Research Article Open Access
Dictyostelium Genes Dysregulated in an O-Glycosylation Mutant Identified by mRNA Differential Display
Motonobu Yoshida1*, Naoya Sakuragi1, Eiji Tanesaka1 and Yutaka Sendai21Department of Agricultural Science, Kinki University, Nakamachi, Nara 631-8505, Japan
2Research Institute for the Functional Peptides, Shimojyo-cho, Yamagata 990-0823, Japan
- Corresponding Author:
- Motonobu Yoshida
Department of Agricultural Science, Kinki University
Nakamachi, Nara 631-8505, Japan
Tel: +81 742 43 5245
Fax: +81 742 43 1155
E-mail: yoshida_m@nara.kindai.ac.jp
Received date: October 27, 2012; Accepted date: November 29, 2012; Published date: December 02, 2012
Citation: Yoshida M, Sakuragi N, Tanesaka E, Sendai Y (2012) Dictyostelium Genes Dysregulated in an O-Glycosylation Mutant Identified by mRNA Differential Display. J Biotechnol Biomater 2:152. doi:10.4172/2155-952X.1000152
Copyright: © 2012 Yoshida M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Seven differentially-expressed cDNA clones were isolated by using an mRNA differential display between a Dictyostelium wild-type AX2 and a mutant HG794 defective in O-glycosylation. Transcript levels for the seven clones were reduced or not detectable in the mutant HG794. Homology search showed that the four cDNA clones, DD-3 and DD-7~9 are novel and that three cDNA clones, DD-4 and DD-5, -6 encode an actin-bundling protein and phosphodiesterase inhibitors, respectively. Full-length cDNAs for DD-3 and -8 were isolated and labeled DD3-3 and DD8-14, respectively. DD3-3 consists of 2,166 bp and DD8-14 of 2,084 bp. DD3-3 was preliminarily reported in a previous paper [1]. SSL850 was named a clone by the “Dictyostelium cDNA Project in Japan”, containing a fulllength cDNA for DD-7 and was labeled DD7-1 of 902 bp. It has 60% homology with discoidin Ia. DD8-14 most likely has no direct role in glycosylation, while DD3-3 and DD7-1 very likely are involved in some aspect of recognition of glycosylation.
Keywords
Dictyostelium; O-glycosylation; Differential display; Regulatory factor; CSA glycoprotein
Introduction
Evidence has accumulated that carbohydrates in glycoproteins or glycolipids are involved in cell-cell adhesion, differentiation, recognition, modulation, cancer invasion and inflammation [2]. Glycoproteins may be N-glycosylated and/or O-glycosylated or may carry N- and/ or O-linked carbohydrates. For N-linked carbohydrates a number of methods have been developed which determine the structures by rather simple biochemical and tissue culture techniques. In contrast, no enzyme is known which would remove O-linked carbohydrates from glycoproteins irrespective of their structure. In addition, at present no efficient inhibitor of O-linked carbohydrates biosynthesis is known. In light of these points, it is quite difficult to analyze the structure and function of O-linked carbohydrates by a biochemical approach. Nowadays, a genomic approach would be more suitable in the research of O-linked carbohydrates than a biochemical one.
In this study we conducted an mRNA differential display between a wild-type and a mutant defective in O-glycosylation of Dictyostelium discoideum to identify the genes involved in O-glycosylation. There are few cases of application of differential display to mutants [3]. In this study, a set of 80 arbitrary primers was used, and the effectiveness of this method was shown by isolation of the gene for a phosphodiesterase inhibitor defective in the mutant HG794 [4].
In D. discoideum, EDTA-resistant cell contact is specifically expressed at the aggregation-competent stage [5]. It is formed through a cell adhesion molecule, csA, with an apparent molecular mass of 80 kDa. The csA glycoprotein is probably involved in multicellular formation in the aggregation-competent stage. The csA glycoprotein retains two types of carbohydrates: N-linked carbohydrate I, which is sulfated [6], and O-linked carbohydrate II, which is mainly recognized by wheat germ agglutinin [7]. Murray et al. [8] have isolated a mod B mutant HL220 defective in O-linked carbohydrate II. Due to a lack of carbohydrate II, the mutant HL220 cells show much weaker EDTA-resistant cell contact and synthesize the csA glycoprotein with an apparent molecular mass of 68 kDa [7,9,10]. It is conceivable that O-linked carbohydrate II plays an important role in EDTA-resistant cell contact [11]. A complementation test between the mod B mutant HL220 and another mutant HG794 without carbohydrate II suggests that more than two genes could be involved in O-glycosylation by carbohydrate II [12]. By using an mRNA differential display between the mutant HL220 and a wild-type AX2, we have isolated three kinds of genes for a protein tyrosine kinase, an adenylyl cyclase, and a protein kinase C inhibitor [13]. Concerning downregulation of the three kinds of genes, two possibilities were considered: (i) a transcriptional regulatory factor(s) which regulates the transcripts of the three genes is defective in the mutant HL220, or (ii) the mutant HL220 is defective in O-glycosylation of regulatory factor(s) including the cell adhesion molecule, csA, whose carbohydrate II is O-glycosidicallylinked carbohydrates [14,15]. Although O-linked carbohydrate II is different from O-linked N-acetylglucosamine (O-GlcNAc), there are common features between both carbohydrate modifications: the presence of O-glycosidically-linked GlcNAc and O-phosphorylation in O-glycosylated proteins [16,17]. Some evidences suggest that reciprocal modification of O-GlcNAc and O-phosphorylation is indeed functional in transcriptional regulation [18,19]. To determine whether either possibility is likely, we conducted the mRNA differential display between the wild-type AX2 and another mutant HG794 defective in O-glycosylation in place of the mutant HL220.
Materials and Methods
Cell culture and development
A mutant HG794 cell was isolated as reported previously [4]. A parent strain HG592 derived from wild-type AX2, was mutagenized with N-methyl-N’-nitro-N-nitrosoguanidine (MNNG). The MNNG- treated HG592 cells were cultivated for several generations in nutrient medium and the mutant HG794 cells were isolated from them as a carbohydrate II negative mutant by using a cell sorter. Wild-type AX2 and the mutant HG794 cells were cultivated at 22°C in a nutrient medium with 1.8% maltose [20]. Development was begun by washing the cells in 17 mM phosphate buffer, pH 6.1. The cells were resuspended in the same buffer at a density of 1x107/ml. They were agitated on a rotatory shaker at 150 rpm, or spread on an agar plate, and then incubated at 22°C for the indicated times.
Preparation of total RNA
Wild-type AX2 and mutant HG794 cells were developed for 3 h after starvation began. Total RNA from the respective cells was extracted by 4 M guanidine isothiocyanate/1% 2-mercaptoethanol and isolated by a CsCl density gradient centrifugation according to Sumbrook and Russell [21].
Differential display
Differential display was performed using an RNAmapTM kit with 80 primers combination between T12MN and AP1-20 (GenHunter Corp., Brooklene, MA) [22]. Total RNA of 0.2 μg from each cell was reverse-transcribed in 20 μl of the reaction mixture (0.1 unit of Moloney murine leukemia virus reverse transcriptase (RT), 1 μM anchored oligo-dT primer T12MN, and 20 μM dNTPs) at 37°C for 1 h. The reaction was inactivated at 95°C for 5 min. One-tenth of RT products was used as the polymerase chain reaction (PCR) templates in 20 μl of the reaction mixture. The reaction mixture for PCR consisted of 1 μM anchored oligo-dT primer T12MN, 0.2 μM arbitrarily designed 10-mer AP1-20 primer, 2 μM dNTPs, 1 unit Taq polymerase (Roche Diagnostics, Mannheim, Germany), and 1 μCi [α-35S]dATP (GM Healthcare, Buckinghamshire, UK). Eighty set primers were used in combination with 4 kinds of anchored oligo-dT primer T12MN and 20 kinds of arbitrarily designed 10-mer AP1-20 primers. The PCR was performed for 40 cycles of 94°C for 30 s, 40°C for 2 min and 72°C for 30 s, followed by a final elongation step at 72°C for 5 min. Samples were analyzed on a 6% (w/v) polyacrylamide gel; after the gel was dried, the bands were visualized by autoradiography using Kodak AR film (Eastman Kodak Co.). The regions containing differentially-expressed bands between wild-type AX2 and mutant HG794 were cut and placed in 100 μl of sterilized distilled water to rehydrate for 10 min. The cDNAs in bands were eluted from the gel by boiling for 15 min and precipitated by ethanol in the presence of glycogen (10 μg/sample). The precipitated samples were dissolved in 10 μl of sterilized distilled water. Four μl of them was used for reamplification by the PCR cycling in 40 μl of reaction mixture (1 μM T12MN, 0.2 μM AP primer, 20 μM dNTPs, 1 unit Taq polymerase). The PCR products analyzed by electrophoresis with a 1.5% (w/v) agarose gel were subsequently extracted using an EASYTRAPTM DNA purification kit (TaKaRa Shuzo Co. Ltd., Kyoto, Japan), and were cloned into pBluescript II (Stratagene, La Jolla, CA). After ligation into pBluescript II, the isolation of a clone was confirmed by getting the same nucleotide sequences from the respective three colonies. DNA sequencing was performed in both directions using a fluorescent-labeled primer method according to the manufacturer’s protocol. The fluorescent-labeled reaction products were analyzed with a DNA sequencer (Model 373S; Applied Biosystems, Inc., Foster City, CA). The determined sequences were then compared with sequences in the GenBank™ data bank.
Northern blotting analysis
Northern blotting analysis was performed using a cDNA probe, which was labeled with digoxigenin (DIG) by a random hexamer procedure using a DIG DNA labeling kit (Roche Diagnostics). About 5.5 μg of total RNAs extracted from wild-type AX2 and mutant HG794 cells was subjected to 1% agarose/formaldehyde gel electrophoresis and transferred onto Hybond™ N+nylon membrane (GM Healthcare) with a vacuum blotter GENOPIRATOR (ATTO Co. Ltd., Tokyo, Japan). The RNAs were then fixed to the membrane by exposure to UV irradiation prior to hybridization with cDNA probes. The membrane was prehybridized for 1 h at 65°C in hybridization buffer (6x SSC: 0.9 M NaCl, 90 mM sodium citrate, pH 7.0), 5X Denhardt’s solution (0.5% Ficoll, 0.5% polyvinylpyrrolidone, 0.5% bovine serum albumin), 0.1% SDS, and 100 μg/ml heat-denatured salmon sperm DNA. The membrane was incubated overnight with a cDNA probe. After hybridization, the membrane was washed twice with 2X SSC, 0.1% SDS for 30 min at 65°C. Next, the membrane was briefly washed in TTBS of 10 mM Tris-HCl, 500 mM NaCl, pH 7.4 containing 0.1% Tween 20 and treated with the blocking solution of TTBS containing 0.2% casein for 30 min at room temperature, and incubated with anti-DIG-AP diluted 1:10,000 of blocking solution. After incubation for 1 h at room temperature, the membrane was washed at least 3 times with TTBS and equilibrated for 5 min in substrate buffer of 100 mM diethanolamine, 1 mM MgCl2, 0.02% sodium azide, pH 10. The equilibrated membrane was incubated in the substrate buffer containing 100 μg/ml of CSPD (disodium 4-chloro-3-(methoxyspiro {1,2-dioxetane-3, 2’-(5’-chloro) tricyclo [3.3.1.13.7] decan}-4-yl) phenylphosphate, TROPIX, Bedford, MA) as fluorescent substrate. The messenger RNAs that reacted with a cDNA probe were visualized by exposure to Hyperfilm-ECL (GM Healthcare).
Gene cloning
For a cDNA library, poly (A)+ RNA was purified from total RNA of AX2 cells starved for 3 h using a poly (A)+ RNA purification kit (GM Healthcare) according to the manufacturer’s specifications. The cDNAs were made from 5 μg of poly (A)+ RNA using a cDNA synthesis kit (GM Healthcare). They were blunted, ligated to EcoRI adaptors, kinased, and size-fractionated by column chromatography. Fractions containing cDNAs of longer than 500 base pairs were collected, precipitated by ethanol and ligated into ZAP II arms (Stratagene, La Jolla, CA). The ligated DNAs were packaged using a GIGA Pack III Gold packaging kit (Stratagene, La Jolla, CA). To identify the missing 5′ end of DD-3, DD-7, and DD-8, the cDNA library was screened using 32P-labeled DD- 3, -7, and -8 as a probe, respectively. Random labeling was performed with a Radprime DNA labeling system (Invitrogen, Carlsbad, CA, USA) and 20 μCi [32P]dCTP (GM Healthcare) according to the protocol of the manufacturer. Hybridization was carried out at 42°C overnight in 6x SSPE containing 0.05% nonfat dried milk and 50% formamide. Filters were first washed in 2X SSC, 0.1% SDS at room temperature and then in 1X SSC, 0.1% SDS at 68°C. Positive clones were sequenced with a DNA sequencer (Model 373S). The determined sequences were then compared with the sequences registered in the GenBank™ data bank. Open reading frames were predicted by using GENETXY- MAC 8.0. Homology searches of nucleotide and deduced amino acid sequences were carried out using software from the National Center for Biotechnology Information (NCBI) and Simple Modular Architecture Research Tool (SMART).
Results and Discussion
Identification and isolation of differentially-expressed cDNA clones
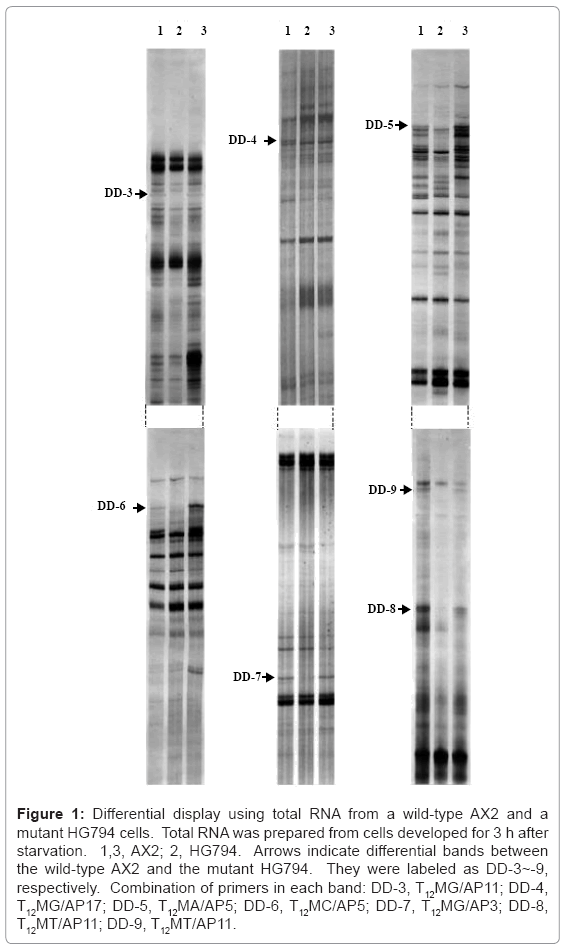
We tried to isolate novel genes involved in O-glycosylation by carrying out an mRNA differential display between a Dictyostelium wild-type AX2 and a mutant HG794 defective in O-glycosylation. At first, we identified 27 differentially-expressed bands, followed by cloning the bands to pBluescript II, based on size ranging from 300 to 500 bp. As a result, seven clones were isolated and labeled DD-3~9, respectively (Figure 1).
Figure 1: Differential display using total RNA from a wild-type AX2 and a mutant HG794 cells. Total RNA was prepared from cells developed for 3 h after starvation. 1,3, AX2; 2, HG794. Arrows indicate differential bands between the wild-type AX2 and the mutant HG794. They were labeled as DD-3~-9, respectively. Combination of primers in each band: DD-3, T12MG/AP11; DD-4, T12MG/AP17; DD-5, T12MA/AP5; DD-6, T12MC/AP5; DD-7, T12MG/AP3; DD-8, T12MT/AP11; DD-9, T12MT/AP11.
The results from the homology search showed that four clones, DD-3 and DD-7~9 are novel and three clones, DD-4~6, are known. The product encoded by DD-4 is an actin-bundling protein, and products of DD-5 and -6 are phosphodiesterase inhibitors. In a previous paper [13], we have isolated three genes, a protein tyrosine kinase, an adenylyl cyclase, and a protein kinase C inhibitor whose expression is developmentally regulated and is functional in a signaling system. Since the transcript level for a phosphodiesterase inhibitor is developmentally regulated and a phosphodiesterase inhibitor is functional in a signaling system [23], it was highly correlated with the properties of the three genes isolated in a previous paper [13]. A parent strain HG592 used for isolation of the mutant HG794 shows reduced activities of an extracellular phosphodiesterase and a phosphodiesterase inhibitor [9,12]. These phenotypic characteristics are assigned to linkage group II [24]. Therefore, the reduced transcripts for a phosphodiesterase inhibitor, DD-5 and -6 were most likely derived from the parent strain HG592. That DD-5 and -6 encoding a phosphodiesterase inhibitor were isolated by differential display indicates the effectiveness of this method for isolating genes that are defective in mutants.
This is one of the reasons why wild-type AX2 was used instead of the parent strain HG592 in differential display of this study. The parent strain HG592 is defective in a signaling system including an extracellular phosphodiesterase and a phosphodiesterase inhibitor, but expresses a 80-kDa csA with N-linked carbohydrate I and O-linked carbohydrate II. On the other hand, the mutant HG794 expresses the 68-kDa csA without O-linked carbohydrate II. The product for DD-4 is an actin-bundling protein that is enriched in cell contact regions [25]. One of the major glycoproteins for O-glycosylation is a cell adhesion molecule, csA. A correlation might exist between an actin-bundling protein and O-glycosylation modification. However, four novel genes were assumed to be candidates required for O-glycosylation. Therefore, we tried to isolate a full-length cDNA clone for the novel DD-3, -7, -8, -9 genes to clarify the entire structure of each gene as a candidate gene for O-glycosylation.
Isolation and characterization of full-length cDNA clones
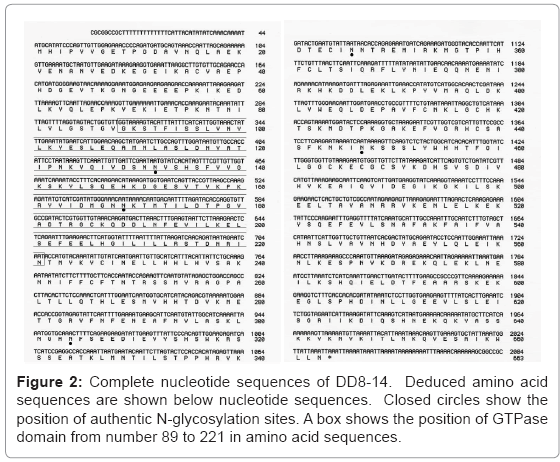
We isolated full-length cDNA clones for DD-3, -7, and -8 by using a respective clone as a probe and the cDNA library, prepared newly from the wild-type AX2 cells that were allowed to develop for 3 h. However, a full-length cDNA for DD-9 was not isolated because of the inferiority of the A-rich probe. A full-length cDNA clone was isolated and labeled as DD3-3 (AB120173) with 2,166 bp for DD-3 as partially reported in a previous paper [1] and DD8-14 (AB120174) with 2,084 bp for DD-8 (Figure 2). During screening using DD-7 as a probe, we found that SSL850, received from Tsukuba University as a clone of the “Dictyostelium cDNA Project in Japan”, contained a full-length cDNA for DD-7. SSL850 was labeled DD7-1 (AB120175) and compared with a complete DD7-1 nucleotide sequence of 902 bp with the SSL850 nucleotide sequence of 914 bp available to the public at; http://www. csm.biol.tsukuba.ac.jp/cDNA project html (Figure 3). The amino acid sequence deduced from DD7-1 were 60% homologous to that of discoidin Ia [26], an endogenous C-type lectin, including RGD of the cell attachment sequences, while the amino acid sequence deduced from DD8-14 has GTPase domain, containing a predicted MMR_HSR1 motif [27]. As reported in a previous paper [1], the homology search showed that part of a deduced amino acid sequence from DD3-3 was 41% homologous to clone AAL56441.1 from Oikopleura dioica [28]. It is of potential interest that DD3-3-like proteins are found in other organisms such as Branchiostoma floridae, Nematostella vectensis, Ciona intestinalis, and Strongylocentrotus purpuratus [29-32]. From point of view of molecular evolution and phylogeny, the function of DD3-3 is most intriguing, and would be expanded to a new field other than glycosylation.
Figure 3: Complete nucleotide sequences of DD7-1. Deduced amino acid sequences are shown below nucleotide sequences, and authentic N-glycosylation sites are represented by closed circles. In (A) a box shows discoidin domain from number 16 to 149 in amino acid sequences. A bold line indicates the position of RGD, cell attachment sequence. In (B) comparison of amino acid sequences among DD7-1, SSL870, and discoidin Ia (Dis) was shown, and common amino acids among DD7-1, SSL870, and Dis were indicated with a box.
Motif searches for DD3-3, DD7-1, and DD8-14 showed that the amino acid sequences deduced from DD3-3 contain five authentic sites for N-glycosylation and two phosphorylation sites; those from DD7-1 contain four authentic sites for N-glycosylation and one cell attachment site and those from DD8-14 contain five authentic sites for N-glycosylation and one ATP/GTP phosphorylation site.
Northern blotting analysis for DD3-3, DD7-1, DD8-14
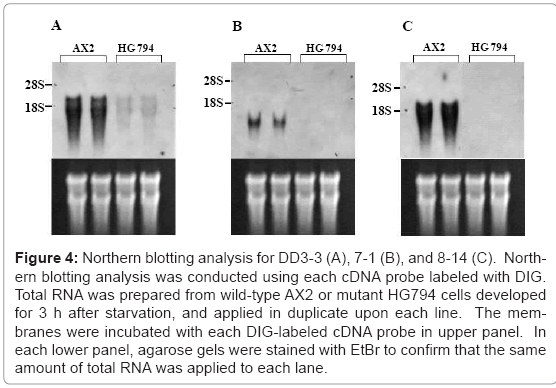
The results from a Northern blotting analysis showed that transcript levels for DD3-3, DD7-1, and DD8-14 were reduced in the mutant HG794 (Figure 4). The parental strain HG592 for mutant HG794 showed the same expression pattern as wild-type AX2. As shown in Figure 5, transcripts for DD3-3, and DD7-1 were detected in the growth phase and the transcript levels were not regulated developmentally for 12 h after starvation, although those for DD-3 increased considerably and those for DD7-1 decreased remarkably at 6 h after starvation began. On the other hand, the transcript for DD8-14 was scarcely detected in the growth phase, and the transcript level was regulated developmentally with an expression peak at 3 h after starvation (Figure 5). In a previous paper [11] we have reported that carbohydrate II-related precursor is synthesized during the growth phase. That O-glycosylation modification by carbohydrate II occurs during the growth phase should coincide with the results of a Northern blotting analysis. DD8-14 might not be an appropriate candidate gene for O-glycosylation because the transcript for DD8-14 was barely detected in the growth phase, and it was developmentally regulated. Therefore, DD8-14 most likely has no direct role in glycosylation. The product encoded by DD7-1 might be a soluble protein because it does not contain any signal sequences. It is known that O-glycosylation occurs in the Golgi apparatus [15]. However, the results by an immunofluorescence microscopy and an immunoelectron microscopy using antibody against DD7-1 product showed that DD7-1 product localized on cell membrane surface and in the Golgi apparatus as well as cytoplasm. DD3-3 is a good candidate for O-glycosylation because it has a signal sequence for membrane attachment and the transcript was expressed invariably after starvation (Figure 5).
Figure 4: Northern blotting analysis for DD3-3 (A), 7-1 (B), and 8-14 (C). Northern blotting analysis was conducted using each cDNA probe labeled with DIG. Total RNA was prepared from wild-type AX2 or mutant HG794 cells developed for 3 h after starvation, and applied in duplicate upon each line. The membranes were incubated with each DIG-labeled cDNA probe in upper panel. In each lower panel, agarose gels were stained with EtBr to confirm that the same amount of total RNA was applied to each lane.
Figure 5: Northern blotting analysis of DD3-3 (A), DD7-1 (B), and DD8-14 (C). Northern blotting analysis was conducted using each cDNA probe labeled with DIG. Total RNA from wild-type AX2 cells developed for 0 h (0), 3 h (3), 6 h (6), 9 h (9), and 12 h (12) was applied to each lane. The membranes were incubated with each DIG-labeled cDNA probe in upper panel. In each lower panel, agarose gels were stained with EtBr to confirm that the same amount of total RNA was applied to each lane.
As reported in a previous paper [13], the mutant HL220 might be defective in a regulatory gene or in an O-glycosylation of regulatory factor(s), which can control the transcription of genes for a protein tyrosine kinase, an adenylyl cyclase, and a protein kinase C inhibitor. Indeed, the mutant HL220 is defective in O-linked carbohydrate II. Although carbohydrate II containing O-glycosidically-linked GlcNAc is different from O-linked N-acetylglucosamine (O-GlcNAc), both carbohydrates retain common features such as the presence of O-glycosidically-linked GlcNAc and O-phosphorylation in O-glycosylated proteins [14-17]. The lack of O-glycosylation in the mutants HL220 and HG794 might be due to a defect in a regulatory gene or O-glycosylation modification of regulatory factor(s). In this study we conducted an mRNA differential display between the wild- type AX2 and another mutant HG794 defective in O-glycosylation to determine whether either possibility is likely in these mutant cells. Should the mutation of a regulatory gene for O-glycosylation lead to O-glycosylation deficiency in mutants HL220 and HG794, then genes as isolated in a previous study might be detected in this study. Combined with previous results [13], it is conceivable that mutant HG794 is defective in O-glycosylation of regulatory factor(s) rather than mutated in regulatory genes. Among novel genes; DD3-3, DD7-1, and DD8-14, a knockout mutant for DD3-3 showed downregulation for a specific phosphodiesterase [1]. This defect might result in the upregulation of O-phosphorylation, and reciprocally the downregulation of O-glycosylation of regulatory factor(s) as suggested by Hart [18]. The properties of products for the other novel gene DD7-1 are probably related to glycosylation and regulation. These results suggest that modification of regulatory factor(s) is defective in both mutants HG794 and HL220, coinciding with the fact that multiple genes were isolated by mRNA differential display of both mutants. In future work, functions for novel genes should be clarified.
Acknowledgements
We thank the “Dictyostelium cDNA Project in Japan” for a gift of SSL850 clone, and Drs. Peter Hare and Gregory Jedd for critical reading of the manuscript, and Y. Taniguchi and S. Hamada for their technical assistance. This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, No.18580341, and by Special Coordination Funds for Promoting Science and Technology of the Science and Technology Agency of the Japanese Government.
The nucleotide sequences reported in this paper have been submitted to the DDBJ, EMBL, and GenBank under accession numbers AB120173, AB120174, and AB120175.
References
- Sakuragi N, Ogasawara N, Tanesaka E, Yoshida M (2005) Functional analysis of a novel gene, DD3-3, from Dictyostelium discoideum. Biochem Biophys Res Commun 331: 1201-1206
- Fukuda M (2000) Cell surface carbohydrates: cell type-specific expression In: Fukuda M, Hindsgaul O (eds) Molecular and cellular glycobiology Oxford University Press, New York, pp 1-61
- Scutt CP, Vinauger-Douard M, Fourquin C, Ailhas J, Kuno, N, et al. (2003) The identification of candidate genes for a reverse genetic analysis of development and function in the Arabidopsis gynoecium. Plant Physiol 132: 653-665
- Yoshida M, Iizuka Y (1989) Isolation of an aggregation-less mutant of Dictyostelium discoideum with the expression of contact site A glycoprotein. Cell Struct Funct 14: 625-636
- Beug H, Katz FE, Gerisch G (1973) Dynamics of antigenic membrane sites relating to cell aggregation in Dictyostelium discoideum. J Cell Biol 56: 647-658
- Stadler J, Gerisch G, Bauer G, Suchanek C, Huttner WB (1983) In vivo sulfation of the contact site A glycoprotein of Dictyostelium discoideum. EMBO J 2: 1137-1143
- Yoshida M, Stadler J, Bertholdt G, Gerisch G (1984) Wheat germ agglutinin binds to the contact site A glycoprotein of Dictyostelium discoideum and inhibits EDTA-stable cell adhesion. EMBO J 3: 2663-2670
- Murray BA, Wheeler S, Jongens T, Loomis WF (1984) Mutations affecting a surface glycoprotein, gp80, of Dictyostelium discoideum. Mol Cell Biol 4: 514-519
- Gerisch G, Hagmann J, Hirth P, Weinhart U, Westphal M (1985) Early Dictyostelium development: control mechanisms bypassed by sequential mutagenesis. Cold Spring Harb Symp Quant Biol 50: 813-822
- Loomis WF, Wheeler SA, Springer WR, Barondes SH (1985) Adhesion mutants of Dictyostelium discoideum lacking the saccharide determinant recognized by two adhesion-blocking monoclonal antibodies. Dev Biol 109: 111-117
- Yoshida M (1991) Function of the carbohydrates in contact site A glycoprotein of Dictyostelium discoideum affected by tunicamycin. Comp Biochem Physiol B 98: 563-568
- Yoshida M, Ishida S, Iizuka Y (1991) Mutants of Dictyostelium discoideum with altered carbohydrate moieties of contact site A. Cell Struct Funct 16: 383-390
- Yoshida M, Sendai Y, Sakuragi N, Hotta Y (2000) Analysis of a mod B mutant in Dictyostelium discoideum using mRNA differential display. Plant Cell Physiol 41: 239-243
- Yoshida M, Takahashi K, Ohmori Y, Hayashi A (1997a) Characterization of a glycosylphosphatidylinositol-anchor in a cell adhesion molecule, csA, from Dictyostelium discoideum. J Carbohydr Chem 16: 647-653
- Yoshida M, Yokota S, Ouchi S (1997) Characterization and distribution of O-glycosylated carbohydrates in the cell adhesion molecule, contact site A, from Dictyostelium discoideum. Exp Cell Res 230: 393-398
- Schmidt JA, Loomis WF (1982) Phosphorylation of the contact site A glycoprotein (gp80) of Dictyostelium discoideum. Dev Biol 91: 296-304
- Lefebvre T, Ferreira S, Dupont-Wallois L, Bussiere T, Dupire MJ, et al. (2003) Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins--a role in nuclear localization. Biochim Biophys Acta 1619: 167-176
- Hart GW (1997) Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem 66: 315-335
- Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocyto- plasmic proteins: signal transduction and O-GlcNAc. Science 291: 2376-2378
- Watts DJ, Ashworth JM (1970) Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J 119: 171-174
- Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual Cold Spring Harbor Laboratory Press, New York
- Liang P, Pardee AB (1992) Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257: 967-971
- Wu L, Franke J (1990) A developmentally regulated and cAMP-repressible gene of Dictyostelium discoideum: cloning and expression of the gene encoding cyclic nucleotide phosphodiesterase inhibitor. Gene 91: 51-56
- Wallraff E, Welker DL, Williams KL, Gerisch G (1984) Genetic analysis of a Dictyostelium discoideum mutant resistant to adenosine 3':5'-cyclic phosphorothioate, an inhibitor of wild-type development. J General Microbiol 130: 2103-2114
- Fechheimer M, Murdock D, Carney M, Glover CVC (1991) Isolation and sequencing of cDNA clones encoding the Dictyostelium discoideum 30,000 dalton actin bundling protein. J Biol Chem 266: 2883-2889
- Berger EA, Clark JM (1983) Specific cell-cell contact serves as the developmental signal to deactivate discoidin I gene expression in Dictyostelium discoideum. Proc Natl Acad Sci USA 80: 4983-4987
- Bassler J, Kallas M, Hurt E (2006) The NUG GTPase reveals and N-terminal RNA-binding domain that is essential for association with 60 S pre-ribosomal particles. J Biol Chem 281: 24737-24744
- Seo HC, Kube M, Edvardsen RB, Jensen MF, Beck A, et al. (2001) Miniature genome in the marine chordate Oikopleura dioica. Science 294: 2506
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, et al. (2002) The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298: 2157-2167
- Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, et al. (2006) The genome of the sea urchin Strongylocentrotus purpuratus. Science 314: 941-952
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, et al (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317: 86-94
- Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, et al. (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453: 1064-1071
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14942
- [From(publication date):
December-2012 - Dec 08, 2025] - Breakdown by view type
- HTML page views : 10245
- PDF downloads : 4697