Research Article Open Access
Effect of the Essential Oils from Parsley and Fennel Seeds on the Growth of Lactobacillus Casei Subsp Rhamnosus
Ouis Nawel1*, Hariri Ahmed2 and El abed Donia zed11Laboratory of Chemistry, Department of Chemistry, Faculty of Sciences, University of Oran Es- Sénia, BP 1524 El M’naouer, 31.100, Oran, Algeria
2Bioconversion Laboratory, Microbiology Engineering and Health Safety, Faculty of Science the Nature and Life, University of Mascara BP.763, Sidi Said, Mascara, 29000, Algeria
- Corresponding Author:
- Ouis Nawel
Laboratory of Chemistry, Department of Chemistry
Faculty of Sciences, University of Oran Es- Sénia
BP 1524 El M’naouer
31.100, Oran, Algeria
Tel: + 213 45 82 09 27
Fax: + 213 45 82 09 27
E-mail: nawel_chim@yahoo.fr
Received date: January 03, 2012; Accepted date: March 20, 2012; Published date: March 22, 2012
Citation: Nawel O, Ahmed H, abed Donia zed El (2012) Effect of the Essential Oils from Parsley and Fennel Seeds on the Growth of Lactobacillus casei subsp rhamnosus. J Biotechnol Biomaterial 2:130. doi:10.4172/2155-952X.1000130
Copyright: © 2012 Nawel O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
In this paper the key odorants effects of parsley ( Petroselinum crispum Hoffm) and fennel ( Foeniculum vulgare ) in the growth of Lactobacillus casei subsp rhamnosus , were studied. Parsley and fennel essential oils were obtained by hydrodistillation with 0.14% yield from parsley and 1% from fennel. All experiments were carried out in a 2L jar fermenter with an initial volume of 1.5L at 42°C, and the constant, adjusted to 200 rpm. Lactobacilli suspensions were incubated at 42°C for 12hr at 200 rpm and then a 10% (v/v) of the bacterial suspension was added to the cultures. The culture pH was maintained at 6.25 by automatic addition of 25% (w/w) NH 4 OH. For that purpose, batch cultures on MRS medium containing 15 g.L -1 of glucose were realized: the first fermentation is considered as a control, the second is characterized by the addition of 20 μL of the parsley essential oil in the growth exponential phase and the third contains additionally 20 μL of the fennel essential oil. The results show that Lactobacillus casei subsp rhamnosus can produce up to 10.96 g.L -1 and 13.78 gL -1 of lactic acid on the control fermentation and on the second fermentation, respectively. The addition of the fennel essential oil provokes an inhibition of the Lactobacillus casei subsp rhamnosus growth which is translated by the obtained weak lactic acid concentration of 5.14 g.L -1 .
Keywords
Parsley; Fennel; Essential oil; Lactic acid production; Lactobacillus rhamnosus
Introduction
By their aromatic properties, the essential oil in plants present great interests in food, pharmaceutical, cosmetic and perfume technologies. The parsley (Petroselinum crispum Hoffm) and the fennel (Foeniculum vulgare L.) belonging to the Apiaceae family are considered as aromatic and medicinal plants used often in traditional medicine for their diuretic, vermifuge, emmenagogue and purgative properties [1,2]. Parsley is known for its antidiabetic [3], antimicrobial, antihypertensive, anticoagulant, antihyperlipidaemic, antihepatotoxic, membrane protective [4] and antioxidant [5] effects. Phytochemical analysises show the presence of avonoids, carotenoids, ascorbic acid, myristicin, apiole, terpenoids and coumarins [6-10].
The fennel is known for a long time as a medicinal plant used for the treatment of several stomach affections (aerophagia or diarrhoea) and obesity. Presently, the ripe fruit is still widely used in Arabian folk medicine systems as a diuretic, stimulant, appetizer, digestive and infantile febrifuge. The active principles have not yet been fully investigated in food and biotechnology. Because of the wide use of this plant, it was of interest to evaluate the activity of its essential oil. Searches made were concerned by the study of stigmasterol, stigmasterol palmitate, coumarin, phenol acids, essential oils, carbohydrates and hydroalcohol extracts [11-16]. The natural extracts stemming from this plant contain a variety of phenolic derivatives and essential oils with an inhibition bacteria proliferation power.
The lactic acid is a natural organic acid. It and its derivatives are widely used in food, pharmaceutical, leather and textile industries [17]. Furthermore, since lactic acid has an excellent reactivity that stems from the fact that it possesses both carboxylic and hydroxyl groups, it can undergo a variety of chemical conversions into potentially useful chemicals such as propylene oxide, propylene glycol, acrylic acid, 2,3-pentanedione and lactate ester [18,19]. Recently, there has been an increased interest in L-lactic acid production because it could be used as a raw material for the production of polylactic acid, a polymer used as special medical and environmental-friendly biodegradable plastic, and hence a substitute for synthetic plastics derived from petroleum feed stocks [20,21].
The objective of this work is the study of the effect of the parsley and fennel essential oil addition on the kinetics of the lactic acid production by Lactobacillus casei subsp rhamnosus.
Materials and Methods
Plant material
The plant materials (Petroselinum crispum and Foeniculum vulgare) were collected from the Mascara region in the northwest of Algeria.
Essential oil isolation
100g of seeds (Parsley or Fennel) were subjected to hydrodistillation for 5 hours. The distillate was extracted with pentane and dried over anhydrous sodium sulphate. The organic layer was then concentrated at 30°C using a rota vapour apparatus and the resulting essential oils were stored at 4°C.
Bacterial strain and media
The Lactobacillus casei subsp rhamnosus (LBC80 10D) strain used in all our experiments was supplied by the Rhone Poulenc group (France). It was maintained on MRS medium in the presence of 10% glycerol and preserved at -20°C. The MRS medium used in growth and inoculum preparations contained the following components (in g.L-1): 10 Soya peptone, 10 beef extract, 5 yeast extract, 2K2HPO4, 5 NaCH3CO2, 3H2O, 2 triammonium citrate, 0.2MgSO4, 7H2O, 0.05 MnSO4, H2O, 15 glucose and 1mL Tween 80. The pH was adjusted to 6.25 by 25% (w/w) NH4OH aqueous solution prior to sterilization at 108°C for 15min. The reactivation phase is realized after two successive transplantations at 42°C during 2 hours on liquid MRS medium [22].
Fermentation conditions and methods
All experiments were carried out in a 2L jar fermenter (Applikon Biocontroller ADI1030) with an initial volume of 1.5L at 42°C. The agitation speed was set at 200 rpm to insure complete mixing of the fermentation medium. The inocula were incubated at 42°C for 12hr at 200 rpm before their transfer to the fermenter in a 10%. The culture pH was maintained at 6.25 by automatic addition of 25% (w/w) NH4OH solution using a computer coupled peristaltic pump during the 24 hours of fermentation. The samples were withdrawn at desired intervals and frozen for further analysis. The fermentation batch (Control) of Lactobacillus casei subsp rhamnosus for production of L-lactic acid at a glucose concentration of 15 g.L-1 was carried out. Other cultures led in the same conditions were also prepared containing 20 μL of the parsley or fennel essential oil added during the exponential growth phase. The evolution of the biomass, the residual glucose amount and the lactic acid production are followed in regular time intervals.
Microbiologic method
Gram colouring is made regularly to control at the same time any risk of contagion and the presence of spores.
Analytical methods
The biomass is determined by measurement of the optical density (OD) at 570 nm by a spectrophotometer HITACHI 4-2000.
Culture samples were centrifuged (13200g at 4°C for 5 min), diluted and filtered. Residual glucose and lactic acid concentrations were determined by Multi parameter Medical Analyzer. The enzymatic kit used for the lactic acid dosage is the PAP Ref-61 192 and for the glucose dosage it is the Elitech diagnosis ref - GPSL-0500.
Data processing treatment
The calculation of the fermentation kinetic parameters requires a preliminary data processing (smoothing) of the rough experimental data with the software KALEIDAGRAPH. This data processing is based on the technique of the averages slipping by using a second degree polynomial.
Calculation of the fermentation kinetic parameters
The various analyses carried out allow the following time evolution of the component concentrations present in the culture medium:
[Biomass: X(OD) = f (t), sugars: S = f (t) and the metabolite produced (lactic acid): P = f (t)].
From these raw data it is possible to calculate the fermentation kinetic parameters in the batch culture by the calculation of the volumetric growth rate (r’’’x in g.L-1.h-1) and the specific growth rate (μ in h-1):
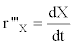
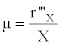
The calculation of the volumetric sugar consumption rate (r’’’s in g.L-1.h-1) and specific sugar consumption rate (Qs in g.g-1.h-1) is also possible:
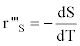
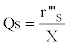
as well as the volumetric lactic acid production rate (r’’’p in g.L-1.h-1) and the specific lactic acid production rate (Ql.a in g.g-1.h-1) :
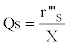
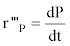
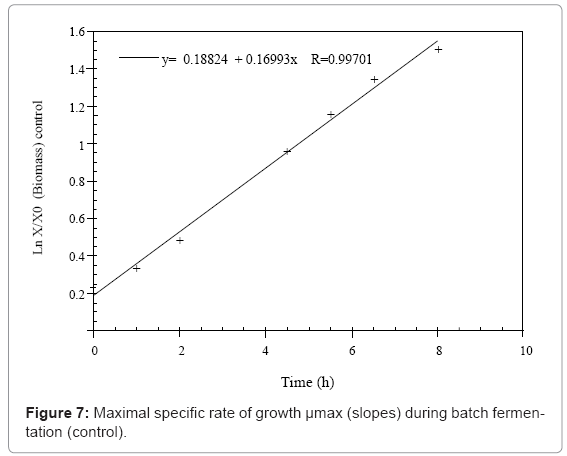
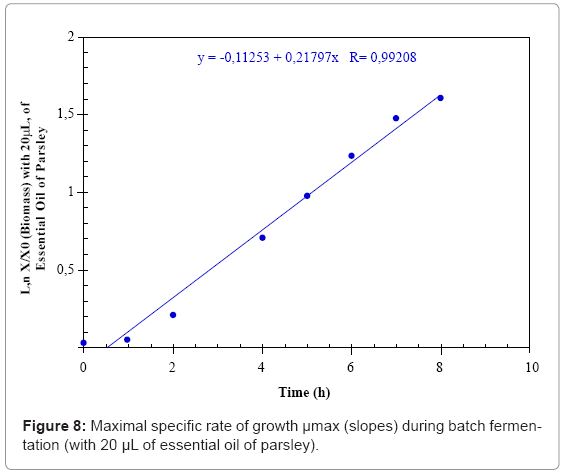
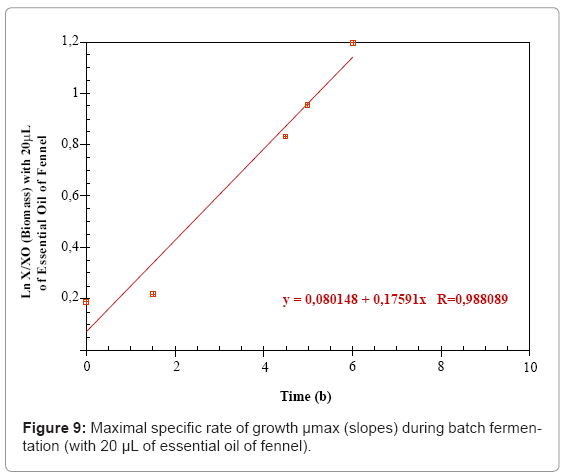
The maximal specific growth rate (μmax) was determined from the slopes of the plotted linear curve: lnX/X0= f (t).
Outputs in biomass and lactic acid (the slopes)
The biomass (Yx/s) and products (Yp/s) yields are defined as the mass ratios in biomass and metabolites formed per gram of consumed carbonaceous substrate. During this fermentation, we were interested in the yields of sugar conversion into biomass and lactic acid.
X0, S0, L0, represents the concentrations in respectively biomass, substrate and metabolites (lactic acid) produced at time t0 of the fermentation and X, S, L at time t of the fermentation.
The approach used to determine the outputs consists in plotting linear curves: [(X–X0) = f (S0–S)] and [(L.A–L.A0) = f(S0–S)]. The slopes obtained from these straight lines represent respectively the outputs of sugar conversion into biomass and lactic acid.
Results and Discussion
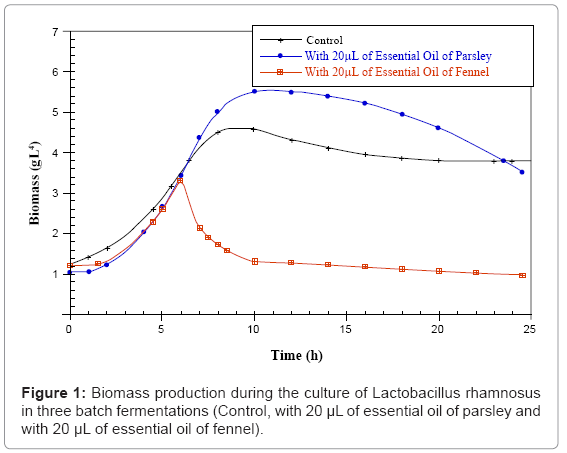
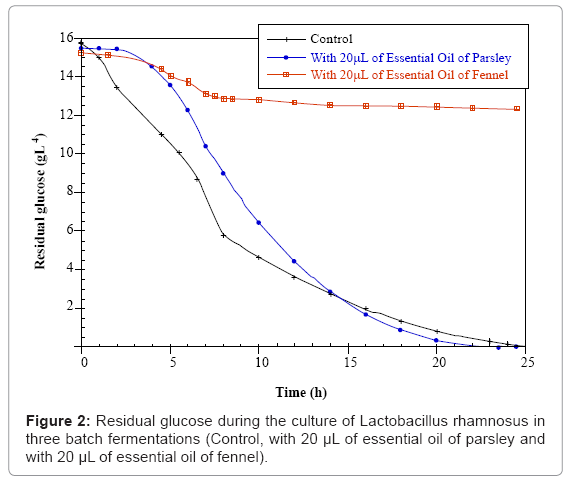
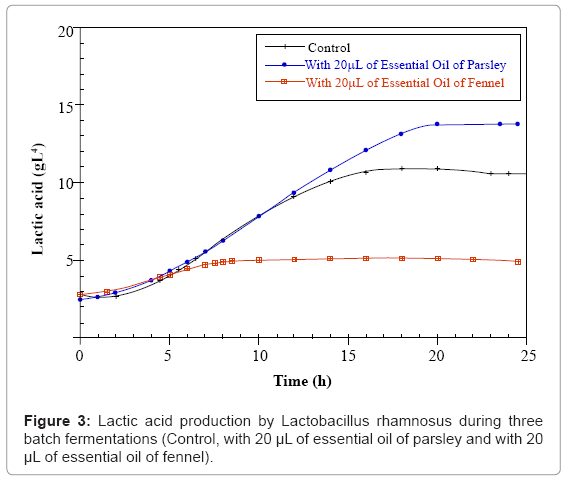
Parsley and fennel oils were obtained with respectively 0.14% and 1% yield based on dry weight. The results of the three batch fermentations of Lactobacillus casei subsp rhamnosus control; with 20 μL of parsley or fennel essential oil are presented in figures 1-3 respectively. We notice that the development of the concentrations in biomass, residual sugars and lactic acid occurs. The initial concentration in glucose (15g.L-1) was the same for the three samples. They were inoculated with the same quantity of biomass (1.2 g.L-1). According to the obtained results, it is observed that the Lactobacillus casei subsp rhamnosus growth for the three fermentations is characterized by a short duration of the latency phase which indicates that the inoculated cells were in full exponential phase.
For the control fermentation, it is observed a weak biomass initial concentration of 1.2 g.L-1 which increases after 6 hours of fermentation until 3.5 g.L-1, then until 4.575 g.L-1 after 8 hours of culture (at the end of the exponential phase). The growth end is due to the glucose exhaustion in the culture medium which reaches a final value of 0.008 g.L-1 after 24 hours of fermentation. During the stationary phase (after 8 hours of culture), the strain consumes the glucose and uses it only for the cellular maintenance. In parallel, the lactic acid production starts from a value of 3 g.L-1 and reaches 10.96 g.L-1 of lactate after 24 hours of fermentation. During this fermentation that lasted 24 hours the quantity of consumed sugar is 15 g.L-1.
However for the parsley fermentation, the Lactobacillus casei subsp rhamnosus biomass starts with an initial concentration of 1.2 g.L-1 and the parsley essential oil was added during the full exponential phase after 6 hours of culture corresponding to a biomass quantity of 3.5 g.L-1. After 10 hours of fermentation, cellular concentration increases at a value of 5.51 g.L-1. For the production of lactic acid the Lactobacillus casei subsp rhamnosus culture reaches the maximum value of 13.78 g.L-1after 24 hours of fermentation. In parallel and since there is an increase in the biomass and lactic acid amounts, there is sugar consumption and it remains at the end of the fermentation 0.007 g.L-1 of glucose.
For the fennel fermentation, the addition of the essential oil is operated after 6 hours of fermentation (3.5 g.L-1 of biomass). A lyses and a remarkable decrease of the cellular biomass is noticed in correlation with the fennel essential oil addition. The quantity of biomass decreases to a final value of 0.98 g.L-1 after 24 hours of fermentation. The medium content in glucose remains high till the end of the fermentation (12.33 g.L-1) and the quantity of lactic acid produced is sharply lower than that of the control culture (5.14 g.L-1) (Figures 1,2,3).
The comparison between the three batch fermentations of Lactobacillus casei subsp rhamnosus indicates that the addition of the parsley essential oil has a positive and very significant effect, since the growth rate and the lactate quantity are improved, as well as the quantity of consumed sugar that is more important compared to the fermentation case with the fennel essential oil addition.
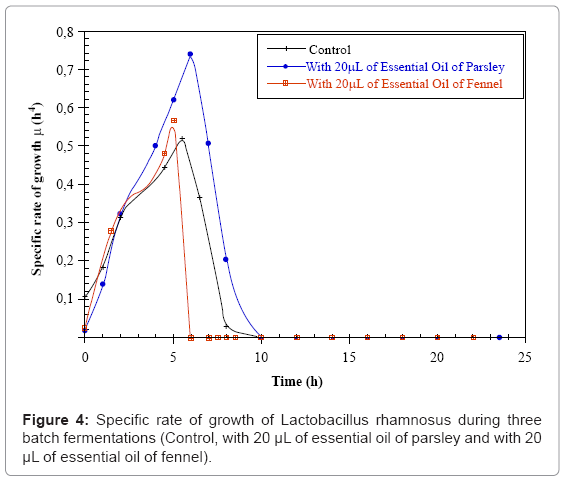
The specific rate of Lactobacillus casei subsp rhamnosus growth (μ) in the control MRS medium starts at 0.11 h-1 and reaches its maximum value 0.5 h-1 after 6 hours of fermentation, then it decreases down to 0 h-1 after 10 hours of culture. For the parsley fermentation, μ starts with an initial value of 0.02 h-1 increasing to 0.74 h-1 after 6 hours of fermentation. In the fennel fermentation, the initial μ is 0.02 h-1 and increases to 0.54 h-1 after 6 hours of fermentation μ decreases abruptly to 0 h-1 at soon as the essential oil is added (Figure 4). This means that the fast increase in the bacterial mass during fermentation is due to the substrate consumption which is very important during this phase. We notice a progressive reduction of the bacteria growth rate which can be due to bacterial lysis.
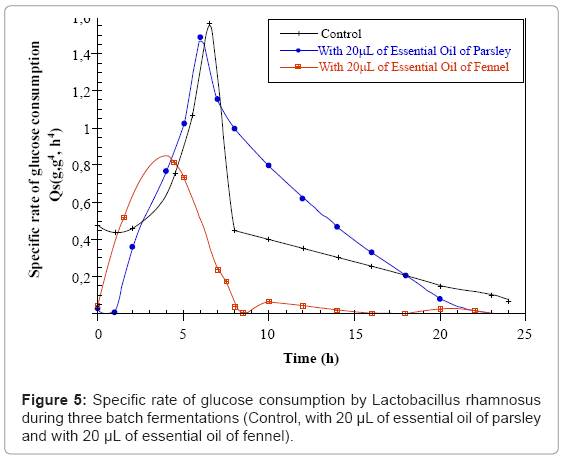
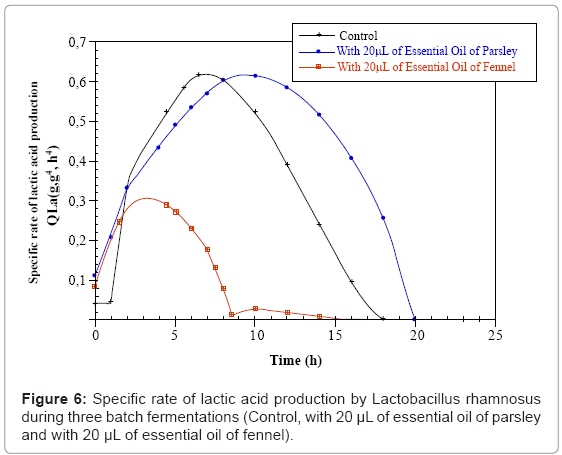
The maximal sugar consumption specific rate (Qs max) is of the order of 1.5 g.g-1.h-1 for the control and parsley cultures and decrease down to 0.73 g.g-1.h-1 for the fennel fermentation. The maximal (Ql.a.max) lactic acid production specific rate is of the order of 0.61 g.g-1.h-1 for the control as well as parsley cultures. However it decreases considerably down to 0.29 g.g-1.h-1 in the fennel culture (Figures 5 and 6).
The growth kinetics may be characterized by a maximal growth specific rate (μmax) which is equal to 0.17 h-1 in the MRS medium (control) and increases up to 0.22 h-1 in the presence of the 20 μL of parsley essential oil. The third fermentation is characterized by a μmax of 0.17 h-1 before the addition of the fennel essential oil that decreases to 0 h-1 when the treatment is applied (Figures 7- 9 and Table 1).
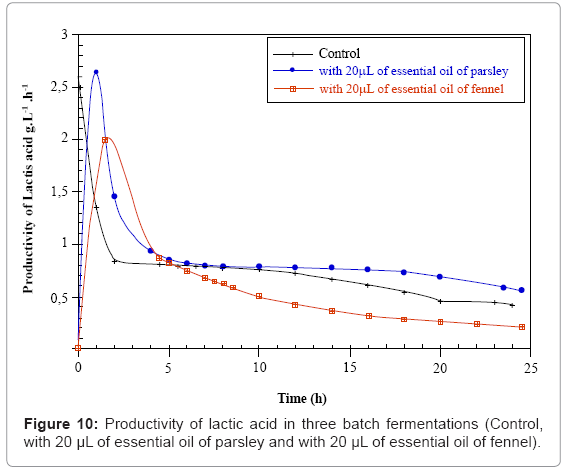
The lactic acid productivities is identical for both control and parsley cultures, and of the order of 2.64 g.L-1.h-1, but weaker for the fennel fermentation (1.98g.L-1.h-1) (Figure 10 and Table 1).
| Parameters | Fermentations | Control | With 20µL of essential oil of parsley | With 20µL of essential oil of fennel |
| Biomass max (g.L-1) | 4.57 | 5.51 | 0.98 | |
| Lactic acid max (g.L-1) | 10.96 | 13.78 | 5.14 | |
| Residual glucose (g.L-1) | 0.008 | 0.007 | 12.33 | |
| µmax (h-1) | 0.17 | 0.22 | 0.17 | |
| Qsmax (g.g-1.h-1) | 1.56 | 1.50 | 0.73 | |
| Q.l.a max (g.g-1.h-1) | 0.61 | 0.61 | 0.29 | |
| Yx/s (g.g-1) | 0.84 | 0.41 | 0.54 | |
| Yp/s (g.g-1) | 0.71 | 0.87 | 0.47 | |
| Productivity in lactic acid (g.L-1.h-1) | 2.64 | 2.64 | 1.98 | |
Table 1: Kinetics parameters of three batch fermentations.
For the control fermentation, the yield of sugar conversion into biomass is 0.84 g.g-1 and 0.71 g.g-1 into lactic acid. It seems that the majority of the sugar is used for the cellular maintenance and reproduction and the remainder for the lactic acid production. The end of the sugar consumption and the Lactobacillus casei subsp rhamnosus growth in the culture containing the fennel essential oil explains the weak yield obtained for lactic acid (0.47 g.g-1). The addition of the parsley essential oil in the culture stimulates the lactic acid production with a conversion yield of 0.87 g.g-1. According to the results on table 1, one finds a positive correlation between the lactic acid production and the sugar consumption rate. This result informs about the fate of consumed sugar: the majority of the consumed sugar is used for the lactic acid production (Table 1).
Conclusion
Healing plants represent a plentiful source of bioactive natural substances among which the essential oils. The objective of this work consists in the estimation of the effect of the parsley (Petroselinum crispum Hoffm) and fennel (Foeniculum vulgare) essentials oil over the kinetics of lactic acid production by Lactobacillus casei subsp rhamnosus. The results of the analysis carried out show a stimulating effect of the Petroselinum crispum Hoffm essential oil in the kinetics of lactic acid production and growth by Lactobacillus casei subsp rhamnosus and an inhibition effect of the Foeniculum vulgare essential oil from its contact with the strain.
Acknowledgements
The authors would like to thank the Pr. Annie Marc responsible for the laboratory GPBA-LSGC (Nancy-INPL-France) for their assistants in the realization of this study.
References
- López MG, Sánchez-Mendoza IR, Ochoa-Alejo N (1999) Comparative study of volatile components and fatty acids of plants and in vitro cultures of parsley (Petroselinum crispum (Mill) Nym ex Hill). J Agric Food Chem 47: 3292-3296.
- Marczal G, Balogh M, Verzar-Petri G (1997) Phenol-ether components of diuretic effect in parsley, I. Acta Agron Budap 26: 7-13.
- Manderfeld MM, Schafer HW, Davidson PM, Zottola EA (1997) Isolation and identification of antimicrobial furanocoumarins from parsley. J Food Prot 60: 72-77.
- Fejes S, Blázovics A, Lemberkovics E, Petri G, Sz''oke E, et al. (2000) Free radical scavenging and membrane protective effects of methanol extracts from Anthriscus cerefolium L. (hoffm.) and Petroselinum crispum (Mill.) nym. ex A.W. Hill. Phytother Res 14: 362-365.
- Nielsen SE, Young JF, Daneshvar B, Lauridsen ST, Knuthsen P, et al. (1999) Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Brit J Nutr 81: 447-455.
- Anand NK, Sharma ND, Gupta SR (1981) Coumarins from Apium petroselinum seeds. Natl Acad Sci Lett 4: 249-251.
- Davey MW, Bauw G, Montagu MV (1996) Analysis of ascorbate in plant tissue by high performance capillary zone electrophoresis. Anal Biochem 239: 8-19.
- Pino JA, Rosada A, Fuentes V (1997) Herb oil of parsley (Petroselinum crispum Mill.) from Cuba. J Ess Oil Res 9: 241-242.
- Tomas F, Mataix JJ, Corpena O (1972) Flavonoid glucosides in Petroselinum sativum. Rev Agroquim Technol Aliment 12: 263-268.
- Tunali T, Yarat A, Yanardag R, Ozçelik F, Ozsoy O, et al. (1999) Effect of parsley (Petroselinum crispum) on the skin of STZ induced diabetic rats. Phytother Res 13: 138-141.
- Beaux D, Fleurentin J, MortierF (1997) Diuretic action of hydroalcohol extracts of Foeniculum vulgare var dulce(D.C.) roots in rats. Phytother Res 11: 320-322.
- Harborne JB, Saleh NAM (1971) Flavonol glycoside variation in fennel Foeniculum vulgare. Phytochemistry 10: 399-400.
- Hegnauer R (1973) Chemotaxonomie der Pflanzen, 6 tomes, Birkh auser Verlag, Basel, Stuttgart.
- Ravid U, Putievsky E, Snir N (1983) The volatile components of oleoresins and essential oils of Foeniculum vulgare in Israel. J Nat Prod 46: 848-851.
- Tanira MOM, Shah AH, Mohsin A, Ageel AM, Qureshi S (1996) Pharmacological and toxicological investigations on Foeniculum vulgaredried fruit extract in experimental animals. Phytother Res 10: 33-36.
- Trenkle K (1971) Recent studies in Foeniculum vulgare. Organic acids, especially phenyl carbonic acids. Planta Med 20: 289-301.
- Hujanen M, Linko YY (1996) Effect of temperature and various nitrogen sources on L (+)-lactic acid production by Lactobacillus casei. Appl Microbiol Biotechnol 45: 307-313.
- Litchfield JH (1996) Microbial production of lactic acid. Adv Appl Microbiol 42: 45-95.
- Yun JS, Wee YJ, Ryu HW (2003) Production of optically pure L(+)-lactic acid from various carbohydrates by batch fermentation of Enterococcus faecalis RKY1. Enzyme Microb Technol 33: 416-423.
- Amass W, Amass A, Tighe B (1998) A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polymers, blends of biodegradable polymers and recent advances in biodegradation studies. Polym Int 47: 89-114.
- Datta R, Tsai SP, Bonsignore P, Moon SH, Frank JR (1995) Technological and economic potential of poly(lactic acid) and lactic acid derivatives. FEMS Microbiol Rev 16: 221-231.
- Amrane A, Prigent Y (1994) Mathematical model for lactic acid production from lactose in batch culture: Model development and simulation. J Chem Technol Biotechnol 60: 241-246.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16607
- [From(publication date):
March-2012 - Dec 08, 2025] - Breakdown by view type
- HTML page views : 11872
- PDF downloads : 4735