reversible immortalization of rat and porcine hepatocytes has also been reported [18,19].
Reversible Immortalized Pancreatic ß-Cells
The successes achieved over the last few decades with islet transplantation of whole pancreata and isolated islets suggest that diabetes can be cured by replenishing deficient β-cells [20,21]. It is logical that replacing the islet tissue itself is a better approach than simply replacing the lost insulin. However, the clinical benefits of islet transplantation are obtained in only a small minority of patients and are not permanent [22]. Our strategy was to transform human primary β-cells with immortalizing genes of SV40Tag and hTERT, and to screen for clones that are not tumorigenic and that express insulin and β-cellassociated factors. Suitable clones could then be expanded, and Cremediated excision of the immortalizing genes would allow for the removal of tumorigenic potential and recovery of the primary β-cell function. Our reversibly immortalized pancreatic β-cell clone (NAKT- 15) secreted insulin in response to glucose stimulation and non-glucose secretagogues, resulting in the expression of proteins characteristic of β-cells (such as Isl-1, Pax 6, Nkx 6.1, Pdx-1, prohormone convertase (PC) 1/3 and PC 2), and secretory granule proteins (such as chromogranin A and synaptophysin). The NAKT-15 cells did not senesce after more than fifty passages in culture, and were continuously expanded. The transplantation of reverted NAKT-15 cells into streptozotocin (STZ)- induced diabetic severe combined immunodeficiency (SCID) mice resulted in perfect control of blood glucose within two weeks, and the mice remained normoglycemic for more than thirty weeks [23].
Reversible immortalization of murine pancreatic β-cells has also been realized using regulatory elements of the bacterial tetracycline (tet) operon for the conditional expression of SV40Tag oncoproteins in transgenic murine β-cells [24]. The tet-on regulatory system was used to generate β-cell lines that divide in the presence of the tet derivative doxycycline (dox), and undergo growth arrest in its absence. The cells produce and secrete high amounts of insulin, and can restore and maintain euglycemia in syngeneic STZ-induced diabetic mice in the absence of dox. Moreover, reversible immortalization of rat pancreatic β-cells has also been reported using tricistronic retroviral vectors, in which Cre-ER, SV40Tag or hTERT and a reporter gene are flanked by the same pair of LoxP sites [25]. The Cre-ER protein was induced to translocate from the cytoplasm to the nucleus by 4-hydroxytamoxifen, in order to excise SV40Tag, hTERT and the Cre-ER gene itself without the need for secondary gene transfer. Reversible immortalization of human pancreatic β-cells via the lentivector-mediated transfer of specific genes has also been reported [26].
The promising results afforded by islet transplantation, coupled with the shortage of cadaver pancreata relative to the potential demand, have provided strong impetus to search for new sources of insulin-producing cells. Alternative sources of islets have been sought in stem cells [27-29], porcine islets [30-33], and β-cell expansion with growth factors [34,35]. However, the differentiation of embryonic and pancreatic stem cells and expansion of differentiated β-cells in vitro is limited [36]. The expansion of primary β-cells by growth factors is also hampered by the senescence of the cells [37]. Establishing reversibly immortalized pancreatic β-cells, is one step toward overcoming the limitations of transplanting primary pancreatic β-cells to control diabetes.
Reversible Immortalization of Mammalian Cells for the Delivery of Functional Molecules
The use of ex vivo gene therapy strategies and cell replacement therapy in the clinical field is often limited by the low number of cells harvested from biopsies, as well as the poor proliferation and premature senescence of these cells in vitro. The proliferative potential of human myogenic precursors declines considerably during early postnatal growth [38,39], in parallel with progressive reduction in the telomere length, which occurs in the first two decades of life [40]. To overcome this problem, Berghella et al. [41] employed Cre-mediated excision of a LoxP-flanked SV40Tag sequence to establish reversibly immortalized human myogenic cells. The clonal isolates of the SV40Tag-positive myogenic cells exhibited modified growth characteristics, and a significantly extended life span while maintaining full myogenic potential. The transient expression of Cre recombinase allowed for excision of the entire provirus, with up to >90% efficiency, although 10% of the “unexcised” cells may still be cancerous. As a result, it is important to improve the excision efficiency, in order to remove these remaining cells. The reverted cells, which were injected into the regenerating muscle of SCID/bg-immunodeficient mice, underwent terminal differentiation in vivo, giving rise to clusters of hybrid fibers, with an efficiency comparable to that of control untransduced cells. This approach may eventually lead to the ex vivo production of an adequate number of myogenic cells to reconstitute at least a few essential muscles in dystrophic patients via transplantation.
Transplantation of primary adrenal chromaffin cells has been used to deliver functional molecules for a variety of therapeutic indications [42]. However, a serious limitation is the need to harvest fresh cells from donors, requiring safety screening for each batch of cells, and a resultant mixture of cell types that is incompletely characterized and nonhomogeneous. Eaton et al. [43] described the generation of chromaffin cell lines using the temperature-sensitive allele of SV40Tag. The cells are able to reverse neuropathic pain after being transplanted in the spinal subarachnoid space [44]. Even with 100% disappearance of SV40Tag in the grafts within a few weeks after transplantation, the oncogene expression in vivo remains a potential possibility, and the use of such cells is not an appropriate strategy for safe clinical application in humans. The same group developed a strategy based on reversible immortalization of primary adrenal chromaffin cells, with a retroviral vector expressing the temperature-sensitive allele of SV40Tag, excisable by means of the Cre/LoxP recombination system [45]. The immortalized cells expressed immunoreactivity for all catecholamine enzymes, including tyrosine hydroxylase (TH), dopamine beta-hydroxylase (DbetaH) and phenylethanolamine-N-methyltransferase (PNMT). When chromaffin cells reverted by the Cre expression were transplanted into a model of neuropathic pain and partial nerve injury, the grafts were equally able to reverse the behavioral hypersensitivity induced by the injury. The use of Cre/Lox site-directed recombination of SV40Tag in chromaffin cells that are able to deliver neuroactive molecules may overcome the limitations of these cells for transplantation.
Bioartificial Organs Produced by Reversibly Immortalized Mammalian Cells
These cell lines are useful in the development of bioartificial organs, as well as cell transplantation and ex vivo gene therapy. One of our long-term goals is to develop bioartificial organ systems that closely mimic the function of normal organs in vivo. Recently, heterotypic cell interactions between parenchymal cells and nonparenchymal neighbors have been recognized to be central to the function of many organ systems. Pure cultures of hepatocytes recapitulate several key liver functions, but fail to provide adequate levels of a few important detoxifying enzymes, including cytochrome p450-associated enzymes (CYPs). Hepatic stellate cells are believed to play an essential role in the known crosstalk between hepatocytes and other liver cells, such as endothelial cells [46,47]. Extending the reversible immortalization system to other cell types present in the human liver would allow for the study of cell-cell interactions, and further contribute to the development of bioartificial livers. We established reversibly immortalized human hepatic stellate cells via the retroviral transfer of hTERT flanked by a pair of LoxP sequences [48]. TWNT-1, an immortalized human stellate cell line, is highly differentiated and exhibits the functions of human stellate cells, including the uptake of acetylated low-density lipoprotein,s and synthesis of collagen type I and hepatocyte growth factor. The efficient excision of retrovirally transferred hTERT cDNAs was achieved via the TAT-mediated transduction of Cre recombinase, a new technology for transducing proteins into cells [49-51]. When cocultured with TWNT- 1 cells, NKNT-3 increases the protein expressions of the detoxifying cytochrome P450-associated protein isoenzymes 3A4 and 2C9, in addition to urea synthesis. This finding supports the contention that heterotypic cell interactions are important for enhancing the production of liver-specific enzymes by hepatocytes in vitro [52,53].
In both the developing and mature adult liver, hepatocyte-toendothelial cell interactions are imperative for the coordination of the sophisticated liver functions [54]. Therefore, we also applied the Cre/LoxP system to human liver endothelial cells [55,56]. Liver endothelial cells were transfected with a retroviral vector that expresses the SV40Tag, flanked by a pair of LoxP recombination targets. One of the transduced clones, HNNT-2, extended the life span from passages 10 to 40; however, complete immortalization was not achieved [50]. This finding is explained by the absence of spontaneous activation of endogenous telomerase, known to be an essential participant in cellular immortalization processes in SV40Tag-transduced cells [57-59]. To enhance the immortalization potential, we used another retroviral vector expressing hTERT flanked by a pair of LoxB target sequences. One of the clones, TMNK-1, expressed EC markers, including factor VIII, vascular endothelial growth factor receptors (flt-1, KDR/Flk- 1) and CD34. TMNK-1 exhibited uptake of Di-I-acetylated-lowdensity lipoprotein and angiogenic potential in Matrigel assays. Following lipopolysaccharide treatment, TMNK-1 produced tumor necrosis factor (TNF)-alpha and interleukin (IL)-6, and exhibited an increased expression of intracellular adhesive molecule-1, vascular cellular adhesive molecule-1 and VE-cadherin. Efficient excision of the retrovirally-transferred hTERT and SV40Tag cDNAs was achieved via the TAT-mediated transduction of Cre recombinase [56].
Recently, much attention has been paid to a novel therapy for liver failure, using a hybrid bioartificial liver support device that incorporates living liver cells. Researchers in various fields have considered the following cells for potential use in bioartificial livers: human embryonic stem (ES) cells, somatic stem cells, differentiated tissue cells and cells derived from tissues of different animal species, particularly pigs. We recommend that researchers adopt a reversible immortalization system that uses the Cre/LoxP site-specific recombination reaction targeting human hepatocytes, and other liver-related cells in their final differentiated state. This system has allowed us to establish a safe human liver cell line for generating bioartificial livers, that is capable of differentiation at a low cost and on a large scale.
Reversible Immortalization of other Cell Types
Reversibly immortalized human melanocytes created using a retroviral vector expressing SV40Tag-EGFP flanked by a pair of LoxP recombination targets have been reported to provide melanocytes with rapid replicative potential in vitro [60]. Following the transplantation of reverted melanocytes into an established vitiligo animal model, the pigmentation formed black macula within three months without tumorigenicity. The pathological results showed that there was significant melanocyte and melanin accumulation in the epidermis with some hair follicles in the transplanted area, which confirmed that the reverted cells display melanogenesis in vivo. Reversibly immortalized human melanocytes may be useful as a successful repigmentation method for depigmented skin disease therapy.
Reversibly immortalized human renal proximal tubule epithelial cells (RPTECs) have been established using two lentivirus vectors carrying hTERT and SV40Tag flanked by LoxP sites [61]. The transduced RPTEC clones continued to proliferate, while retaining the biochemical and functional characteristics of the primary cells. The clones exhibited contact-inhibited, anchorage- and growth factor-dependent growth, and did not form tumors in nude mice. The transient Cre expression observed in these cells resulted in efficient proviral deletion, the upregulation of some renal-specific activities and decreased growth rates. Ultimately, the cells underwent replicative senescence, indicating intact cell cycle control. These data suggest that reversible immortalization of human RPTECs can lead to the largescale production of RPTECs that retain most tissue-specific properties.
A reversibly immortalized murine dental papilla cell line (mDPCET) has been generated by combining the traditional strategy of “Cre/LoxP-based reversible immortalization”, with a tamoxifenregulated Cre recombination system [62]. Tamoxifen-mediated reversible immortalization allowed for the expansion of primary mDPCs that led to the production of odontoblast-like cells that retained most odontoblast-specific properties, representing a safe and ready-touse method due to its simple manipulation.
Reversible immortalization of cardiomyocytes has also been reported [63]. The immortalized cardiomyocytes exhibited the morphological features of dedifferentiation (an increased expression of vimentin and reduced expressions of troponin I and Nkx2.5), along with the continued expression of cardiac markers (alpha-actin, connexin-43 and calcium transients). After the immortalization was reversed, the cells returned to their differentiated state. This strategy for the controlled expansion of primary cardiomyocytes has the potential to provide large amounts of an individual patient’s own cardiomyocytes for cell therapy, and the cardiomyocytes derived using this method may constitute a useful cellular model for studying cardiogenesis.
We previously reported the in vitro amplification of human umbilical vein endothelial cell (HUVEC) populations during the first phase of reversible immortalization resulting from the retroviral transfer of the SV40Tag gene, which was subsequently excised via Cre/LoxP-mediated site-specific recombination [55]. The transduced HUVECs exhibited the morphological characteristics of endothelial cells, and were maintained in the culture up to passage 40. The cells expressed endothelial cell markers, including factor VIII, VEGF receptors (Flt-1 and KDR/Flk-1) and CD34, and endocytosed acetylated low-density lipoproteins. The formation of capillary-like structures in the cells was observed in a Matrigel assay. The complete elimination of the transferred SV40Tag gene was achieved in virtually 100% of the cells, following infection with a recombinant adenovirus expressing Cre recombinase and subsequent selection. The reverted cells maintained their differentiated endothelial cell phenotype. Qiu et al. [64] showed similar data. These studies provide a means of expanding primary endothelial cells of various sources for basic studies and possible cell and gene therapy.
Some groups have also reported the reversible immortalization of progenitor cells. Nishioka et al. [65] and our group collaboratively reported the reversible immortalization of human marrow-derived mesenchymal stem cells (MSCs), with the potential to differentiate into mesenchymal tissues, such as bone, cartilage, adipose tissue and bone marrow stroma, using a retroviral vector carrying SV40Tag, which can be excised via Cre/LoxP site-specific recombination. One of the MSCs cell lines, HMSC-1, retained the original surface characteristics and differentiation potential, and exhibited a higher proliferative capacity than the parental cells. Other groups have demonstrated the reversible immortalization of Nestin-positive progenitor cells (NPPCs) obtained from the murine pancreas, using the tet-on system of the SV40Tag expression [66,67]. The reversibly immortalized NPPCs were efficiently induced to differentiate into insulin-producing cells that contained a combination of glucagon like peptide-1 (GLP-1) and sodium butyrate.
Conclusion
Cre/LoxP site-specific recombination has been used for genetic engineering. Employing this technology with the genes of SV40Tag, and/or hTERT, the transient expression of an immortalizing gene induced via gene transfer is used to generate cell lines that propagate rapidly in cell culture, and display an increased life span without entering senescence. The reversible immortalization system allows for the ex vivo amplification of primary cells in culture, facilitating the study of cell transplantation, as well as cell differentiation, the cell cycle and senescence by allowing one to control cell proliferation. However, some immortalized cell lines have been reported to demonstrate an abnormal karyotype [62]. Therefore, further studies are required before such cells can be used in clinical situations.
Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science; and the Ministry of Health, Labour and Welfare.
References
- Sompayrac L, Danna KJ (1991) The amino-terminal 147 amino acids of SV40 large T antigen transform secondary rat embryo fibroblasts. Virology 181: 412-415.
- Ahuja D, Sáenz-Robles MT, Pipas JM (2005) SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24: 7729-7745.
- Ali SH, DeCaprio JA (2001) Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol 11: 15-23.
- Lilyestrom W, Klein MG, Zhang R, Joachimiak A, Chen XS (2006) Crystal structure of SV40 large T-antigen bound to p53: interplay between a viral oncoprotein and a cellular tumor suppressor. Genes Dev 20: 2373-2382.
- Brodsky JL, Pipas JM (1998) Polyomavirus T antigens: molecular chaperones for multiprotein complexes. J Virol 72: 5329-5334.
- Sullivan CS, Pipas JM (2002) T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis. Microbiol Mol Biol Rev 66: 179-202.
- Ali SH, Kasper JS, Arai T, DeCaprio JA (2004) Cul7/p185/p193 binding to simian virus 40 large T antigen has a role in cellular transformation. J Virol 78: 2749-2757.
- Kohrman DC, Imperiale MJ (1992) Simian virus 40 large T antigen stably complexes with a 185-kilodalton host protein. J Virol 66: 1752-1760.
- Tsai SC, Pasumarthi KB, Pajak L, Franklin M, Patton B, et al. (2000) Simian virus 40 large T antigen binds a novel Bcl-2 homology domain 3-containing proapoptosis protein in the cytoplasm. J Biol Chem 275: 3239-3246.
- Cotsiki M, Lock RL, Cheng Y, Williams GL, Zhao J, et al. (2004) Simian virus 40 large T antigen targets the spindle assembly checkpoint protein Bub1. Proc Natl Acad Sci U S A 101: 947-952.
- Eckner R, Ludlow JW, Lill NL, Oldread E, Arany Z, et al. (1996) Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol 16: 3454-3464.
- Lill NL, Tevethia MJ, Eckner R, Livingston DM, Modjtahedi N (1997) p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J Virol 71: 129-137.
- Poulin DL, Kung AL, DeCaprio JA (2004) p53 targets simian virus 40 large T antigen for acetylation by CBP. J Virol 78: 8245-8253.
- Henderson S, Allsopp R, Spector D, Wang SS, Harley C (1996) In situ analysis of changes in telomere size during replicative aging and cell transformation. J Cell Biol 134: 1-12.
- Westerman KA, Leboulch P (1996) Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc Natl Acad Sci U S A 93: 8971-8976.
- Renfranz PJ, Cunningham MG, McKay RD (1991) Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell 66: 713-729.
- Totsugawa T, Yong C, Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, et al. (2007) Survival of liver failure pigs by transplantation of reversibly immortalized human hepatocytes with Tamoxifen-mediated self-recombination. J Hepatol 47: 74-82.
- Cai J, Ito M, Westerman KA, Kobayashi N, Leboulch P, et al. (2000) Construction of a non-tumorigenic rat hepatocyte cell line for transplantation: reversal of hepatocyte immortalization by site-specific excision of the SV40 T antigen. J Hepatol 33: 701-708.
- Meng FY, Chen ZS, Han M, Hu XP, He XX, et al. (2010) Porcine hepatocyte isolation and reversible immortalization mediated by retroviral transfer and site-specific recombination. World J Gastroenterol 16: 1660-1664.
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, et al. (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343: 230-238.
- Noguchi H, Iwanaga Y, Okitsu T, Nagata H, Yonekawa Y, et al. (2006) Evaluation of islet transplantation from non-heart beating donors. Am J Transplant 6: 2476-2482.
- Robertson RP (2004) Islet transplantation as a treatment for diabetes-a work in progress. N Engl J Med 350: 694-705.
- Narushima M, Kobayashi N, Okitsu T, Tanaka Y, Li SA, et al. (2005) A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol 23: 1274-1282.
- Milo-Landesman D, Surana M, Berkovich I, Compagni A, Christofori G, et al. (2001) Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic beta cells controlled by the tet-on regulatory system. Cell Transplant 10: 645-650.
- Wu HL, Wang Y, Zhang P, Li SF, Chen X, et al. (2011) Reversible immortalization of rat pancreatic ß cells with a novel immortalizing and tamoxifen-mediated self-recombination tricistronic vector. J Biotechnol 151: 231-241.
- Salmon P, Oberholzer J, Occhiodoro T, Morel P, Lou J, et al. (2000) Reversible immortalization of human primary cells by lentivector-mediated transfer of specific genes. Mol Ther 2: 404-414.
- Noguchi H, Ueda M, Matsumoto S, Kobayashi N, Hayashi S (2007) BETA2/NeuroD protein transduction requires cell surface heparan sulfate proteoglycans. Hum Gene Ther 18: 10-17.
- Noguchi H, Bonner-Weir S, Wei FY, Matsushita M, Matsumoto S (2005) BETA2/NeuroD protein can be transduced into cells due to an arginine- and lysine-rich sequence. Diabetes 54: 2859-2866.
- Noguchi H, Kaneto H, Weir GC, Bonner-Weir S (2003) PDX-1 protein containing its own antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes 52: 1732-1737.
- Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, et al. (2006) Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 12: 301-303.
- Noguchi H, Ueda M, Hayashi S, Kobayashi N, Nagata H, et al. (2007) Comparison of M-Kyoto solution and histidine-tryptophan-ketoglutarate solution with a trypsin inhibitor for pancreas preservation in islet transplantation. Transplantation 84: 655-658.
- Ikeda H, Kobayashi N, Tanaka Y, Nakaji S, Yong C, et al. (2006) A newly developed bioartificial pancreas successfully controls blood glucose in totally pancreatectomized diabetic pigs. Tissue Eng 12: 1799-1809.
- Noguchi H, Ueda M, Nakai Y, Iwanaga Y, Okitsu T, et al. (2006) Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am J Transplant 6: 496-504.
- Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, et al. (2002) A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes 51: 3435-3439.
- Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, et al. (1995) Growth factor/matrix-induced proliferation of human adult beta-cells. Diabetes 44: 1458-1460.
- Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, et al. (2000) In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A 97: 7999-8004.
- Halvorsen TL, Beattie GM, Lopez AD, Hayek A, Levine F (2000) Accelerated telomere shortening and senescence in human pancreatic islet cells stimulated to divide in vitro. J Endocrinol 166: 103-109.
- Webster C, Blau HM (1990) Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet 16: 557-565.
- Decary S, Mouly V, Butler-Browne GS (1996) Telomere length as a tool to monitor satellite cell amplification for cell-mediated gene therapy. Hum Gene Ther 7: 1347-1350.
- Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, et al. (1997) Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther 8: 1429-1438.
- Berghella L, De Angelis L, Coletta M, Berarducci B, Sonnino C, et al. (1999) Reversible immortalization of human myogenic cells by site-specific excision of a retrovirally transferred oncogene. Hum Gene Ther 10: 1607-1617.
- Sortwell CE, Sagen J (1993) Induction of antidepressive activity by monoaminergic transplants in rat neocortex. Pharmacol Biochem Behav 46: 225-230.
- Eaton MJ, Frydel BR, Lopez TL, Nie XT, Huang J, et al. (2000) Generation and initial characterization of conditionally immortalized chromaffin cells. J Cell Biochem 79: 38-57.
- Eaton MJ, Martinez M, Karmally S, Lopez T, Sagen J (2000) Initial characterization of the transplant of immortalized chromaffin cells for the attenuation of chronic neuropathic pain. Cell Transplant 9: 637-656.
- Eaton MJ, Herman JP, Jullien N, Lopez TL, Martinez M, et al. (2002) Immortalized chromaffin cells disimmortalized with Cre/lox site-directed recombination for use in cell therapy for pain after partial nerve injury. Exp Neurol 175: 49-60.
- Begue JM, Guguen-Guillouzo C, Pasdeloup N, Guillouzo A (1984) Prolonged maintenance of active cytochrome P-450 in adult rat hepatocytes co-cultured with another liver cell type. Hepatology 4: 839-842.
- Perrot N, Chesné C, De Waziers I, Conner J, Beaune PH, et al. (1991) Effects of ethanol and clofibrate on expression of cytochrome P-450 enzymes and epoxide hydrolase in cultures and cocultures of rat hepatocytes. Eur J Biochem 200: 255-261.
- Watanabe T, Shibata N, Westerman KA, Okitsu T, Allain JE, et al. (2003) Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation 75: 1873-1880.
- Noguchi H, Nakai Y, Ueda M, Masui Y, Futaki S, et al. (2007) Activation of c-Jun NH2-terminal kinase (JNK) pathway during islet transplantation and prevention of islet graft loss by intraportal injection of JNK inhibitor. Diabetologia 50: 612-619.
- Noguchi H, Nakai Y, Matsumoto S, Kawaguchi M, Ueda M, et al. (2005) Cell permeable peptide of JNK inhibitor prevents islet apoptosis immediately after isolation and improves islet graft function. Am J Transplant 5: 1848-1855.
- Noguchi H, Matsushita M, Okitsu T, Moriwaki A, Tomizawa K, et al. (2004) A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med 10: 305-309.
- Loréal O, Levavasseur F, Fromaget C, Gros D, Guillouzo A, et al. (1993) Cooperation of Ito cells and hepatocytes in the deposition of an extracellular matrix in vitro. Am J Pathol 143: 538-544.
- Rojkind M, Novikoff PM, Greenwel P, Rubin J, Rojas-Valencia L, et al. (1995) Characterization and functional studies on rat liver fat-storing cell line and freshly isolated hepatocyte coculture system. Am J Pathol 146: 1508-1520.
- Bhatia SN, Balis UJ, Yarmush ML, Toner M (1999) Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J 13: 1883-1900.
- Noguchi H, Kobayashi N, Westerman KA, Sakaguchi M, Okitsu T, et al. (2002) Controlled expansion of human endothelial cell populations by Cre-loxP-based reversible immortalization. Hum Gene Ther 13: 321-334.
- Matsumura T, Takesue M, Westerman KA, Okitsu T, Sakaguchi M, et al. (2004) Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation 77: 1357-1365.
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, et al. (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279: 349-352.
- Halvorsen TL, Leibowitz G, Levine F (1999) Telomerase activity is sufficient to allow transformed cells to escape from crisis. Mol Cell Biol 19: 1864-1870.
- Zhu J, Wang H, Bishop JM, Blackburn EH (1999) Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc Natl Acad Sci U S A 96: 3723-3728.
- Ying W, Fei H, Jun D, Xi-chuan Y, Bai-yu Z, et al. (2007) Reversible transfection of human melanocytes mediated by Cre/loxP site-specific recombination system and SV40 large T antigen. Exp Dermatol 16: 437-444.
- Kowolik CM, Liang S, Yu Y, Yee JK (2004) Cre-mediated reversible immortalization of human renal proximal tubular epithelial cells. Oncogene 23: 5950-5957.
- Lin H, Liu H, Sun Q, Yuan G, Zhang L, et al. (2013) Establishment and characterization of a tamoxifen-mediated reversible immortalized mouse dental papilla cell line. In Vitro Cell Dev Biol Anim 49: 114-121.
- Zhang Y, Nuglozeh E, Touré F, Schmidt AM, Vunjak-Novakovic G (2009) Controllable expansion of primary cardiomyocytes by reversible immortalization. Hum Gene Ther 20: 1687-1696.
- Qiu HY, Fujimori Y, Nishioka K, Yamaguchi N, Hashimoto-Tamaoki T, et al. (2006) Postnatal neovascularization by endothelial progenitor cells immortalized with the simian virus 40T antigen gene. Int J Oncol 28: 815-821.
- Nishioka K, Fujimori Y, Hashimoto-Tamaoki T, Kai S, Qiu H, et al. (2003) Immortalization of bone marrow-derived human mesenchymal stem cells by removable simian virus 40T antigen gene: analysis of the ability to support expansion of cord blood hematopoietic progenitor cells. Int J Oncol 23: 925-932.
- Wei P, Li L, Qi H, Zhou HX, Deng CY, et al. (2012) Reversible immortalization of Nestin-positive precursor cells from pancreas and differentiation into insulin-secreting cells. Biochem Biophys Res Commun 418: 330-335.
- García-Escudero V, García-Gómez A, Gargini R, Martín-Bermejo MJ, Langa E, et al. (2010) Prevention of senescence progression in reversibly immortalized human ensheathing glia permits their survival after deimmortalization. Mol Ther 18: 394-403.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15454
- [From(publication date):
June-2013 - Dec 19, 2025] - Breakdown by view type
- HTML page views : 10647
- PDF downloads : 4807