Estimation of Lasting Impact of a Chikungunya Outbreak in Reunion Island
Received: 04-Nov-2011 / Accepted Date: 23-Jan-2012 / Published Date: 27-Jan-2012 DOI: 10.4172/2161-1165.S2-003
Abstract
Background: During 2005-2006, chikungunya (CHIK) emerged as a large epidemic in Reunion Island, infecting approximately one third of the population. This study aimed to assess the long term impact of this outbreak by estimation of the progression of chronic patients over time and calculate the global burden of CHIK in Reunion Island with the Disability Adjusted Life Years (DALY) method.
Methods: To estimate the proportion of chronic patients over time, the data of 8 publications were used in a multivariate linear regression model adjusted on patients’ age after the review of more than 50. The selection criteria were: cohort studies studying the persistence of post-epidemic symptoms based on CHIK epidemics located in the Indian Ocean Territories since 2006. These estimations were used for the calculation of years lived with disabilities (YLD) in DALY. As well, as no disability weight has been estimated for CHIK by the global burden disease group of WHO, the disability weights of dengue were used for the acute phase and of rheumatoid arthritis for the chronic phase to weight YLD. To take into account variations in the parameters of YLD, DALY was simulated 1000 times using the Monte Carlo technique.
Results: The regression model estimated that 58.3% (CI: 51.2-65.3%) of the patients were still symptomatic after 1 year and 0% (CI: 0.0-15.2%) after 5 years. In average, the global disease burden was 65-73 DALY/1000 persons, 55.5% concerning the active population (20-60 years old), and 86% due to the CHIK chronic phase.
Conclusion: Patients may have chronic symptoms up to 5 years after the infection. The total loss of healthy years was approximately 55,000 DALYs. The necessity to provide long-term health care for CHIK infected patients and the heavy global burden of CHIK in Reunion Island underline the importance to target prevention to be more cost-effective.
Introduction
Chikungunya virus (CHIKV) is an arthropod-borne virus transmitted by Aedes mosquitoes, first described in 1954 in Tanzania. CHIK infection is responsible of a two phase disease (acute and chronic). The short acute phase, defined as the first 10 days after the disease onset, appears two to six days after the infective mosquito bite. The symptoms are common with other arbovirus’ infections like Dengue, combining high fever, arthralgias, back pain, rash and headache [1]. CHIK has three major differences with Dengue, CHIK asymptomatic infection is rare, approximately 3.5% [2], CHIK disease was known as nonfatal before the 2006 outbreak, and, more specifically, the CHIK acute phase of infection is frequently followed by a chronic phase defined by the persistence of symptoms after 3 months [3,4]. Most of the CHIK infected patients report the relapses of symptoms within three months after the infection [4], but chronic arthralgia may persist long after [4].
Since 1954 sporadic outbreaks were observed in Africa and Asia up to the 1980s, before a period of relative quiescence. In 2004, a major outbreak began in Eastern Africa before disseminating all around the Indian Ocean [4]. This epidemic arrived in Reunion Island, a French overseas department in South-western Indian Ocean, in 2005, causing a two waves outbreak in 2005-2006. The first wave started from March 2005 resulting approximately in 6,000 persons infected until December 2005, when the second explosive wave began with an exponentially increase of incident cases [5,6]. By July 2006, it was estimated that more than 266,000 persons (from a population of 785,221 [6]) were infected, resulting in a cumulative incidence rate of 34% [7,8]. For the first time deaths were reported during this outbreak. By December 2006, 252 death certificates reported CHIK infection as a direct or indirect cause of death [9]. Moreover different clinical studies confirmed the frequent persistence of arthralgia reported up to 30 months [10]. All these considerations conducted to now consider CHIKV as a major public health issue.
Nevertheless, if many studies have been published on the CHIK infection since the emergence of the disease in Reunion Island, few were focussed on the persistence of CHIK symptoms for different periods of time [2,10-16]. All except one [11] are not populationbased. They were conducted on small samples mildly representative of the population [2,10,12-16]. Those results were not appropriate for the purpose of extrapolations on the population-based long term impact of the outbreak.
Although Soumahoro et al. [17] estimated the cost of the acute epidemic based on the health care services consumption, to the best of our knowledge, no study estimated the global burden of CHIK disease on a population based level in Reunion Island. Considering the large prevalence of the infected population, and data on persistence of disabling arthralgia among infected patients, this question is a matter of interest even five years after the outbreak. The present study is conducted with the aim of estimating the global burden of the 2005- 2006 CHIK outbreak in Reunion Island, by modelling the long-term proportion of chronic patients, and calculating the burden of CHIK disease according to the method proposed by the World Health Organisation (WHO). The WHO global burden of disease (GBD) group uses the disability adjusted life years (DALYs) method to estimate the burden of diseases at national, regional and global levels. The DALY measures the difference between the current situation and an ideal situation where everyone lives up to the age of the standard life expectancy, and in perfect health. It is a summary measure which combines the time lived with disability and the time lost due to premature mortality, associated to a specific health event, to calculate the total loss of healthy years due to this event [18], which justify its appropriateness to estimate the global impact of the CHIK outbreak in Reunion Island.
The purpose of our study was to highlight the after effects of the 2005-2006 CHIK outbreaks in Reunion Island so that the public health authorities should be informed of the long term consequences of such large scale epidemics.
Methods
We estimated the proportion of chronic patients over time adjusted on patients’ age, and then we used these estimates to measure the burden of disease with the DALY method.
Estimation of the percentage of chronic patients over time
To estimate the proportion of patients infected with CHIK related persisting symptoms over time, we looked for the available published literature on the topic. As our focus was the 2005-2006 epidemic in Reunion Island, we limited our search on the territories of the Indian Ocean (as it is expected that the same CHIK virus caused epidemics in the region), and focused on the publications concerning CHIK epidemics since 2006 using PubMed and Google search engines. More than 50 publications were reviewed under this process. Eight were selected based on the following criteria of inclusion: 1) studies based on CHIK epidemics since the year 2006, 2) epidemics in the Indian Ocean Territories, 3) use of similar methodology: clinical research interested in the persistence of post-epidemic symptoms with the course of time, and all using prospective or retrospective cohort data. These studies, representing a total number of 1447 patients, were based on different periods of time ranging from 6 to 30 months after infection [2,10- 16]. Most of them underlined the role of patients’ age on the risk of chronicity. The information collected from these studies is summarized in Table 1.
| Study (author, year) | Method | Number of Patients | Duration* (months) | Chronicity (%) | Age† (years) |
|---|---|---|---|---|---|
| Queyriaux et al. 2008 [2] | retrospective cohort study | 168 | 6 | 93.7 | 40.0 |
| Manimunda et al. 2010 [15] | longitudinal follow-up study | 203 | 9 | 49.0 | 35.0 |
| Sissoko et al. 2009 [13] | retrospective cohort study | 147 | 15 | 57.0 | 52.0 |
| Soumahoro et al. 2009 [14] | retrospective cohort study | 199 | 17 | 53.0 | 44.5 |
| Borgherini et al. 2008 [12] | prospective cohort study | 88 | 18 | 63.6 | 58.3 |
| Gérardin et al. 2011 [11] | retrospective cohort study | 512 | 24 | 43.0 | 36.0 |
| Larrieu et al. 2010 [16] | prospective cohort study | 29 | 26 | 58.0 | 49.5 |
| Marimoutou et al. 2010 [10] | retrospective cohort study | 101 | 30 | 36.7 | 42.0 |
†Average age of patients studied
Table 1: Summary of the literature retained for the estimation of chikungunya infected chronic patients over time.
Two important results can be deduced from these studies: 1) the proportion of chronic patients decreases with time; 2) the chronic symptoms are more frequent and persist longer in older patients. The nature of data (8 results pooling data on age, duration and percentage of chronic patients) led us to choose the multiple linear regression model to estimate the proportion of chronic patients with the course of time, and to project estimation after 30 months (censure of the literature).
To calculate the proportion of chronic post-CHICK patients as a function of time and patients’ age, the model can be written as Y= aX1 +bX2+e; where Y corresponds to the percentage of chronic patients (dependant variable), X1 corresponds to the duration (independent variable), X2 corresponds to the patients’ age (independent variable), “e” is the random error term, “a” and “b” are the regression coefficients of duration and age respectively. The constant (intercept) term was not included in the model as the regression line passed through the origin in this case. The model was applied based on the following assumptions: 1) Y is linear; 2) the errors are uncorrelated, have constant variance and follow normal distribution; and 3) X and e are independent. These assumptions were verified using the non constant variance score and the Durbin-Watson tests (for constant variance and independence of errors in the model). We also verified the assumptions of linear model through the R package “gvlma” (global validation of linear model assumptions).
DALY calculation
DALY is the sum of two components, 1) years of life lost (YLL) due to premature mortality, which is the product of fatal cases due to disease and the standard life expectancy at the age of death, 2) years lived with disability (YLD), which is the product of incident cases of disability due to the disease, duration lived with disability and disability weight (i.e. DALY=YLL+YLD). The disability weight is an important factor for the estimation of YLD. It represents the proportion of impairment caused by the health condition. It reflects the average level of preference that the society gives to a year lived with the disability due to a specific disease as compared to a year lived with full health. It can range from 0 to 1, where 0 is assigned to a state comparable to full health and 1 is assigned to death [18]. As CHIK infection is a two phase disease (acute and chronic) with different impairments on health (i.e. with different disability weights), the simplest DALY formula (DALYs) can be written:
DALYs = YLL+ YLD acute +YLD chronic,
where YLL = (number of deaths)*(standard life expectancy at age of death),
YLD acute= IA*DWA*LA, with
IA=incidence cases with acute disease,
DWA = disability weight for acute phase,
LA= average duration of the acute disease disability;
And YLD chronic= IC*DWC*LC, with
IC=incidence cases with chronic disease,
DWC=disability weight for chronic phase,
LC=average duration of chronic disease disability.
In accordance with the DALY concept presented by the WHO GBD group [19], we also calculated time discounted DALY (denoted by DALYD). The concept of discounting represents the social preference of being healthy the current year rather than in the future. To include this concept in the DALY calculation, the value of a year is generally decreased by a fixed percentage (chosen equal to 0.03 by the GBD [19- 21]). The DALY discounted formula is then:
DALYD = YLL+ YLD acute +YLD chronic,
where YLL = (number of deaths)*(1-e-0.03*(standard life expectancy at age of death))/0.03
YLD acute = IA*DWA*(1-e-0.03*LA)/0.03
YLD chronic = IC*DWC*(1-e -0.03*LC)/0.03
Data collection for DALY estimates
To calculate DALY for CHICK outbreak in Reunion Island, the estimates of the number of incident cases and deaths, the average duration of illness, and the disability weights for CHICK were needed. The estimates of Reunion Population and the proportions of CHICK incident cases and deaths by 10-years age group were taken from the data published by the Interregional epidemiologic surveillance system (CIRE La Reunion) [6]. The number of incident cases and deaths according to the 10-years age groups were estimated by extrapolating these proportions (Table 2). In addition, to estimate YLL, the expected life expectancy at the age of death was taken from the French life table edited by the WHO’s website [22].
| Age group (years) | Population** | Incidence cases | Deaths |
|---|---|---|---|
| 0-9 | 139,770 | 36,894 | 3 |
| 10-19 | 138,211 | 53,165 | 0 |
| 20-29 | 120,435 | 31,673 | 1 |
| 30-39 | 115,148 | 43,507 | 5 |
| 40-49 | 114,233 | 46,378 | 15 |
| 50-59 | 74,492 | 26,104 | 17 |
| 60-69 | 44,512 | 16,184 | 35 |
| 70-79 | 25,591 | 9,136 | 55 |
| 80-89 | 10,815 | 2,610 | 84 |
| 90+ | 2,014 | 348 | 37 |
| Total | 785,221 | 266,000 | 252 |
** On January 1, 2006.
Table 2: Reunion Island population, incidences and deaths due to 2005-2006 chikungunya outbreak according to 10 year age groups.
The WHO’s GBD study group has developed disability weights for the different health states of the more common diseases [18], but none are available for CHIK. As recommended by the GBD study group, we applied the disability weights of a disease with close health impact. As some reported important incapacitating conditions during the acute phase [3,4,23], we considered the disability weights for this phase equivalent to that of the acute phase of dengue, another arboviral infection. To take into account the variation in disabilities, we applied the ranges of disability weights for both uncomplicated and hemorrhagic dengue fever used in the GBD 2004 [21]. For the chronic phase, mainly represented by chronic rheumatisms [3,4,13], the GBD 2004 disability weights for rheumatoid arthritis were chosen [21].
The average duration of the chronic phase and the proportion of chronic patients concerned were deducted through our fitted regression model. Table 3 summarizes the input parameters used for DALY calculations.
| Parameter | Mean value | Range | Source (reference) |
|---|---|---|---|
| Disability weight: Acute phase Chronic phase |
- 0.199 |
0.172-0.583 0.185-0.221 |
GBD 2004 (Dengue fever) [21] GBD 2004 (Rhumatoid Arthritis) [21] |
| Duration of illness: Acute phase Chronic phase |
6 days - |
(1-30)days 6 months-4 years |
Sergon et al. [23] Authors’ estimation through MLR* |
| Proportion of chronic patients | - | 0.132-0.658 | Authors’ estimation through MLR* |
GDB: WHO global burden disease group
Table 3: Input parameters used to estimate DALY attributable to the 2005-2006 chikungunya outbreaks in Reunion Island.
Sensitivity analysis
To capture the potential impact of variation in the DALY parameters, we simulated the DALY 1000 times using the Monte Carlo technique [24,25], varying the parameters at random within the limits of their distributions. The results present the average value of these 1000 simulations, their maximum and minimum, and the estimated average DALY per 1000 persons. The whole procedure was executed running a programme in R software.
Results
The estimated regression coefficients showed a negative association between the duration in months and the proportion of chronic patients (b1 = -1.2535), while age was positively associated to this proportion (b2 = 1.7460). R2 and p values of F statistics (R2: 0.9429, adjusted R2: 0.9239 and p-value: 0.0002) indicate that the model well fitted the data. The assumptions of constant variance, independence of errors, and the global validation of linear model assumptions were verified.
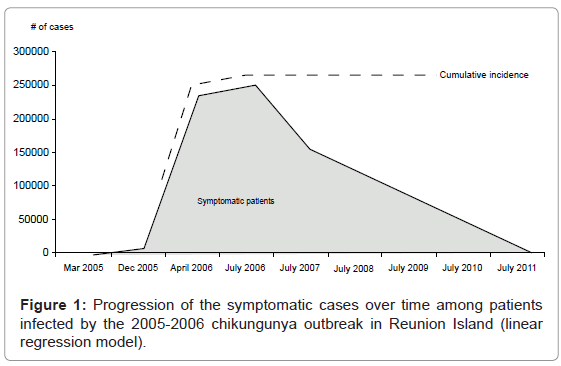
The percentages of chronic post-CHIK patients along with 95% confidence intervals were estimated year wise for a fixed age at 42 years (average age of the patients in the data set of literature). The model estimated that one year after CHIK outbreak, 58.3% (CI: 51.2-65.3%) of patients were still symptomatic. They were 13.2% after 4 years (CI: 0.0-27.7%) and 0% after 5(CI: 0.0-15.2%). Based on the model’s results, Figure 1 presents the progression of CHIK symptomatic patients of the 2005-2006 outbreaks in Reunion Island with the course of time. The area under the curve represents the thousands of people persisting symptomatic after the end of the epidemic (July 2006).
According to the simple DALY method, it is estimated that the population of Reunion Island lost 57,097 years of healthy life. Reported to La Reunion population, this represented 72.7 years/ 1000 persons. These estimations gave a total of 51,219 DALY loss, and 65.3 y /1000 persons with the discounting method. The detailed distributions of YLL, YLD, and DALY by ten years of age are displayed in Table 4a for the simple and Table 4b for the discounting methods. The age wise distribution of DALY showed that reported to 1000 persons, the oldest groups (≥70 years old) were the more concerned by DALY loss, and the 20-29 years old the lowest, with both the simple and discounting methods. Finally, 55.5% of the total DALY concerned the active population (20-60 years old). The detailed analysis of the disease burden showed that the acute phase contributed only 6.5 % to the DALYs, while the extended morbidity (i.e. chronic phase of disease) accounted for 86.5 %.
| AgeGroup (years) | YLLs | YLDs mean | YLDs min | YLDs max | DALYs mean/1000 |
DALYs min/1000 |
DALYs max/1000 |
|---|---|---|---|---|---|---|---|
| 0-9 | 236.4 | 7,340.4 | 557.7 | 20,207.2 | 54.2 | 5.7 | 146.3 |
| 10-19 | 0.0 | 10,577.8 | 803.7 | 29,119.3 | 76.5 | 5.8 | 210.7 |
| 20-29 | 59.5 | 6,301.7 | 478.8 | 17,347.7 | 52.8 | 4.5 | 144.5 |
| 30-39 | 249.0 | 8,656.1 | 657.7 | 23,829.3 | 77.3 | 7.9 | 209.1 |
| 40-49 | 604.5 | 9,227.4 | 701.1 | 25,402.0 | 86.1 | 11.4 | 227.7 |
| 50-59 | 532.1 | 5,193.6 | 394.6 | 14,297.5 | 76.9 | 12.4 | 199.1 |
| 60-69 | 801.5 | 3,220.1 | 244.7 | 8,864.5 | 90.3 | 23.5 | 217.2 |
| 70-79 | 830.5 | 1,817.8 | 138.1 | 5,004.1 | 103.5 | 37.8 | 228.0 |
| 80-89 | 722.4 | 519.4 | 39.5 | 1,429.8 | 114.8 | 70.4 | 199.0 |
| 90+ | 133.2 | 69.2 | 5.3 | 190.6 | 100.5 | 68.8 | 160.8 |
| Total | 4,169 | 52,924 | 4,021 | 145,692 | 72.7 | 10.4 | 190.9 |
Table 4a: Estimates of the disability adjusted life years, due to the 2005-2006 chikungunya outbreak in Reunion Island, with simple method (DALYs)*.
| Age Group (years) | YLLD | YLDD mean | YLDD min | YLDD max | DALYD mean/1000 | DALYD min/1000 | DALYDmax/1000 |
|---|---|---|---|---|---|---|---|
| 0-9 | 90.6 | 6,694.3 | 683.0 | 18,859.1 | 48.5 | 5.5 | 135.6 |
| 10-19 | 0.0 | 9,646.6 | 984.2 | 27,176.3 | 69.8 | 7.1 | 196.6 |
| 20-29 | 27.7 | 5,746.9 | 586.3 | 16,190.2 | 47.9 | 5.1 | 134.7 |
| 30-39 | 129.3 | 7,894.2 | 805.4 | 22,239.4 | 69.7 | 8.1 | 194.3 |
| 40-49 | 350.8 | 8,415.1 | 858.6 | 23,707.0 | 76.7 | 10.6 | 210.6 |
| 50-59 | 345.1 | 4,736.5 | 483.2 | 13,343.5 | 68.2 | 11.1 | 183.8 |
| 60-69 | 579.7 | 2,936.5 | 299.6 | 8,272.8 | 79.0 | 19.8 | 198.9 |
| 70-79 | 667.9 | 1,657.7 | 169.1 | 4,670.0 | 90.9 | 32.7 | 208.6 |
| 80-89 | 636.7 | 473.6 | 48.3 | 1,334.2 | 102.7 | 63.3 | 182.2 |
| 90+ | 126.3 | 63.1 | 6.4 | 177.9 | 94.0 | 65.9 | 151.0 |
| Total | 2,954 | 48,265 | 4,924 | 135,970 | 65.3 | 10.0 | 176.9 |
Table 4b: Estimates of the disability adjusted life years, due to the 2005-2006 chikungunya outbreak in Reunion Island, with time discounting method (DALYD)*.
Discussion
The present study reports that the 2005-2006 CHIK outbreaks in Reunion Island imposed an enormous and long-term impact on the population health. The results of the study showed that patients had chronic symptoms up to 5 years after the infection. The total loss of healthy years was approximately 55,000 DALYs, of which over 55% belonged to the active population (20-60 years old).
Although the number of observations may not seem sufficient to use the multiple linear regression models, the goodness of fit test (F-test) showed that the fitting of the model was satisfying. Moreover, the linear model assumptions (constant variance, homoscedasticity) hold well, and the R software package “gvlma” reconfirmed the validation of these assumptions.
The model is fitted based on existing data up to 30 months [2,10-16], but extended results up to five years based on the assumption of linearity. This assumption may be criticized. The multivariate linear model estimated that around 13% of the patients present persisting chronic symptoms four years after the infection. This result is consistent with the result of an old retrospective study [26] based on the CHIK epidemics in South Africa during 1975-1977, reporting that 87.9% of the CHIK infected patients declared full recovery with no residual symptoms 3-5 years after the infection. As well the percentage of chronic patients was estimated to be 0% after 5 years. This seems in contradiction with the current clinical impression of physicians, who still report numerous consultations of symptomatic post-CHIK patients in 2011, five years after the outbreak. Moreover, the literature suggests that the infection caused by the 2005-2006 CHIK outbreak in Reunion Island was more severe than the previous infections in other countries, and may take a longer time to recover [3,4,16]. A difference of virulence of the CHIKV strains cannot be excluded. On the other hand, the upper 95% confidence interval value was 15% of symptomatic patients at five years. The long-term residual proportion of chronic patients may also correspond to the small percentage of patients that develop authentic inflammatory destructive rheumatisms reported by some authors [15,27-29]. Furthermore, the percentages of chronic patients were estimated by the model fixing the age at 42 years. Patients older than 42 years may report symptoms longer than 5 years after the infection.
We estimated that the CHIK outbreak in Reunion Island imposed 72.7 DALY/1000 persons with the simple and 65.3 DALY/1000 persons with discounting method. This is over 1000 times higher than the DALY rate estimated by Krishnamoorthy et al. [30] for the 2006 CHIK outbreak in India (0.045 DALY/1000 persons with a total estimate DALY=25,588 years). The Indian burden of disease is probably underestimated because of the following reasons: 1) YLLs was not estimated because of non availability of data on CHIK related fatalities, 2) low values were used for input data of YLD calculation (i.e. 6 months was used for the duration of the chronic phase, and the proportion of chronic patients was 12%). Our choice of modelling these two parameters based on the literature probably improved the reliability of our final estimation of DALY. The difference of global burden of the disease between India and Reunion should also be attributed to the difference in prevalence rates. The prevalence rate of the disease in India was very low as compared to Reunion Island (less than 0.12% versus 34%). However, considering the size of the population concerned, this low prevalence represents over a million persons infected in India compared to less than 300,000 in Reunion Island.
Labeaud et al. [31] estimated that the world disease burden of CHIK outbreaks in 2005 was approximately 1,500-1,400,000 DALY, while our analysis reported the Reunion disease burden around 7,800- 150,000 DALY. Being a small part of the world population with less than 800,000 inhabitants, the Reunion Island disease burden (DALY), was comparatively, very high. This over weighted disease burden in Reunion Island may be explained by the CHIKV mutation between 2005 and 2006, responsible for more severe and persisting disabilities.
Compared to other arboviral diseases, CHIK still have a higher global disease burden. Different studies estimated that Dengue has a DALY rate raging from 0.07 to 2.16 per thousand [32-36]. But Beaute et al. [36] reported that 80% the DALY of Dengue in Cambodia belonged to the premature mortality, whereas the present analysis showed that 86% of the disease burden was attributed to the extended phase of CHIK induced morbidity; and only 7.5% to premature mortality. This is coherent with the fact that premature mortality is higher for Dengue and other arboviruses [31], but none of them has long term incapacitating morbidity contrary to CHIK infection.
Our results on both the heavy weight of CHIK disease burden in Reunion Island and the large proportion of DALY due to the chronic phase of the disease are explained by the prolonged persistence of the chronic rheumatisms. This gives the clear message that the health care services should be prepared to provide long-term health care for patients infected by CHIKV. Moreover, prolonged disability may have long term economic impact on health care services. Soumahoro et al. [17] estimated the medical cost associated with the acute phase of the 2005-2006 CHIK outbreaks in Reunion Island. They reported that the social security system had to bear the excess charges of approximately 40 million Euros. They did not include the medical cost of manifestation in the chronic phase of the disease. Marimoutou et al. [10] reported that, in a retrospective cohort study of army policemen in Reunion Island, the CHIK chronic patients sought healthcare services and benefit work stoppage significantly more than non-infected subjects during the two years study period, beginning 6 months and ending 30 months after the acute infection. Moreover our analysis showed that the chronic symptoms may persist up to five years after the acute infection. In the presence of a considerable proportion of CHIK chronic patients, the actual cost of the outbreak should be many times higher than the above said estimate.
This represents a challenge for the public health services for the management of future large scale epidemics. It may be an argument for the public health authorities to improve the prevention and control strategies to avoid such a large scale arbovirus epidemics.
There is no vaccination or specific antiviral treatment currently available for most of the arboviral diseases including CHIK fever. Therefore, the vector control is the only choice to avoid CHIKV infection. The breeding of vectors develop in domestic and peridomestic environment, and may be controlled through container management and source reduction. This task can be achieved by encouraging the participation of community with strong social mobilization and communication [37]. The better knowledge of long term consequences of CHIK infection may help the public health services and the population to improve their implication in the prevention of CHIK outbreaks.
References
- World Health Organization (2008) Guidelines on Clinical Management of Chikungunya Fever.
- Queyriaux B, Simon F, Grandadam M, Michel R, Tolou H, et al. (2008) Clinical burden of chikungunya virus infection. Lancet Infect Dis 8: 2-3.
- Simon F, Parola P, Grandadam M, Fourcade S, Oliver M, et al. (2007) Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore) 86: 123-137.
- Simon F, Javelle E, Oliver M, Leparc-Goffart I, Marimoutou C (2011) Chikungunya virus infection. Curr Infect Dis Rep 13: 218-228.
- http://www.invs.sante.fr/presse/2005/le_point_sur/chikungunya_221205/index.html
- Renault P, Solet JL, Sissoko D, Balleydier E, Larrieu S, et al. (2007) A major epidemic of chikungunya virus infection on Reunion Island, France, 2005-2006. Am J Trop Med Hyg 77: 727-731.
- Gérardin P, Guernier V, Perrau J, Fianu A, Le Roux K, et al. (2008) Estimating Chikungunya prevalence in La Réunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis 8: 99.
- http://www.invs.sante.fr/presse/2006/le_point_sur/chikungunya_reunion050706/ chikungunya_reunion_s26.pdf
- http://www.invs.sante.fr/presse/2006/le_point_sur/chikungunya_061206/index.html
- Marimoutou C, Vivier E, Simon F (2010) Long-lasting overmorbidity and impaired quality of life 30 months after chikungunya infection: Comparative cohort of French gendarmes exposed to chikungunya in 2006 in Reunion Island [abstract 376]. American Society of Tropical Medicine and Hygiene 59th Annual Meeting; Atlanta, Georgia.
- Gérardin P, Fianu A, Malvy D, Mussard C, Boussaïd K, et al. (2011) Perceived morbidity and community burden after a Chikungunya outbreak: the TELECHIK survey, a population-based cohort study. BMC Med 9: 5.
- Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, et al. (2008) Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on reunion island. Clin Infect Dis 47: 469-475.
- Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, et al. (2009) Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis 3: e389.
- Soumahoro MK, Gérardin P, Boëlle PY, Perrau J, Fianu A, et al. (2009) Impact of Chikungunya virus infection on health status and quality of life: a retrospective cohort study. PLoS One 4: e7800.
- Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, et al. (2010) Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans R Soc Trop Med Hyg 104: 392-399.
- Larrieu S, Pouderoux N, Pistone T, Filleul L, Receveur MC, et al. (2010) Factors associated with persistence of arthralgia among Chikungunya virus-infected travellers: report of 42 French cases. J Clin Virol 47: 85-88.
- Soumahoro MK, Boelle PY, Gaüzere BA, Atsou K, Pelat C, et al. (2011) The Chikungunya epidemic on La Réunion Island in 2005-2006: a cost-of-illness study. PLoS Negl Trop Dis 5: e1197.
- Murray CJL, Lopez AD (1996) The Global Burden of Disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020, WHO, Geneva.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL (2006) Global Burden of Disease and Risk Factors. Washington, DC, USA.
- Murray CJL (1996) Rethinking DALYs. The Global Burden of Disease, WHO, Geneva.
- World Health Organization (2008) The global burden of disease: 2004 update. Geneva, USA.
- Sergon K, Yahaya AA, Brown J, Bedja SA, Mlindasse M, et al. (2007) Seroprevalence of Chikungunya virus infection on Grande Comore Island, union of the Comoros, 2005. Am J Trop Med Hyg 76: 1189-1193.
- Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ (1985) Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making 5: 157-177.
- Robert CP, Casella G (2009) Introducing Monte Carlo Methods with R. Springer New York Dordrecht Heidelberg London.
- Brighton SW, Prozesky OW, de la Harpe AL (1983) Chikungunya virus infection. A retrospective study of 107 cases. S Afr Med J 63: 313-315.
- Malvy D, Ezzedine K, Mamani-Matsuda M, Autran B, Tolou H, et al. (2009) Destructive arthritis in a patient with chikungunya virus infection with persistent specific IgM antibodies. BMC Infect Dis 9: 200.
- Bouquillard E, Combe B (2009) A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Joint Bone Spine 76: 654-657.
- Chopra A, Anuradha V, Lagoo-Joshi V, Kunjir V, Salvi S, et al. (2008) Chikungunya virus aches and pains: an emerging challenge. Arthritis Rheum 58: 2921-2922.
- Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK (2009) Burden of chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis 46: 26-35.
- Labeaud AD, Bashir F, King CH (2011) Measuring the burden of arboviral diseases: the spectrum of morbidity and mortality from four prevalent infections. Popul Health Metr 9: 1.
- Cho-Min-Naing (2000) Assessment of dengue hemorrhagic fever in Myanmar. Southeast Asian J Trop Med Public Health 31: 636-641.
- Anderson KB, Chunsuttiwat S, Nisalak A, Mammen MP, Libraty DH, et al. (2007) Burden of symptomatic dengue infection in children at primary school in Thailand: a prospective study. Lancet 369: 1452-1459.
- Meltzer MI, Rigau-Pérez JG, Clark GG, Reiter P, Gubler DJ (1998) Using disability-adjusted life years to assess the economic impact of dengue in Puerto Rico: 1984-1994. Am J Trop Med Hyg 59: 265-271.
- Clark DV, Mammen MP Jr, Nisalak A, Puthimethee V, Endy TP (2005) Economic impact of dengue fever/dengue hemorrhagic fever in Thailand at the family and population levels. Am J Trop Med Hyg 72: 786-791.
- Beauté J, Vong S (2010) Cost and disease burden of dengue in Cambodia. BMC Public Health 10: 521.
- Parks W, Lloyd L (2004) Planning social mobilization and communication for dengue fever prevention and control: a step-by-step guide. WHO, Geneva.
Citation: Yaseen HM, Simon F, Deparis X, Marimoutou C (2012) Estimation of Lasting Impact of a Chikungunya Outbreak in Reunion Island. Epidemiol S2:003. DOI: 10.4172/2161-1165.S2-003
Copyright: © 2012 Yaseen HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16047
- [From(publication date): 10-2012 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 11212
- PDF downloads: 4835