Research Article Open Access
In Vitro Study of Photocurable Embolization Agent for Cerebral Aneurysms
Ayaka Ishikawa1, Yasuhide Nakayama2* and Nobuaki Kambe11Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita 565-0871, Japan
2Division of Medical Engineering and Materials, National Cerebral and Cardiovascular Center Research Institute, 5-7-1 Fujishiro-dai, Suita, Osaka 565-8565, Japan
- Corresponding Author:
- Dr. Yasuhide Nakayama
Division of Medical Engineering and Materials
National Cerebral and Cardiovascular Center Research Institute
5-7-1 Fujishiro-dai, Suita, Osaka 565-8565, Japan
Tel: +81-6-6833-5012 (ex.2624)
Fax: +81-6-6872-8090
E-mail: nakayama@ri.ncvc.go.jp
Received date: February 16, 2012; Accepted date: February 23, 2012; Published date: February 25, 2012
Citation: Ishikawa A, Nakayama Y, Kambe N (2012) In Vitro Study of Photocurable Embolization Agent for Cerebral Aneurysms. J Biotechnol Biomaterial 2:128. doi:10.4172/2155-952X.1000128
Copyright: © 2012 Ishikawa A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The use of liquid embolic agents for the endovascular treatment of cerebral aneurysms has been reported in the neurosurgical literature. A major limitation of these agents is their relatively poor control of hardening ability. In this study, the technical feasibility of a novel gelatin-based photocurable liquid agent for embolization of cerebral aneurysms was evaluated using an experimental aneurysm model. The embolic agent used in this study was an aqueous mixture of photocurable gelatin (gelatin partially derivatized with eosin; 20 wt%; eosin content: 2.8 per gelatin molecule) and polyamine (a polymer of N,N -dimethylaminopropylacrylamide; 5 wt%). Because of its appropriate viscosity at 37 â° C (206 ± 45 mPa•s), the agent could be delivered into the sac of a glass aneurysm model (internal diameter: 3 mm at parent artery part; 5 mm at aneurysm part) by using through a 6 French (Fr)-delivery catheter under the inflation of a balloon catheter at the neck of the aneurysmal sac. After photo irradiation for 40 seconds, the neck of the aneurysm was capped with hydrogel converted from the agent (100%, n=5). Because the colored water was distributed evenly in only a parent tube, complete embolization was observed. Thus, this photocurable agent has potential to be used for the embolization of cerebral aneurysms.
Keywords
Photocurable material; Embolization; Aneurysm; Hydrogel; Liquid embolic agent
Introduction
Improvements in the embolization techniques for the treatment of intracranial aneurysms with Guglielmi detachable coils (GDCs) have led to excellent clinical results [1,2]. However, intrinsic technical problems still remain, especially in the treatment of surgically difficult large or giant aneurysms for which several tenths of the coils are required for complete occlusion. The treatment of broad-based aneurysms even in the case of aneurysms of limited size may cause occlusion of parent vessels if misplaced occlusive material enters and blocks them. Thrombo embolic complications may also occur, and neck remnants may cause regrowth of the aneurysm and rupture. In addition, the placement of a micro catheter in the aneurysm increases the risk of rupture and dislodging the thrombus.
Liquid embolic agents are primarily used for the treatment of arteriovenous malformations (AVMs) [3], duralarteriovenous fistulae [4], and tumors [5]. However, the use of these agents for the occlusion of intracranial aneurysms has not been universally accepted as a standard therapy, despite several studies describing their experimental and clinical use [6-10]. The commonly used liquid embolic agents are n-butyl cyanoacrylate (n-BCA) [11,12]and Onyx [13-15]. A major limitation of using these agents is that it is difficult to control the hardening of the liquid embolic agent when it reaches the neck of the aneurysm. The use of n-BCA for the treatment of brain AVMs requires experience and skill because the intranidal flow and polymerization of n-BCA are rapid and largely unpredictable; Onyx, on the other hand, is less adhesive and polymerizes slowly. Therefore, there is a need for alternative liquid embolic agents that do not require high levels of skill and solvent systems that cause adverse effects in vivo. Further, the use of toxic solvents along with these agents should be eliminated because such solvents limit injection speed or the use of appliances.
Gelatin-based hydrogels are useful biomedical materials [16-25]. Photo-cross-linking is a major hydrogelation method for gelatin, and it offers several advantages such as spatial and temporal control of the photocuring process, curing at room temperature, ease of fashioning, and minimal heat production [18-25]. Several studies have investigated the biomedical applications of photo-cross-linkable gelatins as a matrix in drug delivery [19,20], tissue adhesive glue [21,22] and wound dressing after surgery [23]; as a scaffold material in regenerative medicine [24,25]; and as a coating material for implantable medical devices [19,26].
In this study, an application of the novel photocurable agent for the embolization of cerebral aneurysm was attempted. The agent was an aqueous mixture of photocurable gelatin and polyamine. The photocurable gelatin was gelatin partially derivatized with eosin and polyamine was a polymer of N,N-dimethyaminoproplyacrylamide. Upon photoirradiation, the agent transformed from liquid state into a hydrogel within several tenths of a second via a radical coupling reaction leading to the formation of cross-linked hybrid polymer network. The feasibility of the photocurable agent as the embolic agent was evaluated using a glass aneurysm model.
Materials and Methods
Preparation of the photocurable agent
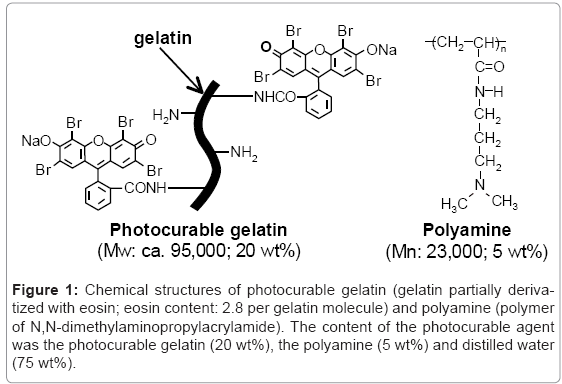
The photocurable gelatin (Figure 1) was synthesized by the condensation reaction between gelatin (CP-925; Jellice Co., Sendai, Japan; obtained from swine skin, molecular weight (Mw): ca. 9.5 × 104) and Eosin Y (sodium tetrabromofluorescein, λmax = 522 nm, ε = 9.9 × 105 dm3 mol-1cm-1 in water) [26]. The number of eosin groups derivatized into a gelatin molecule (degree of derivatization: DD) was 2.8 per molecule, as determined by ultraviolet-visible spectrophotometry (UV-vis spectra) (UV-1700, Shimadzu Co., Kyoto, Japan). Polyamine, [poly (N,N-dimethylaminopropylacrylamide)] (Figure 1) was synthesized by a conventional radical polymerization [26]. The number-average molecular weight (Mn) and poly dispersity index (Mw/Mn) determined by gel permeation chromatography (GPC) analysis (HPLC-8020 calibrated with poly (ethylene glycol), column: TSK gel α-3000 and α-5000, Tosoh Co., Tokyo, Japan) were ca. 23,000 and 2.2, respectively. The photocurable agent was prepared by mixing of the photocurable gelatin (20 wt %) and the polyamine (5 wt %) in deionized water. The viscosity of the agent was measured at 37°C by using a rotating viscometer (TV-22, TOKIMEC, Tokyo, Japan).
Figure 1: Chemical structures of photocurable gelatin (gelatin partially derivatized with eosin; eosin content: 2.8 per gelatin molecule) and polyamine (polymer of N,N-dimethylaminopropylacrylamide). The content of the photocurable agent was the photocurable gelatin (20 wt%), the polyamine (5 wt%) and distilled water (75 wt%).
Measurement of gel yield
For the measurement of gel yield gelation of the photocurable agent (weight of the solid content: Wsolid) was performed on a polystyrene petri dish by irradiation using a clinically used 80-W halogen lamp (irradiation wavelength: 400 to 520 nm, Tokuso Power Light, Tokuyama Co., Tokyo, Japan) for predetermined times (40 or 120 s). The reactants were immersed in distilled water (ca. 40 mL) at room temperature for 12 h to remove unreactedagent. The hydrogel obtained was weighed after vacuum drying (Wdry). The gel yield (%) was calculated using the following equation: gel yield (%) = Wdry/ Wsolidx 100.
Measurement of elastic modulus
The apparent elastic modulus of the hydrogel was determined by the compression method using an apparatus originally designed by Murayama and Omata. The system comprising a 1-mm diameter rodlike stainless steel probe is able to measure the distance that the probe penetrates into the hydrogel as a function of the force applied.
In vitro embolization
The embolization by the photocurable agent was performed using a glass aneurysm model as follows. The tip of commercially available 6 French (Fr)-delivery catheter was maneuvered to the neck of the aneurysmal sac of a glass aneurysm model (internal diameter (ID): 3 mm at parent artery part; 5 mm at aneurysm part). A balloon catheter (Navigater III, Kaneka Co., Osaka, Japan; balloon size: diameter, 3.9 mm; length, 9.7 mm) was inflated at the neck of the aneurysm. The photocurable agent was delivered to the aneurysmal sac through the delivery catheter by syringe and then photoirradiated using an 80-W halogen lamp (Tokuso) for 40 s. The 2 catheters were then removed and the extent of embolization was evaluated by pouring green colored water into the glass tube. The working fluid in the pulsatile flow circuit model was 0.9% saline. Pulsatile flow and flow rate were set at 14 bpm and 98 mL/min, respectively.
Results
Characterization of the photocurable agent
The photocurable agent was an aqueous mixture of photocurable gelatin (20 wt %) and polyamine (5 wt %) (Figure 1). The photocurable gelatin was synthesized by derivatization of eosin (a visible light reactive xanthene dye) to the amino groups of lysine residues in gelatin using a condensation agent. The number of eosin groups derivatized was 2.8 groups per gelatin molecule. On the other hand, the polyamine was synthesized by conventional radical polymerization of N,Ngelatin dimethylaminopropylacrylamide. Because photocurable gelatin was hard to soluble in water due to its high viscosity, heating for at least 1 h at 60°C was needed in the preparation of the photocurable agent. The viscosity of the obtained photocurable agent was 206 ± 45 mPa•s at 37°C and maintained under 37°C (Table 1), whereas under room temperature the liquid state of the agent was converted to gel thermally within 30 min.
| Viscosity of photocurable agent at 37°C | 206 ± 45 mPa·s | ||
| Gel yield | 1mma | 40 Sb | 85.30% |
| 1mma | 40 Sb | 82.5%c | |
| 3 mma | 40 Sb | 64.80% | |
| 3 mma | 120 Sb | 80.40% | |
| Strength of hydrogel | 43.5± 21.5 kPa | ||
aThickness of photocurable agent.
bIrradiation time.
cThe hydrogel was prepared by reaction condition of c.
Table 1: Characteristics of photocurable agent and hydrogel.
The yield of the gel obtained by 40 s of irradiation was 85.3% in 1 mm thickness of the agent on a petri dish, 82.5% in 2 mm, and 64.8% in 3 mm (Table 1). Repeatable irradiation was effective for complete hydrogelation even at deep layer of the agent (80.4% in 3 mm thickness by 120 s of irradiation). Because the data of gel yield was reproducible (n=5, SD<5%), only the average values have been described. The compressive force of the hydrogel produced from 2-mm thickness agent by 40 s of irradiation was 43.5 ± 21.5 kPa (Table 1).
Embolization in glass aneurysm model
To evaluate the technical feasibility of employing a novel photocurable agent for embolization of cerebral aneurysms a glass aneurysm model was prepared (Figure 2a and 2b). Since the photocurable agent had appropriate viscosity at 37°C, it was easily delivered into the aneurysmal sac of the glass model through the delivery catheter, and stored in the sac under occluding both the neck of the aneurysm and the parent artery with the balloon catherter (Figure 2c and 2d). After completely filling the sac with the agent by syringe (Figure 2e), the delivery catheter was easily removed from the neck because there was little adhesion between the catheter and the agent. Irradiation of this agent with visible light for 40 s from the neck side resulted in a color change of the agent special at the neck portion from red to yellow (Figure 2f), indicating the decomposition of eosin and the conversion to hydrogel. The irradiated agent was observed to be insoluble even after it was brought in contact with flowing water when the balloon catheter was removed (Figure 2g). The neck of the aneurismal sac was capped with the hydrogel. The green colored solution poured into the aneurysm model was uniformly distributed only at the parent tube without inflow into the sac, indicating embolization (Figure 2h). On the other hand, the photocurable agent was dissolved easily in water in the parent tube if the balloon catheter was removed before irradiation.
Figure 2: The embolization process by using the photocurable agents in a glass aneurysms model. (a, b) A glass aneurysm model with parent artery (ID: 3 mm) and aneurismal sac (ID: 5 mm). The working fluid was 0.9% saline (pulsatile flow: 14 bpm, flow rate: 98 mL/min). A 6 Fr-delivery catheter was navigated to the neck of the aneurysmal sac. (c, d) The photocurable agent was delivered into the sac through the catherter under the inflation of a balloon catheter at the neck. (e) The inside of the sac was filled with the agent. (f) The light was irradiated to the agent from the neck side after removing the delivery catherter. (g) The balloon catherter was removed. (h) The flow of the green-colored solution was uniformly distributed only at the parent tube without inflow into the sac.
Discussion
This developed agent offers 2 key benefits. First, the agent is photocurable, unlike conventional embolic substances. It is a liquid at 37°C, and on photoirradiation, it can be easily converted to a hydrogel within several tenths of a second. This agent offers the following advantages: spatial and temporal control of the photocuring process, curing at 37°C, ease of fashioning, and minimal heat production. The most effective property of photocuring is in situ hydrogelation, by which three-dimensional form can be easily molded to the desired shape for fitting the shape of aneurysms. As demonstrated in (Figure 2), inside of the aneurysmal sac was completely occluded by capping the neck of the aneurysm with the hydrogel converted from the agent.Although entire hydrogelation was not obtained by 40 s of irradiation because of lack of the light intensity or irradiation time, only capping of the neck of the aneurysm was effective to obtain satisfactory embolization. However, for safety and reliable embolization complete hydrogelation of the agent poured in the sac may be needed, which will obtain by repetition or highly intensity of photoirradiation.
On the other hand, no space in the sac without the agent was observed macroscopically in this in vitro study. In in vivo application, the filling of the agent into the aneurysm may be easily detected by mixing a contrast medium into the agent. Therefore, the leak to parent artery will be prevented.
in vivo photoirradiation method is critical for hydrogelation of the photocurable agent. In this experimental model, irradiation from outside of the glass model was preliminary performed. Previously, we demonstrated hemostais via laparoscopic procedure by using the similar gelatin-based photocurable agent [21]. Gelation on a rat liver surface by irradiation of light transmitted through an optical fiber could be monitored by a TV, in which uniform; continuous gelatinous layer was formed on a rat liver surface. Therefore, it is considered that in vivo endovascular irradiation can be performed by using the optical fiber. In the system, it may be needed that the fiber will equip into the transparent balloon catheter in providing a satisfactory light source in the blood stream. Photoirradiation endovascular devise used in laser balloon angioplasty can be effective for our system [27].
The second benefit of this photocuring agent is that it requires no organic solvents. The major component of the agent is water (75 wt %). In our previous report [21], in vivo degradation of this agentrelated hydrogel was evaluated through the histological examination. At 1 month, significant fragmentation and shrunk volume of the gel photocured in a rat liver surface were noticed along with infiltration of surrounding connective tissues. Inflammatory cells were observed at the local site of the injury. In addition, in the separate study, cells that were seeded on thephotocurable gelatin-coated surface showed good proliferation. Negligible cytotoxicity of eosin–gelatin was confirmed when culturing was performed in the presence of a large amount of eosin–gelatin. These previous results indicate that the photocurable material had little toxicity at least in acute phase. Chronic inflammatory changes may be negligible because the aneurysms will be filled with fibrous connective tissue (organized thrombus) with biodegradation of hydrogel.
Hydrogelation at the outside of the sac may induce distal embolisms. Therefore, sufficient sealing the neck of the sac by balloon catherter is needed to avoid leakage the agent to parent artery. In addition, offing of hydrogels breaked at the surface of the sac will also leave high risks. This is a serious problem. As mentioned above, the cells seeded on the photocurable agent showed good proliferation. Therefore, it is expected that neck of the embolized aneurysm will be covered with vascular tissue in early stage after treatment.
The glass model used in this study was similar in shape to a cerebral aneurysm, and was not mechanically and biologically compatible with natural tissues. The pulsatile flow and flow rate conditions were target for intracranial arteries with internal diameter of 3 mm, in which average flow is 300~400 mL/min and heart rate is about 60. The kinetic viscosity of blood is 4.44 × 10-6 m2/s, which is approximately 4 times that of saline solution (1.00 x 10-6 m2/s). Therefore, the flow conditions in the circuit between the two different fluids, which is defined by the Reynolds number (500~600) and the Womersly number (about 2), were adjusted for evaluation using saline solution.
The thrombus formation may occur on the hydrogel surface at the neck of the sac after blood contacting. In the treatment of aneurysms with GDCs, thrombus forms at the entire body of the sac including the neck. Therefore, it is considered that the thrombus formation on only the hydrogel surface will be clinically acceptable. On the other hand, the photocurable agent could be mixed with antithrombogenic agents such as heparin or argatroban. Previously, the mixture was applied for blood-contacting luminal surface design of the covered stents [28,29]. One month after implantation, the surface of the stents was fully covered with a confluent of endothelial cells with no significant intimal hyperplasia. In addition, in preliminary in vivo experiments gelatinous hydrogel-coated metallic stents immobilized with drug or adenovirus were implanted in rabbit common carotid arteries. Within 3 weeks of implantation, drug permeation and gene expression in the vascular tissues were observed without little thrombus formation. Therefore, there is little thrombus formation on the gelatin-based, highly wateradsorbed surface [30].
Conclusion
The photocurable agent developed here could be delivered into the aneurysm model through the catheter, and the neck of the aneurysm was capped by photoirradiation in short time. The control of hardening ability in liquid embolization agents was extremely improved. Although more detail and in vivo study must be performed prior to clinical application, we are confident that this photocurable agent has the potential to be used as a cerebral embolization agent.
References
- Cloft HJ, Kallmes DF (2002) Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta-analysis. AJNR Am J Neuroradiol 23: 1706-1709.
- Dovey Z, Misra M, Thornton J, Charbel FT, Debrun GM, et al. (2001) Guglielmi detachable coiling for intracranial aneurysms: the story so far. Arch Neurol 58: 559-564.
- Natarajan SK, Ghodke B, Britz GW, Born DE, Sekhar LN (2008) Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with onyx: single-center experience and technical nuances. Neurosurgery 62: 1213-1212.
- Shi ZS, Ziegler J, Gonzalez NR, Feng L, Tateshima S, et al. (2008) Transarterialembolization of clivalduralarteriovenous fistulae using liquid embolic agents. Neurosurgery 62: 408-415.
- Gemmete JJ, Ansari SA, McHugh J, Gandhi D (2009)Embolization of vascular tumors of the head and neck. Neuroimaging Clin N Am 19: 181-198.
- Nishi S, Taki W, Nakahara I, Yamashita K, Sadatoh A, et al. (1996) Embolization of cerebral aneurysms with a liquid embolus, EVAL mixture: report of three cases. Acta Neurochir (Wien) 138: 294-300.
- Nishi S, Nakayama Y, Hashimoto N, Matsuda T (1998) Basic fibroblast growth factor impregnated hydrogel microspheres for embolization of cerebral arteriovenous malformations. ASAIO J 44: M405-M410.
- Wang J, Pang Q, Liu Z, Sheng X (2009) A new liquid agent for endovascular embolization: initial clinical experience. ASAIO J 55: 494-497.
- Takao H, Murayama Y, Yuki I, Ishibashi T, Ebara M, Irie K, Yoshioka H, Mori Y, Vinuela F, Abe T (2009) Endovascular treatment of experimental aneurysms using a combination of thermoreversiblegelation polymer and protection devices: feasibility study. Neurosurgery 65: 601-609.
- Lubicz B, Piotin M, Mounayer C, Spelle L, Moret J (2005)Selective endovascular treatment of intracranial aneurysms with a liquid embolic: a single-center experience in 39 patients with 41 aneurysms. AJNR Am J Neuroradiol 26: 885-893.
- n-BCA Trail Investigators (2002) N-butyl cyanoacrylateembolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol 23: 748-755.
- Pollak JS, White RI Jr (2001) The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol12: 907-913.
- Jankowitz BT, Vora N, Jovin T, Horowitz M (2008) Treatment of pediatric intracranial vascular malformations using Onyx-18. J Neurosurg Pediatr 2: 171-176.
- Corkill RA, Mitsos AP, Molyneux AJ (2007) Embolization of spinal intramedullaryarteriovenous malformations using the liquid embolic agent, Onyx: a single-center experience in a series of 17 patients. J Neurosurg Spine 7: 478-485.
- Weber W, Kis B, Siekmann R, Jans P, Laumer R, et al. (2007) Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery 61: 244-252.
- Liu Y, Chan-Park MB (2010) Abiomimetichydrogel based on methacrylateddextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 31: 1158-1170.
- Zhong H, Matsui O, Xu K, Ogi T, Sanada J, et al. (2009) Gene transduction into aortic wall using plasmid-loaded cationized gelatin hydrogel-coated polyester stent graft. J Vasc Surg 50: 1433-1443.
- Hu X, Ma L, Wang C, Gao C (2009) Gelatin hydrogel prepared by photo-initiated polymerization and loaded with TGF-beta1 for cartilage tissue engineering. Macromol Biosci 9: 1194-1201.
- Nakayama Y, Ji-Youn K, Nishi S, Ueno H, Matsuda T (2001) Development of high-performance stent: gelatinous photogel-coated stent that permits drug delivery and gene transfer. J Biomed Mater Res 57: 559-566.
- Okino H, Nakayama Y, Tanaka M, Matsuda T (2002) In situ hydrogelation of photocurable gelatin and drug release. J Biomed Mater Res 59: 233-245.
- Nakayama Y, Matsuda T (1999) Photocurable surgical tissue adhesive glues composed of photoreactive gelatin and poly (ethylene glycol) diacrylate. J Biomed Mater Res 48: 511-521.
- Li C, Sajiki T, Nakayama Y, Fukui M, Matsuda T (2003) Novel visible-light-induced photocurable tissue adhesive composed of multiply styrene-derivatized gelatin and poly (ethylene glycol) diacrylate. J Biomed Mater Res B Appl Biomater 66: 439-446.
- Ohya S, Sonoda H, Nakayama Y, Matsuda T (2005) The potential of poly (N-isopropylacrylamide) (PNIPAM)-grafted hyaluronan and PNIPAM-grafted gelatin in the control of post-surgical tissue adhesions. Biomaterials 26: 655-659.
- Zhu A, Zhao F, Ma T (2009) Photo-initiated grafting of gelatin/N-maleicacyl-chitosan to enhance endothelial cell adhesion, proliferation and function on PLA surface. Acta Biomater 5: 2033-2044.
- Hoshikawa A, Nakayama Y, Matsuda T, Oda H, Nakamura K, et al. (2006) Encapsulation of chondrocytes in photopolymerizablestyrenated gelatin for cartilage tissue engineering. Tissue Eng 12: 2333-2341.
- Fukaya C, Ishikawa A, Nakayama Y, Murayama Y, Omata S, et al. (2009) Development of a Photocurable gelatin-based gelation material for application to periodontal regeneration. J Photochem Photobiol A Chem 199: 255-260.
- Jenkis RD, Spears JR (1990) Laser balloon angioplasty. A new approach to abrupt coronary occlusion and chronic restenosis. Circulation 81: IV101-IV108.
- Nakayama Y, Nishi S, Ishibashi-Ueda H (2003) Fabrication of drug-eluting covered stents with micropores and differential coating of heparin and FK506. Cardiovasc Radiat Med 4: 77-82.
- Nishi S, Nakayama Y, Ishibashi-Ueda H, Matsuda T (2003) Occlusion of experimental aneurysms with heparin-loaded, microporousstent grafts. Neurosurgery 53: 1397-1404.
- Nakayama Y, Kim JY, Nishi S, Ueno H, Matsuda T (2001) Development of high-performance stent: gelatinous photogel-coated stent that permits drug delivery and gene transfer. J Biomed Mater Res 57: 559-566.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15012
- [From(publication date):
February-2012 - Dec 19, 2025] - Breakdown by view type
- HTML page views : 10258
- PDF downloads : 4754