Case Report Open Access
A Case of Rare Cutaneous Mycobacteriosis and Central Nervous System Post-Transplant Lymphoproliferative Disorder in a Female Patient after Kidney Transplantation
Dorota Miszewska-Szyszkowska1*, Olga Tronina1, Katarzyna √?¬?lubowska1, Natalia Miko√?¬?ajczyk-Korniak1, Dominika D√?¬?borska-Materkowska1, Renata Wieczorek-Godlewska1, Agnieszka Furma√?¬?czyk-Zawiska1, Joanna Pazik1, Ewa Rzadkiewicz2, Dariusz Lipowski2, Magdalena Durlik1 and Piotr Rzepecki3*
1Department of Transplantation Medicine, Nephrology and Internal Diseases, Medical University of Warsaw, Poland
2Department of Infectious Diseases for Adults, Medical University of Warsaw, Poland
3Department of Hematology with Bone Marrow Transplantation Unit, Military Institute of Medicine, poland
- *Corresponding Author:
- Dorota Miszewska-Szyszkowska
Department of Transplantation Medicine
Nephrology and Internal Diseases
Medical University of Warsaw
59, Nowogrodzka st, Warsaw
02-006, Poland
Tel: 48-600322573
E-mail: dorotaszyszkowska@gmail.com
Received date: Jun 05, 2016; Accepted date: Jun 24, 2016; Published date: June 27, 2016
Citation: Miszewska-Szyszkowska D, Tronina O, √?¬?lubowska K, Miko√?¬?ajczyk-Korniak N, D√?¬?borska-Materkowska D, et al. (2016) A Case of Rare Cutaneous Mycobacteriosis and Central Nervous System Post-Transplant Lymphoproliferative Disorder in a Female Patient after Kidney Transplantation. J Infect Dis Ther 4:285. doi:10.4172/2332-0877.1000285
Copyright: © 2016 Miszewska-Szyszkowska D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Introduction
Progress in various medical fields made it possible to control chronic diseases which once quickly led to death, and — despite serious diagnoses — long-term and relative comfortable survival of patients may be achieved. Undoubtedly, one such a field is transplantology: modern immunosuppressive pharmacotherapy enables organ recipients to survive as long as several decades. The costs of aggressive treatment, leading to impairment of both cell-mediated and humoral immunity, include for instance serious infectious and neoplastic complications, which are rarely seen in the general population and difficult to diagnose. They include mycobacteria other than tuberculosis (MOTT) infections and post-transplant lymphoproliferative disorder, in a rare location, i.e. primary involvement of the central nervous system.
Below we present a case of rare bacterial and neoplastic complications, which occurred in a patient 7 years after kidney transplantation. Both in this case, as in other patients treated with immunosuppressants, we can assume that the total load of immunosuppression over the years has an impact on the increased incidence of infections, including those with atypical etiology or location, formerly known rather as an indicator for example AIDS. It should be noted that the patient was also treated with immunosuppressants prior to transplantation of the kidney, due to an autoimmune disease - that prolonged exposure to drugs.
Case Report
A Caucasian woman born in 1962, a professionally active dentist was diagnosed in 1990 with systemic lupus erythematosus complicated with class IV lupus nephritis. The patient was treated with glucocorticosteroids and cyclophosphamide. Because of progression of chronic kidney disease to its end-stage form, peritoneal dialysis therapy was performed in the years 2003–2005. In April 2005, the patient underwent kidney transplantation from a deceased donor. The recipient did not produce cytotoxic antibodies; she received treatment according to the following regimen: glucocorticosteroids, cyclosporine A, mycophenolate mofetil. In early peritransplantation period, she required surgical treatment of lymphorrhea and, as a result of grade IIB acute rejection, she received methylprednisolone infusions. Creatinine concentration settled at 1.1–1.4 mg/dl.
Until 2012, no significant medical problems had been observed, except for occasional urinary tract infections, which were treated in the outpatient setting. The patient remained professionally active. In spring 2012, she travelled as a tourist to Thailand and Cambodia.
In September 2012, the patient presented to hospital with dermatitis and panniculitis in the form of well-defined, reddened and painful lesions in the area the lower and upper extremities as well as the face persisting for several weeks and unsuccessfully treated with cephalosporin. A slight increase in inflammatory markers was observed (CRP: 40 mg/l), while blood and urine cultures remained sterile, with tests for the presence of autoantibodies also yielding negative results. Based on a consultation with an infectious disease specialist, a suspicion of erysipelas was raised for differentiation against systemic lupus erythematosus or systemic vasculitis. The patient received antibiotics (penicillin derivatives, quinolone, metronidazole and finally macrolide); the basic dose of glucocorticosteroids was increased. Improvement of skin lesions and CRP normalisation were achieved.
After three weeks, the patient was hospitalised again for myasthenia, diarrhoea and wandering arthralgia. Relatively low leukocytosis was diagnosed in spite of using large glucocorticosteroid doses, along with deficiency of complement component C3 and impaired renal function with an increase in serum creatinine concentration up to 2.3 mg/dl. A suspicion was raised of lupus exacerbation, and intravenous methylprednisolone infusions were administered. At the same time, a genetic test revealed high CMV viral load — 228,000 copies of virus/ml; ganciclovir was administered to good effect. Normalisation of serum creatinine concentration was achieved, but it was accompanied by intensification of skin lesions and inflammatory lesions in the knee joint area. Tests for autoantibodies were once more negative. Borrelia burgdorferi, Yersinia or Chlamydia infection was ruled out. The hospitalization was complicated with bilateral pneumonia, successfully controlled with cephalosporin, macrolide and metronidazole.
After three months, the patient was hospitalised in a rheumatology ward for exacerbation of skin lesions and arthralgia. At one time, the presence of cANCA was detected, ANA at a titre of 1:160 as well as weakly positive lupus anticoagulant. Since systemic vasculitis was suspected, glucocorticosteroid dosage was increased again.
After subsequent two months, the patient presented with fever and intensification of skin lesions; blood autoantibodies: negative, CRP: 280 mg/l. Escherichia coli sepsis was diagnosed of unknown origin successfully treated with ceftazidime. Furthermore, latent CMV infection reactivated, with a viral load of 41,000 copies of the virus/ml. In light of infection complications, an attempt was made at reducing the glucocorticosteroid dose, which led to intensification of skin lesions; mycophenolate mofetil was temporarily discontinued. In a collected skin specimen histopathological signs of abscess were found, with negative tests for mycobacteria and fungi. Because of a history of pneumonia, the patient was consulted by a pulmonologist, who sustained the previous suspicion of active lupus.
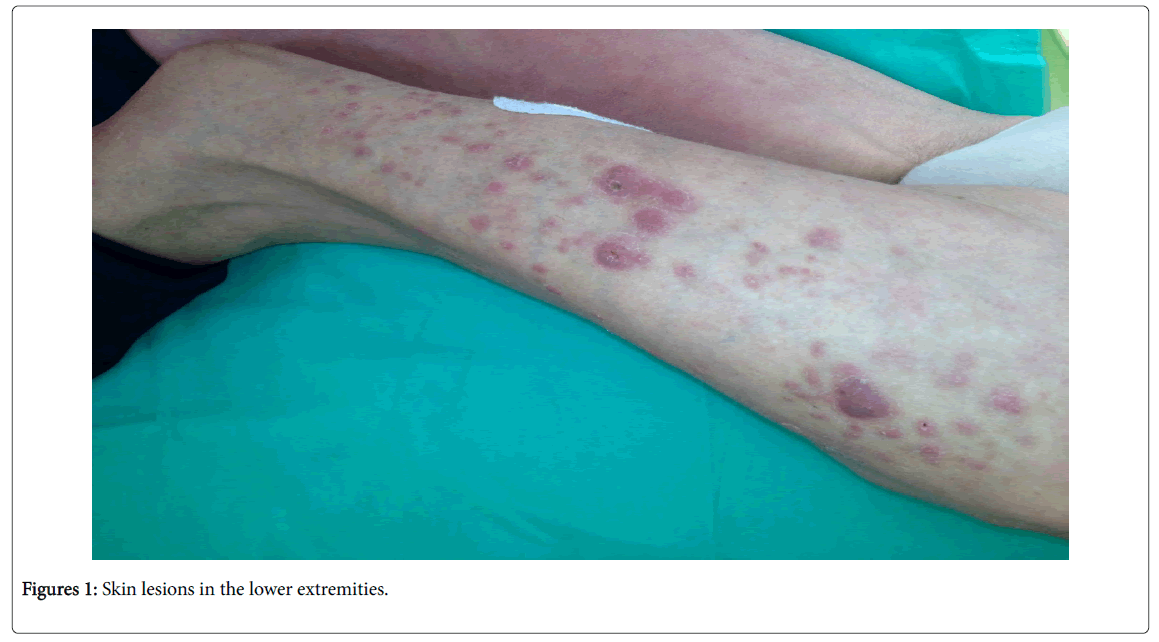
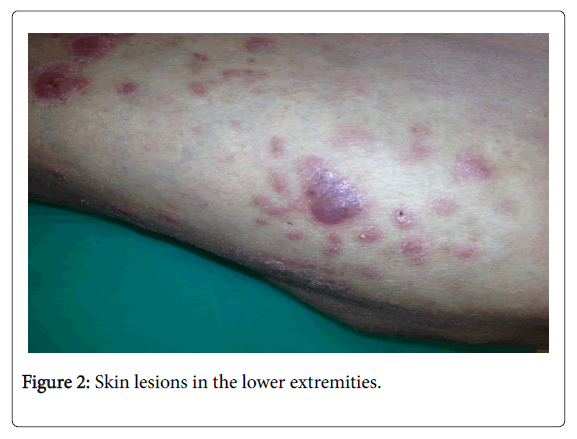
In summer 2013, the patient was hospitalised in a pulmonology ward. A skin specimen was collected again, with diagnosis of fibrous histiocytoma; Quantiferon blood test: negative. Appearance of new skin lesions was observed, including purulent ones (Figures 1 and 2).
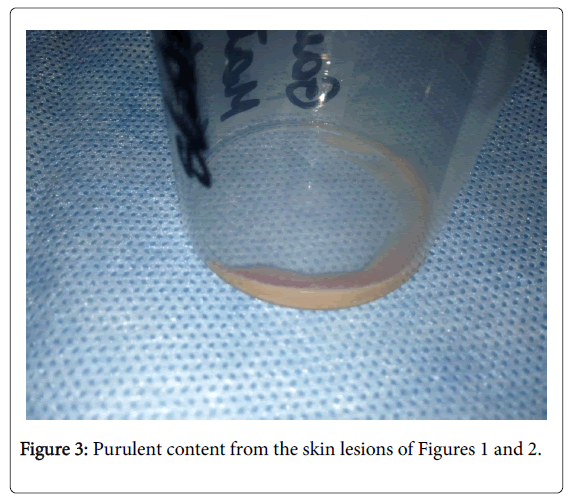
Because of inefficacy of the current treatment, skin specimens and purulent content were collected again in September (Figure 3), and empirical antimycobacterial treatment was initiated with rifampicin and ethambutol as well as clarithromycin. Cyclosporine and mycophenolate mofetil were discontinued, while maintaining glucocorticosteroid monotherapy. Information was received of the presence of numerous acid-fast mycobacteria in the skin material, which were later assessed as atypical.
At the same time, emerging left-sided hemiparesis was observed, accompanied by cerebellar syndrome, without meningeal signs. A CNS MRI scan revealed a 16 × 15 mm purulent lesion near the anterior horn of the right lateral ventricle, with mass effect, modelling and slight compression of the anterior horn as well as a smaller 5 mm focus in the posterior part of the left thalamus (Figure 4). A suspicion of neuroinfection was raised in the course of specific process.
Cerebrospinal fluid examination showed cytosis: 17 cells/mm3, protein: 1.19 g/l, glucose: 3.25 mmol/l, lactates: 1.95 mmol/l, granulocyte smear; fungal antigens and genetic test for mycobacteria: negative.
While waiting for the identification of skin mycobacteria, the patient was treated with rifampicin, isoniazid and ethambutol as well as carbapenem, trimethoprim/sulfamethoxazole and clarithromycin; she also required anti-oedema treatment with dexamethasone. An improvement in neurological conditions was observed, accompanied by regression of skin lesions. Creatinine concentration remained stable at approx. 2.5 mg/dl.
An attempt at discontinuing carbapenem for 2 weeks (discharge home) caused recurrence of fever and skin lesions. The mycobacteria were finally identified as MOTT — Mycobacterium peregrinum , in vitro resistant to streptomycin, ethambutol, rifampicin and isoniazid, while susceptible to ofloxacin, ethionamide, capreomycin and amikacin.
Two months after CNS lesions had been diagnosed, in a follow-up MRI scan in November 2013, a marked progression of the lesions was observed, with appearance of new foci. In light of suspected CNS mycobacteriosis, stereotactic brain biopsy was not performed because of the risk of disseminating infection along the biopsy path. Several blood EBV DNA tests performed yielded negative results. During further follow-up, the patient’s condition was stable, and she stayed at home for approx. 2 months, receiving rifabutin, moxifloxacin and ethionamide. The patient experienced marked regression of skin lesions and previously described lung abnormalities after bilateral pneumonia.
In February 2014, a follow-up CNS MRI scan was performed, revealing progression of the lesions compared to the examination from three months earlier. Because of inefficacy of the antimycobacterial treatment, the patient was referred to a neurological ward, where she underwent brain biopsy. Five tissue fragments were collected. An immunohistochemical analysis revealed: strong CD20/+/ membranous reaction, overexpression of BCL2/+/ and EBV/+/ — heterogeneous cytoplasmic reaction in most cells, associated with EBV infection, few cells with outstanding cytoplasmic reaction; nuclear Ki67 reaction observed in 70% of cells. Lymphomatoid granulomatosis was diagnosed, according to the WHO definition: DLBCL — diffuse large B-cell lymphoma. BCL2 overexpression indicated primary CNS involvement in the course of large B-cell lymphoma.
Involvement of other organs was ruled out with bone marrow punch biopsy and PET-CT. Despite the diagnosis made, the patient’s clinical condition remained fairly good, with no neurological deficits observed. A modified chemotherapy was introduced based on high intravenous doses of cytosine arabinoside and rituximab. Methotrexate was not administered due to chronic kidney disease. After three days of cytarabine treatment, a sudden deterioration of the general condition was observed, including disturbances of consciousness and hemiparesis. A CT scan revealed above all infratentorial herniation of the cingulate gyrus. After initiation of anti-oedema treatment and improvement in general condition, rituximab was administered. On subsequent days, the patients’ general condition deteriorated again. It was decided that the patient’s condition resulted from underlying disease progression and resistance to the treatment used. The patient died in March 2014, with functioning graft.
Discussion
In the presented case, rare conditions have been described: cutaneous mycobacteriosis of Mycobacterium peregrinum etiology and primary CNS lymphoma, both resulting from long-term immunity impairment in a patient with a history of systemic lupus erythematosus treated with immunosuppressants, dialysis therapy and 9 years of immunosuppression following kidney transplantation.
Atypical mycobacteria (MOTT — mycobacteria other than tuberculosis, NTM — nontuberculous mycobacteria) are organisms commonly found in the environment, most often in bodies of water and soil; they can form a biofilm on bathroom fittings, where they achieve several times higher concentrations than those found in the natural environment. So far, more than 125 NTM species have been identified, with approx. 50% of them having pathogenic potential in humans [1]. Until recently, no evidence had been known of their transmission between humans, but in the recent years cases have been described of infections in a population of cystic fibrosis patients within a facility [2]. One needs to note that not every patient with the presence of MOTT requires treatment due to colonisation of the respiratory, gastrointestinal and urogenital systems. Incidence is difficult to assess due to lack of a global MOTT infection registry; data are based on case reports as well as reports from different facilities and they indicate incidence of approx. 0.4% in kidney recipients, approx. 3% in heart recipients and approx. 8% in patients after lung transplantation [2]. Until 2014, 293 cases had been described of NTM infections among vascularised organ recipients [2]. The most commonly affected sites, except for the respiratory system, include the skin and subcutaneous tissue of the extremities and in proximity of wounds; paricatheter locations are also important (central venous catheters, Tenckhoff catheters). M. peregrinum belongs to so-called rapidly growing mycobacteria (RGM) [3], which means they grow quickly on media (within 7 days), unlike more frequently isolated slowly growing mycobacteria, e.g. M. avium or the most commonly observed in Central Europe – M. kansasii . M. peregrinum belongs to the M. fortuitum complex, responsible for 1–2% of all RGM infections. M. fortuitum exhibits in vitro resistance to typical antimycobacterial drugs, but — which distinguishes atypical mycobacteria from typical mycobacteria (M. tuberculosis) — this does not necessarily translate into in vivo resistance [3]. Increasing resistance to macrolides is observed, with maintained susceptibility to aminoglycosides, fluoroquinolones, carbapenems and trimethoprim/sulfamethoxazole [4], which is confirmed in the presented case by periods of skin lesion remissions during antibiotic treatment. What attract attention are comorbidities diagnosed in certain patients, such as CMV, Escherichia coli, Aspergillus, Nocardia or Pneumocystis jiroveci infections [4]. Among the reported M. fortuitum infection cases, the most common ones are catheter-related infections and infections arising from disruption of skin integrity. The optimum treatment time for cutaneous mycobacteriosis has not been definitively determined, but it is assumed the treatment should last for at least 6 months [5]. The ATS/IDSA recommendations on mycobacteriosis treatment in different patient populations are still applicable [6]. Mycobacteriosis has not been named so far a revelatory of a neoplastic disease.
Although post-transplant lymphoproliferative disorder affects only 1% of kidneys recipients (compared with 20% patients after small intestine transplantation) [7], it is — in light of the statistically most numerous kidney transplant population — an increasing diagnostictherapeutic challenge across the world. Primary CNS lymphoma accounts for 5–15% of all PTLD cases; it is diagnosed at a later time after transplantation than systemic lymphoma (on average 31 vs. 6 months) [8]. At the same time, it is an extranodal form of lymphoma associated with the worst prognosis, with lesions in the brain often being of diffuse, multi-focal nature [8]. In 90% of cases, these are large B-cell lymphomas (DLBCL). Lymphoma is a perfect “imitator”, which is often falsely diagnosed as an abscess in imaging examinations (marginal enhancement) [7], which proved true also in the presented case. It remains a mystery why lymphoma is localised in the CNS, where there are no lymphoid cells or lymphatic vessels. One of several theories that would explain the pathogenesis of this disease is one assuming that lymphocytes are taken up by the CNS during an inflammatory process and undergo neoplastic transformation, while another hypothesis suggests neoplastic B cells may have receptors for ligands on the surface of the CNS endothelium [9]. While the generalised form of lymphoma manifests morphological diversity, CNS PTLD usually has monomorphic nature. In 80% of cases, CNS PTLD is associated with EBV infection [8], although this pertains mostly to early forms of PTLD (with one year after transplantation); late PTLD cases are more strongly related to cumulative immunosuppressive load than to EBV status [7]. In the presented case, long-term treatment with calcineurin inhibitor was certainly a factor — both tacrolimus and cyclosporine increase risk of PTLD [10]. It is worth stressing that reports of primary CNS lymphomas as a special form of PTLD are limited in number [11]. This hampers development of the optimum treatment regimen. It is based on reduction of immunosuppression, which is insufficient in an emergency situation (central nervous system involvement), and one would need to wait several weeks for any effect. Standard lymphoma chemotherapy (CHOP) cannot be applied here because of poor penetration of the drugs through the blood-brain barrier, while intrathecal treatment is contraindicated in cerebral oedema. The treatment is based on cytosine arabinoside and methotrexate as well as glucocorticosteroids and rituximab, although one needs to remember about its low (1%) concentration in the cerebrospinal fluid [9,11].
Conclusion
The patient whose case has been descried was hospitalised 12 times in various wards during a 1.5-year period. For many months, she received high glucocorticosteroid doses for suspected active autoimmune disease. One year had passed from the appearance of the first skin lesions to diagnosis of mycobacteriosis, and lack of symptoms suggesting CNS disease might have been secondary to glucocorticosteroid treatment. The presented case illustrates diagnostic problems encountered while diagnosing rare diseases, the co-existence of which in one person seems unique, even in the selected population of post-transplant patients.
References
- Keating MR, Daly JS, AST Infectious Diseases Community of Practice (2013) Nontuberculous mycobacterial infections in solid organ transplantation. Am J Transplant 13: 77-82.
- Knoll BM (2014) Update on nontuberculous mycobacterial infections in solid organ and hematopoietic stem cell transplant recipients. Curr Infect Dis Rep16:421.
- Daley CL (2009) Nontuberculous mycobacterial disease in transplant recipients: early diagnosis and treatment. CurrOpin Organ Transplant 14:619-624.
- Han XY, De I, Jacobson KL (2007) Rapidly growing mycobacteria: clinical and microbiologic studies of 115 cases. Am J ClinPathol 128:612-621.
- Piersimoni C (2012) Nontuberculous mycobacteria infection in solid organ transplant recipients. Eur J ClinMicrobiol Infect Dis31:397-403.
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, et al. (2007) An official ATS/IDSA statement: diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am J RespirCrit Care Med 175:367-416.
- Sola-Valls N, Rodriguez NY, Arcal C, Duran C, Oppenheimer F, et al. (2014) Primary brain lymphomas after kidney transplantation: an under-recognized problem? J Nephrol27:95-102.
- KempfCh, Tinguely M, Rushing EJ (2013)Posttransplant lymphoproliferative disorder of the central nervous system. Pathobiology80:310-318.
- Szczepanek D, WĬ?sik-Szczepanek E, Stoma F (2011) Primary central nervous system lymphomas. ActaHaematologicaPolonica 42:367-373.
- Cavaliere R, Petroni G, Lopes MB, Schiff D, The International Primary Central Nervous System Lymphoma Collaborative Group (2010) Primary central nervous system post-transplantation lymphoproliferative disorder. Cancer15:863-870.
- Evens AM, Choquet S, Knoll-Desrosiers AR, Jagadeesh D, Smith SM, et al. (2013) Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transp13:1512-1522.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14251
- [From(publication date):
June-2016 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 13188
- PDF downloads : 1063