Lower Recurrence in HPV-Positive Head and Neck Squamous Cell Carcinoma Mediated by Down-Regulation of Sphingosine Kinase 1
Received: 24-Jun-2011 / Accepted Date: 13-Jul-2011 / Published Date: 23-Jul-2011 DOI: 10.4172/2161-119X.1000101
Abstract
Background: Human papilloma viruses (HPVs) are implicated in a subgroup of head and neck squamous cell carcinoma (HNSCC) with a more favorable prognosis and lower recurrences. The mechanism of the HPV ‘s protective role remains unclear. We observed that HPV+ tumors express lower levels of sphingosine kinase 1 (Sphk1), an important regulator of apoptosis and proliferation in cancer cells. Previously, we showed that both in vitro and in vivo knockdown of Sphk1 increased radiosensitivity in HNSCC cell lines. Our goal is to determine the potential of Sphk1 as a biomarker for recurrence.
Methods: DNA polymerase chain reaction classified HPV status for 16 tumors and 6 primary cell cultures developed from HNSCC. Immunohistochemistry assessed p53, Rb, p16, Sphk1, and TGF-β expression in tumor samples. Western Blot examined Sphk1 expression in primary cell cultures. We gathered clinical information through review of medical records.
Results: All HNSCC patients, both HPV+ and HPV-, presented at advanced stage (III/IV). HPV+ samples had lower p53 and Rb and higher p16 expression. On immunohistochemistry Sphk1 expression was lower in HPV+ (46%) than HPV- (70%) tumors (p=0.014). Primary cell cultures from HNSCC patients displayed lower Sphk1 expression in HPV+ cells on Western Blot. TGF-β showed no difference among these groups (p=0.99). HPV+ patients had fewer recurrences (p=0.001) and lower 5-year local recurrence (p=0.03).
Conclusion: The study showed that Sphk1 expression was lower in HPV+ than HPV- HNSCC. Primary cell cultures showed robust Sphk1 expression in HPV- cells and diminished expression in HPV+ cells. Sphk1 expression was associated with recurrence, suggesting its utility as an early biomarker for detecting recurrence. Despite the small size of the cohort, we observed significant differences between HPV+ and HPV- groups. We identified a novel pathway through which HPV may confer a favorable prognosis in HNSCC. This pathway may have value for further testing in Sphk1-targeted therapies.
Keywords: HPV, HNSCC, p53, Rb, p16, TGF-?, Sphk1.
246853Abbreviations
HPV: Human Papillomavirus; HNSCC: Head and Neck Squamous Cell Carcinoma; Rb: Retinoblastoma, TGF-β: Transforming Growth Factor beta, Sphk1: Sphingoskine kinase 1
Introduction
In the United States, head and neck squamous cell carcinoma (HNSCC) was responsible for 35,000 newly diagnosed cases and 7,600 deaths in 2008 [1]. HNSCC is the 6th leading cancer worldwide by incidence, with approximately 643,000 new cases and 245,000 deaths each year [2,3].
Traditionally tobacco and alcohol are considered the primary causative etiologies, with sex, age, and ethnicity as contributing factors. Recent findings revealed that the cause of HNSCC might be more heterogeneous than previously considered. Numerous studies have convincingly demonstrated the independent causal association between oncogenic human papillomavirus (HPV) and a subclass of HNSCC, biologically distinct from carcinogen-associated cancer [4-6].
Over the last 15 years, the incidence of HNSCC at certain anatomical sites has gradually declined in the western world, largely secondary to a decrease in smoking. However, the incidence of oropharyngeal squamous cell carcinoma (OSCC) has risen steadily, especially in the younger adult population [7]. Patients diagnosed with HPV-positive HNSCC are on average 9 years younger than their HPV-negative counterparts (54.5 vs 63.5 years old) [15,16]. A number of contemporary studies using polymerase chain reaction (PCR) detected HPV genome in a variable proportion of HNSCC, ranging from 4% to 62% [5,6,8-16]. This new development can be ascribed in part to the increase in oropharyngeal HPV infection due to changing sexual behaviors [7,17]. Additionally, nonsmokers with HNSCC are approximately 15 times more likely to have HPV-positive HNSCC than smokers [5,18,19].
Studies of HPV’s role in HNSCC implicate certain molecular events in HPV-driven cellular transformation. Its carcinogenic potential can be attributed to overexpression of the virus’ E6 and E7 oncogenes. E6 binds tumor suppressor p53 to enhance its degradation, while E7 binds retinoblastoma (Rb) to interfere with its ability to inactivate E2F transcription factors [20,21]. The consequence of these two processes is cell cycle entry and loss of p53 mediated apoptosis.
Since Rb is a negative regulator of p16, E7 inhibition of Rb in HPV infection results in up-regulation of p16 [20,22,23], a tumor suppressor that normally inhibits Cyclin D1: CDK4/CDK6 complex to prevent Rb phosphorylation [24]. Overexpression of p16 has been consistently observed in HPV-positive HNSCC and shown to serve as a reliable surrogate marker for HPV positivity [22,23]. This is in contrast to HPVnegative HNSCC, which typically exhibits early loss of p16 expression [25,26]. While the roles of p53 and Rb in HPV-related HNSCC have been investigated extensively, little is known about the significance of p16 overexpression in HPV-related cellular transformation.
Weinberger et al. characterized a subgroup of HPV-positive HNSCC with p16 overexpression that has a more favorable prognosis [23], but the exact mechanism has yet to be described in detail. HPVpositive HNSCC has been shown to be more radiosensitive than HPVnegative HNSCC, possibly accounting for better treatment outcomes [27].
We previously established the intimate association between sphingosine kinase 1 (Sphk1) and HNSCC radiosensitivity [28]. Sphingolipids, particularly sphingosine, ceramide, and sphingosine- 1-phosphate (S1P), have recently emerged as regulatory mediators of apoptosis, cell survival, and proliferation. Ceramide and sphingosine, a ceramide derivative, promote apoptosis and inhibit cellular proliferation. Conversely, S1P promotes proliferation and enhances cell survival [29,30]. Sphk1 regulates levels of ceramide, sphingosine, and S1P through its ability to phosphorylate sphingosine and create S1P, thus functioning as a critical mediator of apoptotic and proliferative states [31-34]. We recently demonstrated that HNSCC tumor tissues overexpress Sphk1 compared to normal surrounding tissue and also showed that blocking the expression of Sphk1 in cancer cells using small interfering RNA (siRNA) increased the radiosensitivity of these cells both in vitro and in vivo [28].
To date no one has investigated the relationship between HPV status and Sphk1 in HNSCC. In this study, we aim to compare gene expression of Sphk1, TGF-β, and genes associated with HPV infection. Specifically, we want to determine the molecular differences between HPV-positive and HPV-negative HNSCC that are responsible for the improved treatment outcome seen in HPV-positive HNSCC. We hypothesize that Sphk1 levels in HPV-positive HNSCC are lower than HPV-negative HNSCC, explaining for the increased radiosensitivity in the former. Furthermore, if Sphk1 levels are indeed affected by the presence of HPV infection, we intend to establish the potential of Sphk1 to serve as a biomarker for multiple recurrences in HPVnegative versus HPV-positive HNSCC.
Materials and Methods
Patient selection
Sixteen patients with head and neck squamous cell carcinoma who underwent surgical resection at the University Hospital-University of Southern California (UH-USC) were selected for this study. Surgeries were performed between May 2009 and January 2010. To minimize the effect of radiation therapy on tissue samples, those selected had either no history of radiation exposure or a minimum of 6 months radiation-free interval. This study was approved by the Institutional Review Board of the UH-USC, Los Angeles, California. Normal and tumor tissues were obtained from 16 patients at UH-USC during surgery, stored on dry ice in the operating room, and immediately transferred to the laboratory. For primary cell culture the tumors from 6 HNSCC patients were placed in DMEM media and transported to the laboratory on ice at 4°C. Each tissue specimen was assigned a unique identifying number in the laboratory database. Tumor recurrence was evaluated at continuity clinic visits, with a minimum of 15 months postoperative follow-up. Demographic data were collected from patient medical records. Outcome variables measured included tumor type, local recurrence, number of repeat operations, radiation therapy, survival, and alcohol and tobacco use. Perioperative data comprised of tumor site and TNM staging (Stages I-IV according classification system set forth by the American Joint Committee on Cancer). The cohort consisted of six patient-pairs matched by tumor site and two unmatched pairs. The laboratory was blinded to the HPV status of the tissue samples.
Polymerase chain reaction
DNA was extracted as described by Jiang et al. [35] and polymerase chain reaction (PCR) was performed using the DNA samples of all 16 tumor samples and 6 primary cell cultures for HPV as previously described [35]. We used HPV consensus primers MY09, MY11, GP5+, and GP6+. Primer sequences are noted in Table 1.
| Assay | Sequence | Target (bp) |
|---|---|---|
| MY | ||
| MY09 | GCTCCMARRGGAWACTGATC | 450 |
| MY11 | GCMCAGGGWCATAAYAATGG | 450 |
| GP+ | ||
| GP5+ | TTTGTTACTGTGGTAGATACTAC | 150 |
| GP6+ | GAAAAATAAACTGTAAATCATATT | 150 |
Uegenerate code: MA orC; riA orG; VV= Aor I; Y = C or I
Abbreviations: bp: base pairs
Table 1: Primer Sequences for MY and GP+ DNA Used in PCR for Detection of HPV.
Immunohistochemistry
Frozen tumor tissues from 16 patients were cut into 5μm sections and kept frozen at -80°C until time of use. The sections were allowed to equilibrate to room temperature before fixation with 10% formalin solution for 10 minutes. To quench endogenous peroxidase activity, sections were incubated in 3% H2O2 for 10 minutes and in blocking buffer (0.1% Gelatin, 0.1% BSA, 2.5% normal donkey serum, 2.5% normal goat serum, 0.3% Triton-PBS) for 30 minutes. After washing with 0.3% Triton-PBS, sections were incubated with primary antibody at 4°C overnight. Monoclonal antibodies to p53 (Bp53-12), Rb (IF8), p16 (1E12E10), and polyclonal antibodies to Sphk1, and TGF-β (Santa Cruz Biotechnology, Santa Cruz, CA) were used; antibody identifiers and working concentrations are listed in Table 2. Negative controls included replacement of the primary antibody with rabbit or mouse IgG of the same species. After washing with 0.3% Triton-PBS three times, we incubated the sections with Horseradish peroxidase (HRP)- conjugated secondary antibody at room temperature for 30 minutes. The specimen were then developed using diaminobenzidine (DAB) (Biocare Medical, Concord, CA), counterstained with Modified Mayer’s hematoxylin, dehydrated, cleared, and mounted. Hematoxylin-eosin stain was done to confirm histological integrity of stromal and tumor tissue for each sample.
| Ab Name | Brand | Cat# | Source | Isotype | Reactivity | Expression localization |
Working concentration |
| p53 (Bp53-12) | Santa Cruz | sc-263 | mouse | Monoclonal IgG2a |
Hm | nuclear | 4 Ilg/ml |
| Rb (IF8) | Santa Cruz | sc-102 | mouse | Monoclonal IgG1 |
Rat, Hm | nuclear | 2.51lg/ml |
| p16 (1 E12E10) | Santa Cruz | sc-81156 | mouse | Monoclonal IgG1 |
Rat, Hm | cytoplasm | 2 Ilg/ml |
| SphK1 | Santa Cruz | sc-48825 | rabbit | Polyclone IgG |
Hm | nuclear, cytoplasm | 2.51lg/ml |
| TGF-Jb | Santa Cruz | sc-146 | mouse | Polyclone IgG |
Ms, Rat, Hm | extracellular | 2.51lg/ml |
Abbreviations: Rb: retinoblastoma, Sphk1: sphingosine kinase 1, TGF-(3: transforming growth factor beta; Hm: human, Ms: mouse, Cat #: catalog number
Table 2: Antibodies and Concentrations used for Immunohistochemistry.
Two blinded observers evaluated all immunohistological specimens. Concordance rate was >90%. We randomly selected 5 highpower fields from each section to evaluate staining. More than 500 cells were examined per specimen and samples assigned a grading of negative, weak, moderate, or strong [36].
Image-Pro Plus statistical analysis program (MediaCybernetics, Bethesda, MD) quantitatively calculated overall percent expression for Sphk1 and TGF-β, including cancer cell nuclei and excluding normal stromal cells. We averaged percent expression from 6 randomly selected high-power fields to calculate overall percentage expression for each specimen.
Primary cell culture
Fresh tumor tissues from 6 patients were obtained from UH-USC. Tumor tissues were finely minced and, after extensively washing with culture medium (DMEM media containing 10% of FBS), the cells were transferred into a 25cm2 culture flasks coated with fibronectin at 37°C, with 5% CO2. The culture medium used consisted of DMEM media supplemented with 10% decomplemented FCS. After passage 5, cells were cultured in culture medium devoid of gentamicin. Controlled trypsinizations removed contaminating fibroblasts. The cells were routinely passed once a week, and the medium was changed twice in between. Early primary culture cells were used to prepare cell lysate.
Western blot
Four million primary culture cells were used to make cell lysates by adding 0.5ml of cold lysis buffer (50mM Tris, pH 8, 150mM NaCl, 1% Triton X-100, 0.5mM EDTA, containing Halt Protease Inhibitor cocktail [Pierce, Rockford, IL]). The cell cultures were homogenized on ice using a PowerGen 125 homogenizer (Fisher Scientific) in 15-second bursts. Homogenized samples were transferred to microcentrifuge tubes and lysates cleared by centrifugation at 14,000 x g for 30 minutes at 4°C. Protein extracts were gently removed to fresh tubes. Total protein content was determined by BioRad Dc colorimetric assay (BioRad, Richmond, CA). Samples of 25-30 μg protein were fractionated on 4% to 20% Tris-glycine polyacrylamide gels and transferred to polyvinylidene difluoride membrane (BioRad) by electroblotting. Western blots were digitized and the relevant protein bands of Sphk1 were normalized with β-actin and quantified (Fluor-S Multi-Imager System; BioRad Laboratories, Hercules, CA) with the normal tissue intensity set to 1.0.
Statistical analysis
Clinical and immunohistological variables between HPV-positive and HPV-negative cohorts were analyzed using Fisher exact comparison test (t-test) and Pearson’s Chi-square test (χ2 test). Self-reported alcohol and tobacco use were classified as dichotomous variables. For overall survival, disease free survival, and local recurrence we used Kaplan- Meier Curve with log rank for determining statistical significance. Survival analyses were performed with 5-year cutoffs, and patients who did not have at least 5-years of follow-up were appropriately censored. All p-values were 2-sided and a p-value of less than 0.05 was considered significant.
Results
Patient characteristics
Sixteen HNSCC tumor tissues were tested using PCR, which identified 8 positive and 8 negative for HPV DNA. Between the two groups, 6 pairs of patients matched by tumor site. The cohort included 10 men and 6 women, ranging from 56-86 in age (mean = 72.2). Eight (50%) patients reported frequent alcohol consumption, 7 (44%) denied alcohol use, and 1 did not answer. Ten (63%) smoked 1 or more pack per day for at least 10 years, 5 (31%) either did not smoke, used less than 1 pack per day, or smoked less than 10 years, and 1 did not answer. The mean follow-up from surgery of tumor collection was 21.2 months for survivors and 19.3 months for the entire cohort.
All patients presented with advanced HNSCC; 6 stage III (38%), 10 stage IV (62%). Eight of the 16 (50%) patients had nodal involvement, 4 HPV-positive and 4 HPV-negative. At the time of tumor sampling, 9 were primary cases (56%) and 7 were recurrent tumors (44%). Of the 7 recurrent tumors, 3 were HPV-positive and 4 were HPV-negative. Six (38%) patients have not had any recurrences since primary definitive treatment, 6 (38%) had one recurrence, 2 (12%) had two recurrences, and 2 (12%) had three recurrences. Multiple recurrences occurred more frequently among HPV-negative patients.
Two patients died during this study, one HPV-positive and one HPV-negative. The HPV-positive patient was an elderly gentleman from Croatia with a lifelong history of heavy smoking and chronic lung disease. He presented at advanced stage IV (T4N2M0) and died within 6 months. The HPV-negative patient underwent treatment for lymphoma the same year he had surgery for HNSCC. He experienced recurrence of lymphoma 5 months after surgery and elected for palliative care.
No statistically significant differences in terms of age (p=0.38), gender, TNM stage (p=0.36), tumor type (primary vs recurrent) (p=0.36), or alcohol and tobacco use existed between HPV-positive and HPV-negative HNSCC patients. HPV-positive HNSCC patients had significantly lower recurrence rates (p=0.001) and fewer repeat surgeries (p=0.03) than their HPV-negative counterparts. Despite only 15 months of follow-up time, HPV-negative patients tended to do worse overall. Demographic variables are summarized in Table 3.
| Characteristics | All patients (n-16) |
HPV positive (n=8) |
HPV negative (n=8) |
p-value |
| Age Mean Range |
72.2 52-86 |
76.3 53-86 |
68.0 52-81 |
0.38 |
| Sex Male Female |
10 6 |
5 3 |
5 3 |
n/a |
| TNM stage I/II III IV |
0 6 10 |
0 4 4 |
0 2 6 |
0.36 |
| Nodal involvement Positive Negative |
8 8 |
4 4 |
4 4 |
n/a |
| Tumor type Primary Recurrent |
9 7 |
5 3 |
4 4 |
0.36 |
| XRT Yes No |
13 3 |
8 0 |
5 3 |
0.2 |
| Number of Recurrences 0 1 2 3 |
6 6 2 2 |
5 2 1 0 |
1 4 1 2 |
*0.001 |
| Number of Surgeries 1 2 ³3 |
8 6 2 |
6 2 0 |
2 4 2 |
*0.003 |
| Survival Yes No |
14 2 |
7 1 |
7 1 |
n/a |
| Alcohol Yes No Not recorded |
8 7 1 |
3 4 1 |
5 3 0 |
0.62 |
| Smoking >1PPD no or <lPPD Not recorded |
10 5 1 |
5 2 1 |
5 3 0 |
1 |
Abbreviations: HPV: human papillomavirus. XRT: radiation therapy, PPD: pack per day
Table 3: Characteristics of Patients with HNSCC.
Expression of p53, Rb, p16, Sphk1, and TGF-β analyzed by immunohistochemistry
We examined p53, Rb, p16, Sphk1, and TGF-β expression. We used immunohistochemistry to search for expression differences of p53, Rb, and p16 between HPV-positive and HPV-negative tumors.
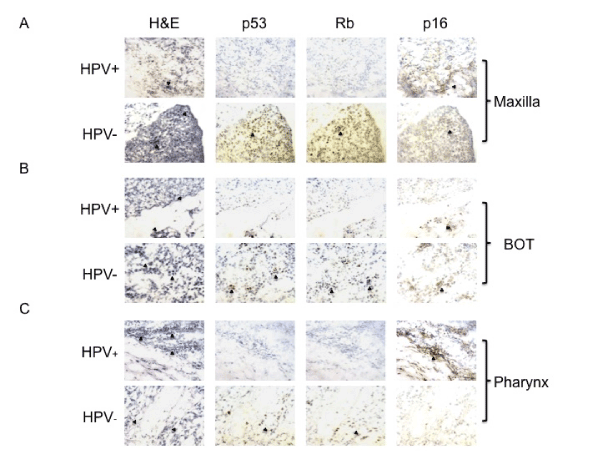
Out of a total of 16 tumors, 8 (53%) stained positively for Rb and 1 had insufficient tissue. All 7 (100%) HPV-negative tumors stained positively for Rb, whereas only 2 of 8 (25%) HPV-positive tumors stained for Rb weakly. Two tumors had insufficient tissue for p53 and p16 staining. As Sphk1 and TGF-β were the focus of this study, we used the available tissue for Sphk1 and TGF-β staining first. Eight of 14 (57%) tumors expressed p53. Similarly, 6 of 7 (85%) HPV-negative tumors demonstrated strong p53 expression, while only 2 of 7 (29%) HPVpositive tumors stained for p53 weakly. We observed p16 expression in 13 of 14 (93%) tumors. Six of 7 (86%) HPV-negative tumors stained for p16 weakly to moderately, and all 7 (100%) HPV-positive tumors expressed p16 strongly. The negative controls showed absolutely no staining (Figure 1A, B, C).
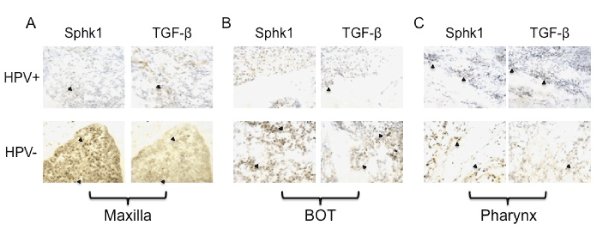
We then used immunohistochemistry to examine Sphk1 and TGF-β for all 16 patient samples. All HPV-positive and HPV-negative tumors demonstrated Sphk1 and TGF-β expression. Overall, Sphk1 staining was stronger and more diffuse in HPV-negative tumors than HPV-positive tumors. TGF-β expression was moderate and approximately equivalent for both groups. Immunohistochemistry results are summarized in Table 4 (Figure 2 A, B, C).
| HPV+ | HPV - | |
|---|---|---|
| Rb | 2/8 (25%) | 6/7 (86%)* |
| P53 | 2/7 (29%)+ | 6/7 (86%)+ |
| P16 | 7/7 (100%) strong+ | 6/7 (86%) weak+ |
Abbreviations: HPV: human papillomavirus, Rb: retinoblastoma, Sphk1:
sphingosine kinase 1, TGFβ: transforming growth factor beta
*One patient had insufficient tissue for determination of Rb staining results
+Two patients did not have enough tissue forp53 and p16 staining
Table 4: Expression of p53, Rb, p15, Sphk1, and TGF-β between HPV+ and HPVPatients Analyzed by Immunohistochemistry.
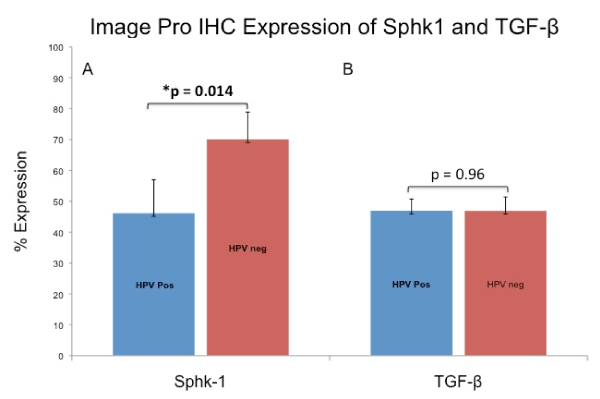
To further assay the difference in expression of Sphk1 between HPV-positive and HPV-negative tumors we quantitatively measured the difference using Image-Pro Plus analytical software (MediaCybernetics, Bethesda,MD). The percentage of cells expressing Sphk1 and TGF-β was calculated for all 16 tumors. In HPV-positive tumors 46% of cancer cells expressed Sphk1, whereas in HPV-negative tumors 70% of its cells expressed Sphk1 (p=0.014). No difference existed for TGF-β expression among the HPV-positive (46.9%) and HPV-negative (46.8%) groups (p=0.99) Figure 3.
Figure 3: Comparison of Sphk1 and TGF-β expression between HPV-positive and HPV-negative tumors. Percent cell expression were quantified using Image Pro Plus software. (3A) Sphk1 expression was significantly lower in HPV-positive tumors (p=0.014) while (3B) TGF-β expression was equivalent between groups (p=0.99).
Western blot detected higher levels of Sphk1 in recurrent tumors
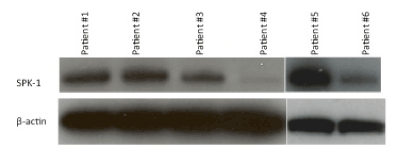
After detecting a significant difference in Sphk1 between HPVpositive and HPV-negative tumors using immunochemistry, we developed primary cell cultures from 6 patients (numbered #1 through #6) with advanced (III/IV) HNSCC to further evaluate Sphk1 expression with Western Blot.
Patients #1, 2, and 5 exhibited considerably higher Sphk1 expression compared to #3, 4, and 6. Figure 4. Patients #1, 2, and 5 experienced tumor recurrence at 11, 8 and 5 months, respectively. Those with weak or absent Sphk1 expression remained recurrence-free at 11 months and beyond.
Figure 4: Western blot for primary cell cultures derived from 6 patients with head and neck cancer. Patients #1, 2, and 5 demonstrated greater Sphk1 expression and had recurrence at 11, 8, and 5 months respectively. Patients #3, 4, and 6 showed lesser Sphk1 expression and had no recurrence at 11 months and beyond.
We then examined the HPV status of the 6 primary cell cultures by performing PCR for HPV from DNA. We found that the patients with greater Sphk1 expression (#1,#2,#5) were HPV-negative, and the patients with lower Sphk1 expression (#3,#4,#6) were HPV-positive.
Survival analysis by HPV status
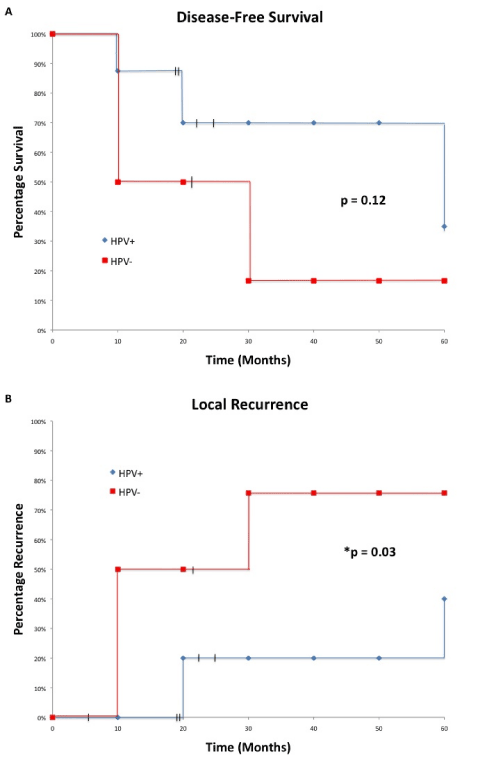
When all HPV-positive cases were compared to HPV-negative cases, HPV status had no prognostic value for overall survival (p=0.96). Only 1 patient from each group died during the study; one died because of recurrent lymphoma and the other due to advanced stage at the time of presentation. For disease-free survival HPV-positive patients fared better than HPV-negative patients (50% vs 12.5%), but the difference was not statistically significant (p=0.12). Patients with HPV-positive HNSCC have improved local recurrence after treatment (p=0.03), with mean time to recurrence at 45 months, compared to 21.9 months for HPV-negative patients. HPV-positive patients had a 5-year local recurrence rate of 43% versus a rate of 87.5% in HPV-negative patients. Survival analyses are summarized in Figure 5A,B. and Table 5.
Figure 5: 5-year survival analysis comparing disease-free survival and local recurrence between HPV-positive and HPV-negative patients. (5A) HPVpositive patients have a better disease-free survival than HPV-negative, but the difference was not significant (p=0.12). (5B) HPV-positive patients had significantly lower local recurrence than their HPV-negative counterparts (p=0.03).
| Mean Time to Death or Recurrence (months) | Recurrence or Death | |||
| % | 95% CI | P-value | ||
| Disease-free survival HPV+ HPV - |
35.3 21.9 |
50% 87.50% |
19% to 81% 64.5% to 100% |
0.12 |
| Local Recurrence HPV+ HPV- |
45 21.9 |
43% 87.50% |
8.5% to 77.5% 64.5% to 100% |
*0.03 |
Abbreviations: HPV: human papillomavirus, CI: confidence interval
Table 5: Survival Analysis of HPV+ and HPV- Patients Based on Disease-Free Survival and Local Recurrence.
Discussion
In this study we investigated expression of Sphk1, TGF-β, and genes associated with HPV-induced HNSCC. We found that HPV-positivity in HNSCC was inversely associated with Sphk1 immunoreactivity. Furthermore, HPV-positive patients with lower levels of Sphk1 experienced significantly improved local recurrence rate and greater quality of life after treatment. These findings suggest that downregulation of Sphk1 may contribute to the improved outcome in HPV-positive HNSCC treatment, possibly through HPV mediated suppression of Sphk1 expression.
The majority of HPV-positive tumors in our cohort did not express p53 or Rb, supporting the widely accepted mechanism that E6 and E7 oncoproteins inhibit these important cell cycle regulators [4,20,21,37]. We also found that HPV-positive tumors expressed p16 more robustly than HPV-negative tumors, which is consistent with observations made by Lundberg et al. and numerous other studies [20,22,23].
We examined the relationship between HPV status and Sphk1 expression in two ways. We first identified a significant difference in Sphk1 expression between HPV-positive and HPV-negative tumors using immunohistochemistry. This was confirmed using Western blot that found recurrent tumors overexpressed Sphk1, while tumors that did not recur had much lower levels of Sphk1. Subsequent PCR revealed that recurrent tumors were more likely to be HPV-negative. HPVpositive HNSCC demonstrated lower levels of Sphk1 expression and fewer recurrences compared to HPV-negative HNSCC. These findings indicate an association between HPV status, Sphk1 expression, and local recurrence and suggest that down-regulation of Sphk1 expression may mediate the improved outcome seen in HPV-induced HNSCC, possibly through increased radiosensitivity [27].
We also evaluated TGF-β expression, normally induced by ionizing radiation [38], to ensure that previous radiation therapy did not confound our findings. Rube et al. investigated the temporal release of TGF-β following tissue irradiation and found that expression peaked immediately after irradiation (6 hours to 1 week) and diminished to very weak or near absent levels at 24 weeks postirradiation [39]. Therefore, we consider it reasonable to conclude that radiation will have negligible effect on outcomes if we selected for patients with at least 6-month interval since last radiation exposure. As expected, no difference in TGF-β expression was detected between HPV-positive and HPV-negative tumors.
Even though this study showed no prognostic value in terms of overall survival with respect to HPV status, many other studies have found an improved outcome for those with HPV-induced HNSCC [9,14,18,19,23,27]. Notably, Fakhry et al. found that HPVpositive patients have half the risk of death compared to their HPVnegative counterparts [18]. An important caveat is the small size of our study group. Despite the size, however, we observed convincing differences between HPV-positive and HPV-negative HNSCC for Sphk1 expression and tumor recurrence. A larger study is currently in progress to determine the mechanisms involved in the association of HPV and Sphk1.
Discoveries from this study show promising potential for treatment of HNSCC. We identified a novel pathway through which HPV-infection may confer a favorable prognosis in HNSCC. The association between HPV status and Sphk1 expression presents Sphk1 as a potential biomarker for detecting recurrence. Further studies will be necessary to delineate the exact mechanisms of Sphk1 suppression in HPV-positive HNSCC.
Additionally, Sphk1 overexpression in recurrent tumors raises the importance of its role in tumor cell survival and radioresistance. Similar Sphk1 overexpression has been identified in numerous other cancers, including colon, prostate, leukemia, and lung cancer [40-44]. We propose that knockdown of Sphk1 may be employed as a novel therapeutic strategy to increase radiosensitivity of cancers. Our previous study showed that Sphk1 inhibition enhanced cell death synergistically when administered in concert with ionizing radiation, allowing for use of sub-maximal radiation dosages that normally would be insufficient to treat cancer [28]. There may be value for future studies using and delivering small interfering RNA to down-regulate Sphk1 expression in tumors.
We also propose a need for stratifying HNSCC by HPV status to better tailor individualized treatment. Patients with HPV-induced HNSCC have more favorable prognosis and could potentially be spared the devastating effects of aggressive therapies. On the other hand, HPV-negative HNSCC with elevated Sphk1 expression may require more aggressive multimodality treatment regimens initially to reduce recurrence and increase survival.
Acknowledgements
We would like to thank Susan MacDonald for collecting patient data. This study was made possible by a grant from Health Research Association and RAM Capital.
References
- Globocan (2008) (IARC) Section of Cancer Information: United States of America.
- Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24: 2137-2150.
- Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94: 153-156.
- Andl T, Kahn T, Pfuhl A, Nicola T, Erber R, et al. (1998) Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res 58: 5-13.
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, et al. (2000) Evidence for a causal association between human papilloma virus and a subset of head and neck cancers. J Natl Cancer Inst 92: 709-720.
- Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, et al. (2003) Human papilloma virus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95: 1772-1783.
- Shiboski C, Schmidt BL, Jordan R (2005) Tongue and tonsil carcinoma: Increasing trends in the U.S. population ages 20-44s. Cancer 103: 1843-1849.
- Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev 14: 467-475.
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, et al. (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261-269.
- Mork J, Lie AK, Glattre E, Clark S, Hallmans G, et al. (2001) Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 344: 1125-1131.
- Franceschi S, Munoz N, Bosch XF, Snijders PJ, Walboomers JM, et al. (1996) Human papillomavirus and cancers of the upper aerodigestive tract: A review of epidemiological and experimental evidence. Cancer Epidemiol Biomarkers Prev 5: 567-575.
- Snijders PJ, Scholes AG, Hart CA, Jones AS, Vaughan ED, et al. (1996) Prevalence of mucosotropic human papillomaviruses in squamous cell carcinoma of the head and neck. Int J Cancer 66: 464-469.
- McKaig RG, Baric RS, Olshan AF (1998) Human papillomavirus and head and neck cancer: Epidemiology and molecular biology. Head Neck 20:250-265.
- Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E (2000) Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int J Cancer 89:300-304.
- Strome SE, Savva A, Brissett AE,Gostout BS, Lewis J, et al. (2002) Squamous cell carcinoma of the tonsils: A molecular analysis of HPV associations. Clin Cancer Res 8:1093-1100.
- Ringstrom E, Peters E, Hasegawa M, Posner M, Liu M, et al. (2002) Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res 8:3187-3192.
- Schwartz SM, Daling JR, Doody DR, Wipf GC, Carter JJ, et al. (1998) Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst 90: 1626-1636.
- Fakhry, C, Gillison ML (2006) Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol 24:2606-2611.
- Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM (2001) Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive group of head and neck carcinoma. Cancer 92: 805-813.
- Leemans CR, Braakhuis BJM, Brakenhoff RH (2010) The molecular biology of head and neck cancer. Nature Reviews Cancer 11: 9-22.
- Munger K, Howley PM (2002) Human papillomavirus immortalization and transformation functions. Virus Res 89: 213-228.
- Lundberg M, Leivo I, Saarilahti K, Makitie AA, Mattila PS (2011) Increased incidence of oropharyngeal cancer and p16 expression. Acta Oto- Laryngologica. Early online: 1-4.
- Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, et al. (2006) Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin ONcol 24: 736-747.
- Serrano M, Hannon GJ, Beach D (1993) A new regulatory motif in cell-cycle control causing specific inhibition of Cyclin D/CDK4. Nature 366: 704-707.
- Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, et al. (1995) 5' CpG island methylation is associated with transcriptional silencing of the tumor suppressor p16/CDKN2/MTS1 in human cancers. Nat Med 1:686-692.
- Reed AL, Califano J, Cairns P, Westra WH, Jones RM, et al. (1996) High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res 56: 3630-3633.
- Pang E, Delic NC, Hong, A, Zhang M, Rose, BR, et al. (2011) Radiosensitization of oropharyngeal squamous cell carcinoma cells by human papillomavirus 16 oncoprotein E6*I. Int J Rad Onco Biol Phys 79(3): 860-865.
- Sinha UK, Schorn VJ, Hochstim C, Chinn SB, Zhu S, et al. (2011) Increased radiation sensitivity of head and neck squamous cell carcinoma with sphingosine kinase 1 inhibition. Head and Neck 33(2): 178-188.
- Ogretmen B, Hannun YA (2004) Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4: 604-616.
- Hannun YA, Obeid LM (2008) Principles of bioactive lipid signaling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139-150.
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, et al. (1996) Suppression of ceramide-mediated programmed cell death by sphingosine-1- phosphate. Nature 381:800-803.
- Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, et al. (1998) Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem 273: 23722-23728.
- Mandala, SM, Thornton R, Tu Z, Kurtz MB, Nickels J, et al. (1998) Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc Natl Acad Sci USA 95:150-155.
- Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, et al. (1999) Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol 147: 545-558.
- Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, et al. (1997) PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol 35(6): 1304-1310.
- Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, et al. (2002) Expression and prognostic roles of ß-Catenin in Hepatocellular carcinoma: Correlation with tumor progression and postoperative survival. Clin Cancer Res 8: 450-456.
- Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch Fx (2002) Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 21(10): 1510-1517.
- Liu RM, Gaston Pravia KA (2009) Oxidative stress and glutathione in TGF-ß- mediated fibrinogenesis. Free Radic Biol Med 48(1):1
- Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, et al. (2000) Dosedependent induction of transforming growth factor ß (TGF-ß) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Rad Onc Biol Phys 47(4): 1033-1042.
- Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, et al. (2009) Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J 23: 405-414.
- Van Brocklyn JR, Jackson CA, Perl DK, Kotur MS, Snyder PJ, et al. (2005) Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol 64: 695 - 705.
- Li J, Guan HY, Gong LY, Spong LB, Zhang N, et al. (2008) Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival. Clin Cancer Res 14: 6996-7003.
- Li W, Yu CP, Xia JT, Zhang L,Weng GX, et al. (2009) Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Canc Res 15: 1393-1399.
- Vadas M, Xia P, McCaughan G, Gamble J (2008) The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction? Biochim Biophys Acta 1781: 442-447.
Citation: Masood R, Delaney SW, Zhu S, Gilde J, Kokot NC, et al. (2011) Lower Recurrence in HPV-Positive Head and Neck Squamous Cell Carcinoma Mediated by Down-Regulation of Sphingosine Kinase 1. Otolaryngol 1:101. DOI: 10.4172/2161-119X.1000101
Copyright: © 2011 Masood R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15761
- [From(publication date): 8-2011 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 10875
- PDF downloads: 4886