A Case of Brunner Gland Adenoma, which Exhibited Dramatic Macroscopic Metamorphosis in 2 Years without Canceration
Received: 30-Nov-2016 / Accepted Date: 06-Dec-2017 / Published Date: 13-Dec-2017 DOI: 10.4172/2161-069X.1000539
Abstract
A 68-year-old Japanese male was shown to have a spherical semipedunculated duodenal submucosal tumor (SMT) with a small central depression. He was followed up under the tentative diagnosis of Brunner gland (BG) hyperplasia (BGH) by biopsy. The tumor was found to have turned inverted dome-shaped with a central depression occupying almost all the top of it 2 years later. The disrupted surface was uneven, more reddened and lobulated by groove-like excavations, in and around which the mucosal pattern was obscured and abnormal vessels were observed. The biopsied specimen was interpreted as BG adenoma (BGA). The tumor was endoscopically resected because of a risk of complicating malignancy, judged to be completely excised, and definitely diagnosed as BGA containing no malignancy by the intensive examinations. Though explicitly proves that, contrary to the reports so far, dramatic macroscopic metamorphosis of BGA does not necessarily herald malignant degeneration within but is apparently due to disequilibrated blood supply, the present case implicitly omens, rather than completely excludes, the future possibility of canceration in the lesion on the grounds of the neoplastic findings.
Keywords: Brunner gland adenoma; Inequality in blood supply; Inverted growth; Macroscopic transformation; Malignant degeneration; Natural history
Introduction
Though used to be arbitrarily called hamartoma, BG nodules greater than 5 mm in diameter are currently considered hyperplasia irrespective of the presence or absence of the admixing fat and bundles of smooth muscle [1-3]. It is only the nodules, whose epithelium is dysplastic, that deserve to be designated adenoma [1,3]. BGH is a rare lesion, which accounts for approximately 1% of the small bowel and 5% of the duodenal tumors [4], but 2.1% of the lesion is composed in part of dysplastic glands [5], which should be classified as BGA. Both tumors are located in the duodenal bulb and descending part of the duodenum, measure 1-8 cm in diameter, generally occur in the middleaged patients and grossly appear pedunculated, semipedunculated, or sessile [2,5,6], while the latter also assumes a form of a flat elevation with a central depression [5].
The former shows no sex preponderance and causes gastrointestinal hemorrhage, obstructive symptoms, or no symptoms at all [2], whereas, in the latter, male predominates [5] but no exact data have been presented concerning the symptoms. Though canceration of them was once questioned [7], such very rare cases have been published [5,8-13] and a definite association was reported of macroscopic transformation of the latter in the natural history with development of malignancy [10,12]. This report presents a case of BGA, which showed dramatic macroscopic metamorphosis during a 2 year-period but was revealed to harbor no carcinoma.
Case Report
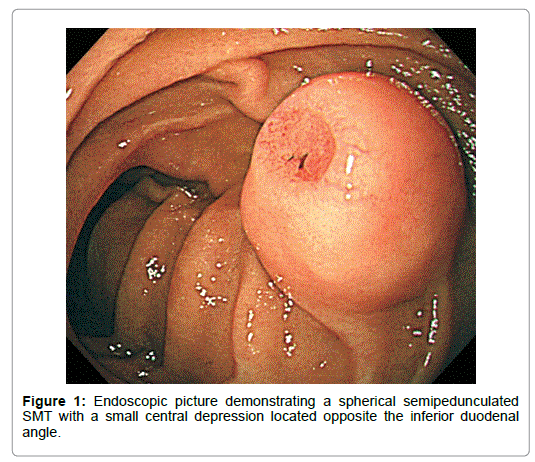
A 68-year-old diabetic Japanese male was incidentally shown to have a medium-sized smooth-surfaced semipedunculated globular tumor accompanied with the bridging folds on the lateral aspect of the inferior duodenal curvature by esophagogastroduodenoscpy (EGD). It was covered with the normal-appearing duodenal mucosa except a small central depression, which was slightly reddened, rather regularly finely granular-surfaced and flat except a shallow Y-shaped reddened cavity in the anal part (Figure 1).
The biopsied specimen from the depression revealed the normal BGs covered with the regenerative mucosa, under which the muscularis mucosae was completely interrupted. His past and family histories were noncontributory except that he suffered from peptic ulcer at 50, when Helicobacter pylori (H. pylori) could not be eradicated. Laboratory data were unremarkable. He was to be followed up under the tentative diagnosis of BGH.
Though appeared neither enlarged nor diminished in volume, the tumor was shown to have the deeper central depression 3 months later. Biopsy demonstrated the cystically dilated BGs with papillary proliferation of the epithelium under the regenerative mucosa. He did not undergo endoscopy another 3 months later due to his incompliance and 1 year and 8 months had passed since the second EGD when he underwent the third. Though his general status was not changed at all then, the tumor showed dramatically different macroscopic features.
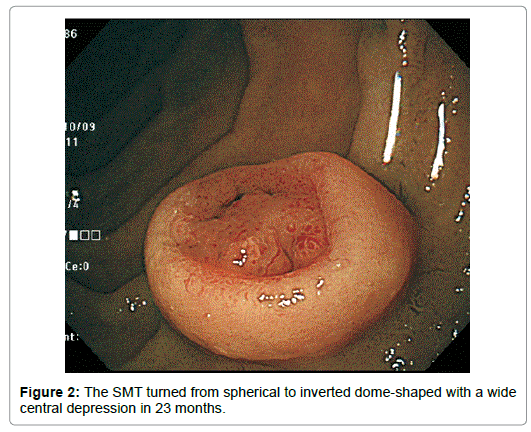
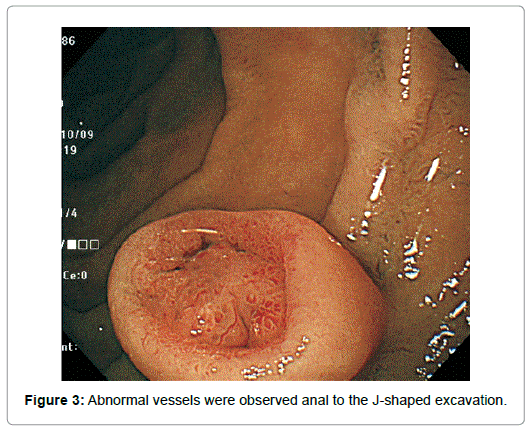
Though appeared neither increased nor decreased in horizontal size, it diminished in height by half. Completely losing the original upper half, it had turned from spherical to inverted dome-shaped with the rim at about the height of the equator of the original tumor with the central depression occupying almost all the top of the lesion except the edge (Figure 2). The tumor remained covered with the normalappearing duodenal mucosa, semipedunculated and accompanied with the bridging folds, however. The disrupted surface was uneven, more reddened and lobulated by the oral J-shaped and anal inverted V-shaped groove-like excavations, in and around which the mucosal pattern was obscured. Abnormal vessels were observed anal to the J-shaped excavation (Figure 3).
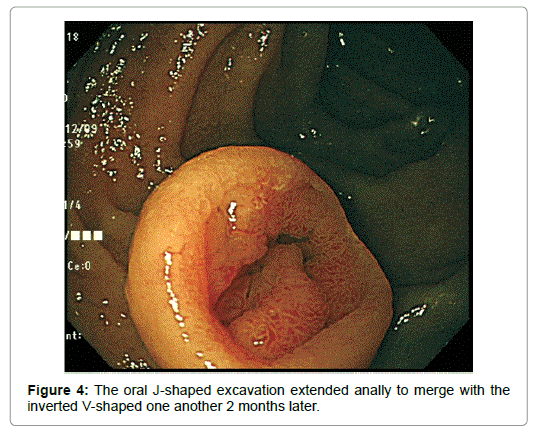
Biopsy demonstrated the densely packed, cystically dilated BGs directly under the regenerative mucosa, which were larger than usual and showed an acinar pattern. The nuclei were round and larger than usual. The glandular cells were cylindrical and the epithelium developed papillary infoldings. They were not stained with p53, only small portion of which was immunoreactive to Ki 67. Taken together, the lesion was diagnosed as BGA (Figure 4).
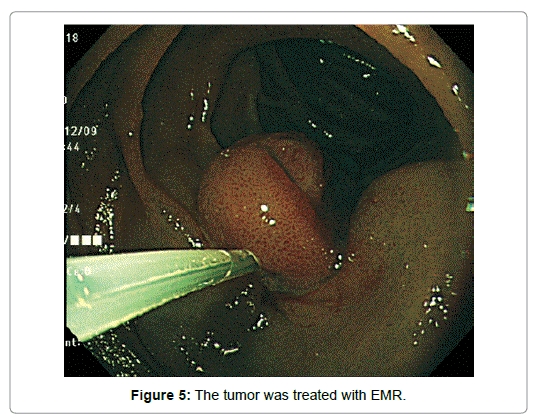
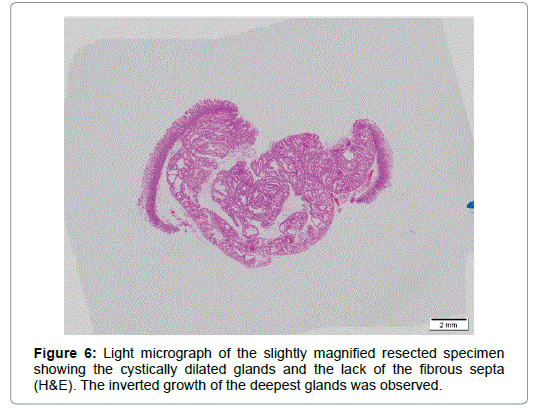
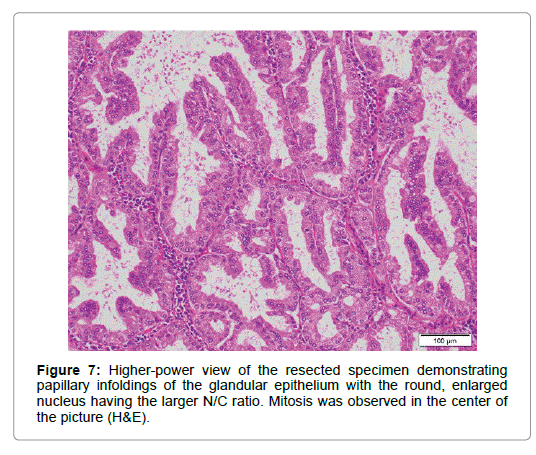
An imminent risk of complicating cancer got it treated with endoscopic mucosal resection (EMR) 2 months later, which, measuring 17 × 12 × 10 mm in diameter with a 13 × 8 mm wide central depression, was judged to be completely excised (Figure 5). At that time the central depression got slightly deeper and the oral J-shaped groove-like excavation extended anally to merge with the inverted V-shaped one, which had turned into a wide, deep serpentine cleft transversely traversing the almost entire surface of the center of the depression. Though became more distinct on the surface of the depression, the mucosal pattern was blurred on that of the cleft, where abnormal vessels were observed (Figure 4). The other features of the tumor remained unchanged.The resected specimen was revealed to be composed of nothing but BGs showing cystic dilatation with no components of the adipose tissue or bundles of smooth muscle at all. The glands situated at the deepest part showed expansive proliferation deeper and deeper, developing the inverted growth. No fibrous septa existed separating the lobules (Figure 6). The glandular epithelium demonstrated papillary infoldings with the round large nucleus having no conspicuous nuclear crowding with stratification, particularly in the central part of the lesion. The nuclear-cytoplasmic (N/C) ratio was larger than usual and mitosis was observed (Figure 7).
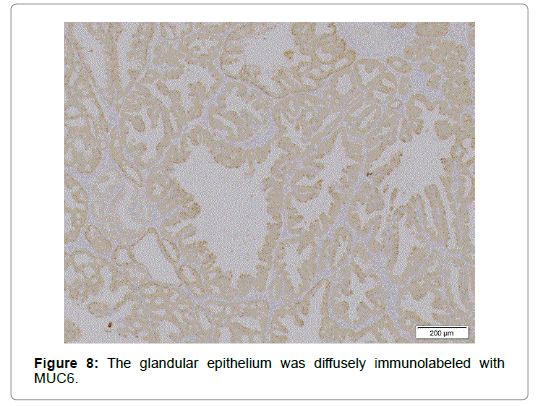
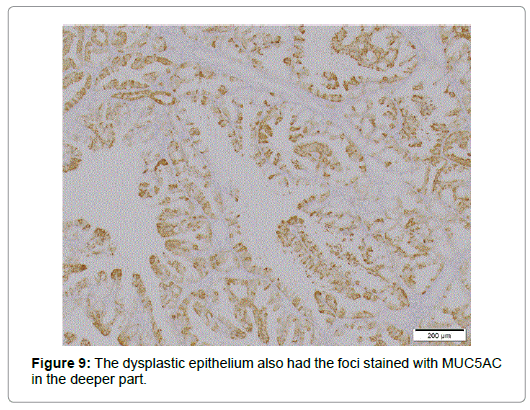
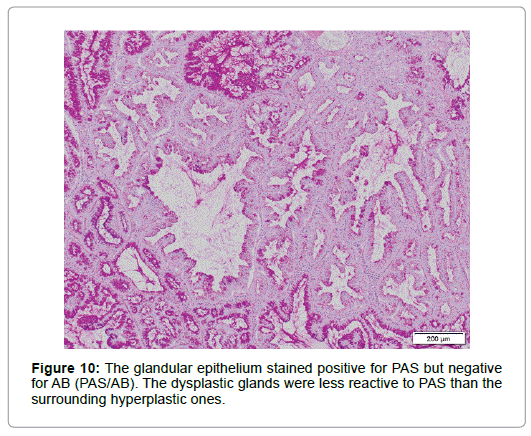
Relatively larger proportion of the cells was Ki 67-positive in the superficial part but only few p53 positive cells were scattered. Though diffusely immunolabeled with MUC6 but not with MUC2 (Figure 8), the lesion, in contrast to the normal BG, also had the foci positive for MUC5AC not only in the superficial but in the deeper part (Figure 9), where no regenerative impact extended, showing the character of a neoplasia. The epithelium showed positivity for PAS but not for AB, which was stained negative for pepsinogen1 and H+K+-ATPase. The dysplastic glands stained less positive for PAS than the surrounding hyperplastic, however (Figure 10). The tumor was differentiated from pyloric gland adenoma (PGA), definitely diagnosed as BGA, in which no malignancy was detected.
Discussion
BGA is a rare disease entity of unknown etiology [5]. It appears pedunculated, semipedunculated, or sessile or assumes a form of a flat elevation with a central depression located in the duodenal bulb and descending part of the duodenum [5,6,14]. The term BGA has rather loosely been used. It is necessary to strictly discriminate from BGH, in which no neoplastic glands are detected irrespective of the presence or absence of the other normal tissues unusually admixed [2,3,5], the true adenoma, which is far less common [5], in order to delve into the true nature of the neoplastic lesion.
The present case was an asymptomatic semipedunculated lesion detected in the inferior duodenal curvature, diagnosed as perfectly authentic adenoma by intensive examinations. The lesion explicitly demonstrated not only the macroscopic but microscopic features of BGA: the reddened lobulated surface with the characteristic groovelike excavations and abnormal vessels [6], papillary infoldings of the glandular epithelium, the enlarged round nucleus with an increased N/C ratio, and vanishment of the fibrous septa between the lobules [3,15]. The diagnosis was further substantiated by the positivity not only for MUC6 but MUC5AC. Though the positivity for the latter in the shallower part might be influenced by regenerative stimuli, that in the deeper part clearly indicated the feature of a neoplasia.
Some authors insist that PGA is differentiated from BGs [16,17] or BGA [18] on the grounds that, while the former not only expresses MUC6 but MUC5AC, the latter group is stained negative for MUC5AC, but it is the neoplastic BGs that showed positivity for a foveolar mucin marker in the present case, which expressed neither pepsinogen1 nor H+K+-ATPase [3,19]. In addition, the present case was positive for PAS, differentiated from PGA [16,18]. The dysplastic glands were, however, less reactive to PAS than the surrounding hyperplastic ones, which suggests that the former fairly lost the feature of the original BG to acquire the neoplastic characteristics, such as positivity for MUC5AC.
BGH or BGA was once not believed to undergo malignant degeneration [7] but the cases complicated with malignancy have been reported [5,8-13]. Since macroscopic transformation of BGA in the natural history is considered indicating canceration within, it is proposed as the hallmark of malignant degeneration advocating endoscopic or surgical intervention [10,12]. The present case was, however, proven to be an exception to this law.
Dramatic macroscopic metamorphosis was observed in the present case from a spherical to hemispherical form with the total disappearance of the upper half, which urged the intervention. Biopsy, though sustained the diagnosis of adenoma, did not explicitly indicate the presence of malignancy in no uncertain terms. The macroscopic transformation could be explained by autolysis of malignancy supposed to be situated only in the upper half. Since even the traces of carcinoma were not detected in the biopsied or resected specimen, however, it was not regarded as the culprit in the transformation.
The upper half might be corroded by gastric acid as in a case of MEN1-associated duodenal gastrinoma contained in the densely packed conglomerated hyperplastic BGs [15] because the patient had the previous history of peptic ulcer and was still infected with H. pylori despite the prior attempt to eradicate the bacterium. It is, however, hardly conceivable that the acid did corrode only the SMT in the inferior duodenal curvature but spared the most vulnerable portion of the duodenum, the bulb. Therefore, peptic ulcer was not considered responsible for effacement of the upper half of the original tumor.
Total loss of the upper half could be caused by autolysis due to the activated substance secreted by the gland itself. The possibility that pepsin or hydrochloric acid digested the part was completely ruled out by the negative expression of the proenzyme or the enzyme activating the proton pump in the resected specimen.
The mechanical stress of the ingested material is not considered to be the cause of the central depression because its maximal effect should be exerted on the oral wall of the SMT but not on the summit.
In the resected specimen the deeper the BGs were situated, the larger they expanded. The deepest glands were seen to have expansively proliferated deeper and deeper, exhibiting the inverted growth: the deeper, larger glands appeared to have deprived the more superficially situated ones of sufficient blood supply. Such an ischemic insult could devastate most easily the farthest point from the trunk of the arteries in the duodenum, the summit of the tumor, and could be conducive to destruction of the shallower glands including the more superficially existing structures, which resulted in formation of a wider central depression and deeper excavations. The surface of the depression was afterwards covered with the regenerative epithelium, which demonstrated distinct mucosal pattern.
It is not autodegradation of malignancy that engendered disruption of the superficial structures resulting in a dramatic change of the macroscopic configuration but it is considered due to inequality in blood supply leading to hypoxemia in the superficial structures, which might be further aggravated by biopsy in the present case. It is not hard to imagine that the following situation occurs in BGA during a natural history: for whosoever hath, to him shall be given, and he shall have more abundance: but whosoever hath not, from him shall be taken away even that he hath [20].
Conclusion
The present case explicitly indicates that, contrary to the reports so far, dramatic macroscopic transformation of BGA in a natural history does not necessarily proclaim the occurrence of carcinoma within but that such a phenomenon is considered rather due to shortage of blood supply engendered by advanced inverted growth of the adenoma. It does not, however, rule out the possible malignant degeneration of BGA. Rather the dysplastic glands described above, which have so proliferated invertedly to induce macroscopic transformation of the tumor in toto due to inequality in blood supply, sufficiently substantiate potential canceration in the lesion.
Conflict of Interests
The authors declare that they have no conflict of interests concerning this article.
References
- Lewin KJ, Riddell RH, Weinstein WM (1992) Brunner’s gland nodules and hamartomas. Gastrointestinal pathology and its clinical implications. New York: Igaku-Shoin, New York pp: 1169-1171.
- Levine JA, Burgart LJ, Batts KP, Wang KK (1995) Brunner’s gland hamartomas: Clinical presentation and pathological features of 27 cases. Am J Gastroenterol 90: 290-294.
- Umizaki Y (2015) Brunner gland hyperplasia. Pathol Clinical Med 33: 94.
- Sakurai T, Sakashita H, Honjo G, Kasyu I, Manabe T (2005) Gastric foveolar metaplasia with dysplastic changes in Brunner gland hyperplasia: Possible precursor lesions for Brunner gland adenocarcinoma. Am J Surg Pathol 29: 1442-1448.
- Botsford TW, Crowe P, Crocker DW (1962) Tumors of the small intestine: A review of experience with 115 cases including a report of a rare case of malignant hemangio-endothelioma. Am J Surg 103: 358-365.
- Goda K (2015) Magnifying endoscopic findings of duodenal lesions. Gastroenterlog Endosc 57: 2478-2488.
- Dixon CF, Lichtman AL, Weber HM, MacDonald JR (1946) Malignant lesions of the duodenum. Surg Gynec Obstet 83: 83-93.
- Christie AC (1952) Duodenal carcinoma with neoplastic transformation of the underlying Brunner’s glands. Br J Cancer 7: 65-67.
- Shorrock K, Haldane JS, Kersham MJ, Leach RD (1986) Obstructive jaundice secondary to carcinoma arising in Brunner’s glands. J R Soc Med 79: 173-174.
- Itsuno M, Makiyama K, Omagari K, Tanaka T, Hara K, et al. (1993) Carcinoma of duodenal bulb arising from the Brunner’s gland. Gastroenterol Jpn 28: 118-125.
- Brookes MJ, Manjunatha S, Allen CA, Cox M (2003) Malignant potential in a Brunner’s gland hamartoma. Postgrad Med J 79: 416-417.
- Koizumi M, Sata N, Yoshizawa K, Kurihara K, Yasuda Y (2007) Carcinoma arising from Brunner’s gland in the duodenum after 17 years of observation: A case report and literature review. Case Rep Gastroenterol 1: 103-109.
- Ohta Y, Saitoh K, Akai T, Uesato M, Ochiai T, et al. (2008) Early primary duodenal carcinoma arising from Brunner’s glands synchronously occurring with sigmoid colon carcinoma: Report of a case. Surg Today 38: 756-760.
- Fujimaki E, Nakamura S, Sugai T, Takeda Y (2000) Brunner’s gland adenoma with a focus of p53-positive atypical glands. J Gastroenterol 35: 155-158.
- Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG (2008) Epithelial tumors of the small intestine: Gastrointestinal pathology. An atlas and text. (3rd edn) Philadelphia: Wolters Kluver| Lippincott, Williams & Wilkins, USA pp: 471-495.
- Chen Z-M, Scudiere JR, Abraham SC, Montgomery E (2009) Pyloric gland adenoma: An entity distinct from gastric foveolar type adenoma. Am J Surg Pathol 33: 186-193.
- Chlumska A, Waloschek T, Mukensnabl P, Martinek P, Kaspirkova J, et al. (2015) Pyloric gland adenopma: A histologic, immunohistochemical and molecular genetic study of 23 cases. Cesk Patol 51: 137-140.
- Adenomatous polyps of the stomach. (2009) In: Gastric adenoma-Surgical pathology criteria, Stanford University, School of Medicine.
- Kushima R, Katsurahara M, Bamba M, Hattori Y, Matsubara A (2015) Expanding on the histopathological understanding and differential diagnosis of fundic gland-type adenocarcinoma. Stomach and Intestine (Tokyo) 50: 1481-1491.
- Sasaki K (2016) Duodenal gastrinoma associated with multiple endocrine neoplasia type1 (MEN1) detected by esophagogastroduodenoscopy (EGD), which was buried under ulcer. J Gastrointest Dig Syst 6: 2.
Citation: Sasaki K, Takano N, Iwama N, Masuda T (2017) A Case of Brunner Gland Adenoma, Which Exhibited Dramatic Macroscopic Metamorphosis in 2 Years without Canceration. J Gastroint Dig Syst 7: 539. DOI: 10.4172/2161-069X.1000539
Copyright: © 2017 Sasaki K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7040
- [From(publication date): 0-2017 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 6027
- PDF downloads: 1013