Research Article Open Access
A Comparative Study of Olmesartan and Valsartan on Insulin Sensitivity in Hypertensive Patients with Diabetes Mellitus or Impaired Glucose Tolerance (OVIS Study)
| Sadatoshi Biro1*, Tetsunori Saikawa2, Takatoshi Otonari3, Yasunori Sawayama4, Masato Ageta5, Hachiro Obata6, Suminori Kono7 and Jun Sasaki8 | |
| 1Tsukasa Health Care Hospital, Kagoshima, Japan | |
| 2Japan Community Health Care Organization Yufuin Hospital, Yufu, Japan | |
| 3Otonari Clinic, Fukuoka, Japan | |
| 4Hara Sanshin Hospital, Fukuoka, Japan | |
| 5Ageta Clinic, Nichinan, Japan | |
| 6Okino Hospital, Kagoshima, Japan | |
| 7Kyushu University, Faculty of Medical Sciences, Fukuoka, Japan | |
| 8International University of Health and Welfare, Graduate School of Pharmaceutical Medicine, Fukuoka, Japan | |
| Corresponding Author : | Dr. Sadatoshi Biro Tsukasa Health Care Hospital Kagoshima, Japan Tel: 81-92-711-1126 E-mail: birou@peace.ocn.ne.jp |
| Received July 08, 2014; Accepted July 23, 2014; Published July 25, 2014 | |
| Citation: Biro S, Saikawa T, Otonari T, Sawayama Y, Ageta M, et al. (2014) A Comparative Study of Olmesartan and Valsartan on Insulin Sensitivity in Hypertensive Patients with Diabetes Mellitus or Impaired Glucose Tolerance (OVIS Study). Clin Pharmacol Biopharm 3:118. doi:10.4172/2167-065X.1000118 | |
| Copyright: © 2014 Biro S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Activation of renin angiotensin system is implicated in insulin resistance. In mega trials, it has been suggested that angiotensin receptor blockers may be beneficial on insulin sensitivity in hypertensive patients. We conducted a multicenter, open-label, parallel-group trial to compare the effects of olmesartan and valsartan on insulin sensitivity and adiponectin levels in 206 hypertensive patients with diabetes mellitus or impaired glucose tolerance. Patients were randomly assigned to either olmesartan 20 mg/day or valsartan 80 mg/day treatment for 24 weeks. Blood pressure, fasting glucose, fasting insulin, glycosylated hemoglobin (HbA1c), homeostasis model assessment for insulin resistance (HOMA-IR), and serum adiponectin levels were measured. The efficacy was evaluated in 197 patients (olmesartan, n=98; valsartan, n=99). At baseline, all parameters except for systolic blood pressure (SBP) and serum triglyceride did not differ between the 2 groups. HbA1c decreased slightly after a 24-week treatment with valsartan, but not olmesartan, while the decrease did not significantly differ in the two groups. There was no difference in the change from the baseline between olmesartan and valsartan groups concerning fasting glucose, fasting insulin, HOMA-IR, and adiponectin levels after 24-week treatment. The decrease in SBP tended to be greater in the olmesartan group than in the valsartan group even with adjustment for the baseline difference. In conclusion, there was no significant difference in insulin sensitivity or adiponectin levels between the olmesartan and valsartan groups. In the standard dose, olmesartan significantly decreased SBP as compared with valsartan.
| Keywords |
| Angiotensin II type 1 receptor blocker; Insulin sensitivity; Adiponectin; Olmesartan; Valsartan |
| Introduction |
| Angiotensin II type I receptor blockers (ARBs), blocking agents of the renin angiotensin system (RAS), have been world-wide prescribed as the first line therapeutic agents in patients with hypertension. ARBs have been shown to have an action of end-organ protection, including cardiac, renal and vascular protection [1,2], as well as a blood-pressure lowering effect. This action has been ascribed to modulation of tissue remodeling by inhibition of chronic RAS activation. In clinical trials, ARBs have significantly reduced the risk of cardiovascular diseases, such as myocardial infarction, heart failure and stroke [2,3]. Furthermore, ARBs have been reported to improve cardiac hypertrophy [4] and proteinuria or albuminuria [5,6]. |
| In recent large studies, ARBs significantly reduced the incidence of new onset diabetes mellitus in patients with or without hypertension who were at high risk of developing diabetes [7,8]. This finding suggests that ARBs may improve the insulin resistance in hypertensive patients with diabetes mellitus. Several [9-14], but not all [15-17], studies have demonstrated that ARBs have improved the glucose metabolism parameters. |
| Adiponectin is one of the adipokines, a variety of biologically active peptides, which are produced and secreted by adipose tissues [18]. This peptide has attracted much attention because it has anti-diabetic, antiinflammatory and anti-atherogenic properties. It is still controversial whether ARBs increase the levels of adiponectin [10,11,14,19-23]. |
| We conducted a comparative trial using valsartan, a classical ARB proved to reduce the incidence of diabetes mellitus in VALUE study [24] and olmesartan, a relatively new ARB, to investigate the effects on insulin sensitivity and adiponectin levels in hypertensive patients with diabetes mellitus or impaired glucose tolerance. |
| Methods |
| Patients |
| Patients were recruited from 34 clinics and hospitals in Kyushu District, Japan, between February 2007 and March 2008. The study subjects were male and female outpatients who had blood pressure higher than the goals recommended by the guidelines for the management of hypertension in Japan. The subjects were aged 20 years or older and had diabetes mellitus or impaired glucose tolerance, i.e., fasting glucose ≥ 110 mg/dl, 1-h plasma glucose ≥ 180 mg/dl or 2-h plasma glucose ≥ 140 mg/dl in an oral glucose tolerance test, or casual plasma glucose ≥ 140 mg/dl. Patients with any of the following conditions were excluded: use of angiotensin converting enzyme inhibitors (ACEIs) or ARBs; type I diabetes mellitus; use of insulin or thiazolidinediones; poor control of diabetes mellitus (≥ 8% of HbA1c); secondary hypertension; severe renal or liver dysfunction; allergy to olmesartan or valsartan; and being inappropriate for participation as assessed by study physician. |
| Study design |
| This was a multi-center, open-label, parallel-group trial. Eligible patients were enrolled at the central registration center and were randomized to receive either of olmesartan (20 mg/day) or valsartan (80 mg/day) treatment for 24 weeks. The doses of the study drugs are approved as standard in Japan. A computer-generated list of 220 random assignments was prepared by a statistician at the registration center using the random permutation block method with equal assignments to the two treatment groups. Allocation to each treatment was made according to the sequence of the randomization list, which was kept confidential throughout the study period. |
| After obtaining informed consent, study physicians reported eligible patients to the registration center by fax and were informed of the assigned treatment during a run-in period of 2-4 weeks. All study physicians followed the allocation correctly. The run-in period corresponded to a typical interval in time between clinic visits. Follow-up measurements were carried out at 12 and 24 weeks; the measurements of body weight, waist circumference and serum adiponectin were scheduled only at 24 weeks. |
| Concomitant use of ARBs other than the study drugs and ACEIs was not allowed during the study. Use of calcium channel blockers was permitted when the target blood pressure level, as defined by the Japanese Society of Hypertension, was not achieved after 8-12 weeks of treatment. Advice on diet and exercise were not specified for the study. Agents prescribed before enrollment was permitted on the condition that the doses were not changed during the study. Adherence to the study drug was assessed by asking patients to report their use of the prescribed dose at weeks 12 and 24 with four options regarding drug use during the interval between clinic visits (daily, 5-6 days per week, 3-4 days per week and 1-2 days per week). Daily use or use on 5–6 days per week was defined as good adherence. The study protocol was reviewed and approved by the ethics committee of each institution. |
| Blood pressure measurements |
| Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by an automatic apparatus (Omron HEM-1000), which was provided for each participating clinic or hospital by the central administration office. Three measurements were repeated with patients sitting on a chair for at least 5 minutes in a relaxed position. The second and third readings were averaged and adopted as the measurements for use. |
| Laboratory measurements |
| Blood samples were collected after an overnight fast at baseline and after 12 and 24 weeks of treatment. All determinations were performed at an external laboratory (SRL, Hachiohji, Japan) where the biochemical measurements were routinely under quality-control procedures. Plasma glucose was measured by the hexokinase method and fasting serum insulin by chemiluminescent enzyme immunoassay. HbA1c was assayed by the latex agglutination immunoassay. The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated by dividing the product of fasting plasma glucose (mg/ dL) and fasting serum insulin (IU/L) by 405. High-sensitive C-reactive protein (hs-CRP) was measured by immunonephelometry, and total adiponectin was measured by ELISA. hs-CRP values of >10 mg/L were discarded because acute inflammation was suspected. The detection limit of hs-CRP concentrations was 0.05 mg/L, and undetectable values were recorded as 0.025 mg/L. |
| Effectiveness outcomes |
| The primary effectiveness outcome measures were changes in fasting glucose, fasting insulin, glycosylated hemoglobin (HbA1c), and homeostasis model assessment for insulin resistance (HOMA-IR) after the 24-week treatment. The effects on adiponectin and blood pressure were of secondary interest. The effects on serum hs-CRP and serum lipids were of subsidiary interest. |
| Tolerability outcomes |
| Tolerability was assessed at each visit and included adverse events either spontaneously reported or elicited by questioning, physical examination findings, and clinical laboratory test results. Study physicians rated the causal relationship of adverse events to study medication as unrelated, suspected or probable. These ratings were finalized by the Safety Monitoring Committee in a blinded manner. Serious adverse events were defined as any untoward medical conditions that resulted in death, hospitalization, life-threatening condition or birth defect. |
| Abnormal laboratory test results were defined as values >1.5 times the upper limit of the reference range. |
| Statistical analysis |
| The change in the parameter from the baseline was of primary interest in the analysis, but the between-subject variation in the change was unknown. We thus used sample-size estimation in the comparison of two means of after-treatment values. We assessed two conditions; one was that HOMA-IR would be 15% lower in olmesartan treatment than in valsartan treatment, and another was that the mean value of HOMA-IR would be 1.4 (SD 0.5) in the latter with reference to a previous study [9]. A required sample size was calculated as 89 for each group, with a two-sided significance level of 0.05 and a statistical power of 0.80. With allowance for a dropout rate of 10%, we decided to recruit a total of 200 patients. |
| The mean ± SD was used as summary statistics of the continuous variables for ease of presentation. The effects on the outcome measurements were evaluated by the change at 12 and 24 weeks from the baseline. The between-group comparison was assessed by Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for dichotomous variables. The difference from the baseline in each group (within-group comparison) was evaluated by Wilcoxon singed rank test. Analysis of covariance was used to control for the change in adiposity or the baseline value. Statistical significance was declared if two-sided P value was less than 0.05. All statistical computations were performed using Stata Release 10.0 (Stata Corporation, College Station, TX, USA). |
| Results |
| Study population |
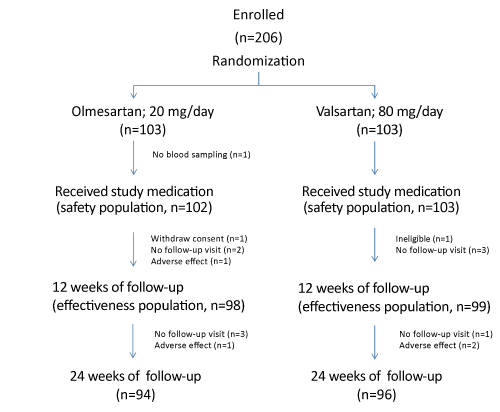
| A total of 206 patients were enrolled and randomly allocated to olmesartan or valsartan treatment (Figure 1). After randomization, it was found that one patient in the olmesartan group did not initiate the medication because blood sampling at 0 week was not done successfully. Of 205 patients who started the medication, one patient was found to have been under insulin therapy, and 7 patients failed to continue the medication in the first 12 weeks because of withdrawal of consent (n=1), adverse effect (n=1), and no follow-up visit (n=5). The remaining 197 patients (98 in the olmesartan group and 99 in the valsartan group) continued the medication at least until the first followup visit at 12 weeks, and 190 patients (94 in the olmesartan group and 96 in the valsartan group) completed the second follow-up visit at 24 weeks. The 197 patients who had at least one follow-up visit were included in the efficacy analysis, and the 205 patients who initiated the medication under study were used in the analysis on safety. |
| Baseline characteristics |
| The baseline characteristics of the patients in the efficacy analysis are shown in Table 1. There were no significant differences in the ratio of men to women, smoking habits, alcohol consumption or comorbid conditions (diabetes mellitus, renal disease, coronary artery disease, heart failure and dyslipidemia) between the two groups. |
| The baseline values for the outcome variables are presented in Table 2. There was no significant difference between the two groups in terms of glucose metabolism parameters, adiponectin and hs-CRP. At baseline, SBP and serum triglycerides were slightly higher in the olmesartan group than in the valsartan group, the differences being statistically significant. |
| Changes in glucose metabolism parameters |
| As shown in Table 3, a statistically significant decrease in HbA1c was observed at 24 weeks in the valsartan group (P=0.03), but not in the olmesartan group (P=0.72), and the between-group difference was nearly statistically significant (P=0.06). None of the other glucose metabolism parameters showed a measurable between-group difference in the change at either 12 weeks or 24 weeks. Waist circumference decreased slightly in both olmesartan and valsartan groups, each with a statistical significance (P<0.01). With adjustment for the change of waist circumference, the adjusted mean change of HbA1c at 24 weeks were almost the same as the unadjusted values (–0.13% versus –0.04%), but the between-group difference was far from the statistical significance (P=0.25). Neither hs-CRP nor adiponectin levels showed a differential change between the two groups. |
| Changes in blood pressure and serum lipids |
| Both SBP and DBP decreased substantially after treatment in both of the two groups (Table 4). The decrease in blood pressure was much greater in the olmesartan group than in the valsartan group at both 12 and 24 weeks. The olmesartan group also showed a significantly greater decrease in pulse. |
| After adjustment for the baseline value, the between-group difference in the change of blood pressure and pulse were attenuated substantially. The adjusted means of the change in SBP were –20.5 mmHg in the olmesartan group and –17.4 mmHg in the valsartan group at 12 weeks (P=0.06), and the corresponding values at 24 weeks were –22.3 mmHg and –18.2 mmHg, respectively (P=0.01). The change of DBP did not attain a statistically significant between-group difference either at 12 weeks (P=0.051) or 24 weeks (P=0.20). Nor did the change of pulse (P=0.08 at 12 weeks and P=0.95 at 24 weeks). |
| Adherence to drug use |
| At weeks 12, information on adherence was available for all of the patients, and proportions of the subjects who reported to have taken the study drug daily (excellent), 5-6 days (good), and 3-4 days (moderate) per week were 85.7%, 9.2%, and 5.1%, respectively in the olmesartan group, and the corresponding values in the valsartan group were 92.9%, 5.1%, and 2.0%, respectively (P=0.25 on the basis of chi-square with 2 degrees of freedom). At weeks 24, 89 patients on olmesartan and 87 on valsartan reported adherence, and proportions of excellent, good, and moderate adherence were 88.3%, 5.3%, and 6.4% respectively in the former group, and 92.7%, 4.2% and 3.1% respectively in the latter group (P=0.52). |
| Adverse events |
| Two cases of serious adverse events were noted in the olmesartan group, including acute respiratory failure and bone fracture, and two serious adverse events were observed in the valsartan group, such as liver abscess and oligohydramnios. |
| Overall, adverse events occurred in 25 (24.5%) and 15 patients (14.6%) in the olmesartan and valsartan groups, respectively, including mild elevation of uric acid, digestive symptoms and upper respiratory infection. Discontinuation of treatment was only necessary in the abovementioned three patients with serious adverse events, except for the patient with bone fracture. |
| Discussion |
| In the present study, there was no significant difference between the olmesartan and valsartan groups in the glucose metabolism as assessed by fasting glucose, fasting serum insulin, HbA1c and HOMA-IR after 24 weeks of treatment and none of them improved insulin sensitivity. Furthermore, no significant difference in the level of adiponectin between the both groups was observed. With respect to the effect of lowering blood pressure, olmesartan (20 mg/day) resulted in a greater decrease in systolic blood pressure than valsartan (80 mg/day) after 24 weeks of treatment in the setting of the standard dose in Japan. |
| On the incidence of new onset diabetes with the use of ARBs, candesartan [25,26], losartan [27] and valsartan [24] have been reported to significantly reduce it in large scaled randomized controlled trials. Two mega trials using telmisartan showed a favorable trend but not statistical significance [28,29]. However, in comparative studies among ARBs on insulin sensitivity, telmisartan has been applied most frequently because of having partial peroxisome proliferator-activated receptor gamma (PPARγ) agonist activity [30]. No direct comparison was carried out on the effects of olmesartan and valsartan on glucose metabolism. Regarding randomized comparative trials using valsartan or olmesartan, only 2 studies were reported. In a comparative trial on telmisartan (40 mg/day, n=74), candesartan (8 mg/day, n=79) and valsartan (80 mg/day, n=74) in hypertensive patients with type 2 diabetes mellitus [17], no significant difference in insulin sensitivity among three ARBs was observed and none of them improved the insulin sensitivity after 3 months of treatment. Another comparative study of telmisartan (80 mg/day, n=34) vs. olmesartan (40 mg/day, n=31) in hypertensive obese patients showed that 3 months treatment of telmisartan significantly improved insulin resistance, but olmesartan did not [23]. |
| As for olmesartan, 2 studies have evaluated the effect on insulin sensitivity except for the comparative study mentioned above. One study demonstrated that 40 mg/day olmesartan treatment for 16 weeks resulted in a significant decrease of fasting plasma glucose, fasting insulin, HOMA index and HbA1c in patients (n=52) with chronic kidney disease without diabetes mellitus [10]. In a crossover study using olmesartan (20 mg/day) and telmisartan (40 mg/day) for 8 weeks, there was no difference between the groups in metabolic parameters (HbA1c and HOMA-IR) and adiponectin levels in Japanese early stage type 2 diabetics with hypertension [31]. On the other hand, 3 studies using valsartan showed an effect of improving insulin sensitivity in maintenance hemodialysis patients (n=10) for 12 weeks treatment [12], in obese patients with impaired glucose tolerance (n=13) for 4 weeks treatment [14], and in normotensive subjects with impaired glucose tolerance (n=40) for 26 weeks therapy [32]. However, in a trial using 80 mg/day valsartan, it did not improve insulin sensitivity in 91 patients with diabetes and mild-to-moderate hypertension [33]. As stated above, the effects of olmesartan or valsartan on insulin sensitivity were still controversial. One of the reasons for inconsistency is that profiles of patients and study designs were different. Another reason may be a small sample-size; the patients mostly numbered less than 100 in one arm. Anyway, further mega trial or meta-analysis will be needed. |
| The possible mechanisms by which ARBs may improve the insulin resistance are hemodynamic effects, increase of glucose transport and improvement of the intracellular signal transduction of insulin [34,35]. Furthermore, these effects may be due to blocking the oxidative stress and the reduction of adiponectin level [36]. Telmisartan and irbesartan have a partial agonist action of PPARγ [30] and are expected to have beneficial effects on insulin resistance by increasing adiponectin levels than the other ARBs without such action. However, supportive data were published in 6 of 12 clinical studies using telmisartan [11,13,15,17,22,23,37-42] and in 1 of 2 studies using irbesartan [11,16], indicating almost same rates comparing with those using other ARBs [9,10,12,14,17,23,32,33]. |
| In our study patients neither olmesartan nor valsartan increased the levels of adiponectin without any difference between them. Also on adiponectin levels, no direct comparative study of olmesartan vs. valsartan was found. For olmesartan, adiponectin levels were examined in 3 studies. In all of them [10,20,23], olmesartan treatment did not increase the levels of adiponectin. There were 3 trials investigating the effects of valsartan on adiponectin levels: Valsartan (160 mg/day) significantly increased it in 13 obese patients with impaired glucose tolerance after 4 weeks treatment [14], valsartan (160 mg/day, for 12 weeks) increased it in 91 hypertensive patients with diabetes mellitus [33], and 80 mg of valsartan also increased it in 20 hypertensive patients with metabolic syndrome after 3 months therapy [19]. Although the reasons for inconsistency with the data in the present study cannot be explained clearly, the reasons may be the difference in the background of study patients and study designs. Further study will be needed. |
| A notable finding in the present study was that the decrease in SBP was significantly greater in the olmesartan group than in the valsartan group in the standard dose. DBP and pulse also showed a slightly greater decrease in the olmesartan group (Table 4). The baseline values of these measurements, especially of SBP, were unexpectedly higher in the olmesartan group than in the valsartan group despite randomized allocation. A greater decrease in blood pressure or pulse would be more likely to occur when the baseline value is higher. The difference in the decrease in SBP was slightly attenuated with statistical adjustment for the baseline value, but the decrease in SBP remained greater in the olmesartan group; adjusted mean decreases derived from analysis of covariance in the olmesartan and valsartan groups were 22.1 mmHg and 17.5 mmHg, respectively (P=0.008). On the other hand, the adjusted mean decrease did not show a measurable difference between olmesartan and valsartan with respect to DBP (12.0 mmHg versus 10.6 mmHg, P=0.26) and pulse (0.7 versus 0.3, P=0.66). In this study design, dose up of the drugs or additional use of calcium channel blockers was allowed when the target blood pressure level was not achieved after 8-12 weeks of treatment. The dose of valsartan was increased to 160 mg/day in 2 patients because of insufficient lowering of blood pressure, but none in the olmesartan group. Addition of calcium channel blocker or β-blocker was observed in 3 patients in the olmesartan group and 4 in the valsartan group. |
| There are some limitations in the present study. The patient number was relatively small and study duration was relatively short. Furthermore, insulin sensitivity was assessed mainly by HOMA-IR in this study. More precise methods such as the glucose clamp technique should be employed. |
| Conclusion |
| In Japanese hypertensive patients with diabetes mellitus or impaired glucose tolerance, there was no difference in the glucose metabolism parameters between olmesartan and valsartan treatment, and none of them improved the insulin sensitivity. These drugs also did not increase the levels of adiponectin without the between-group difference. Olmesartan seems to have a more potent effect of lowering blood pressure than valsartan in the standard dose. |
| Acknowledgements |
| This study was funded by a clinical research grant from the International University of Health and Welfare, Tochigi, Japan. |
| The study’s Executive Committee consisted of J Sasaki (Principal Investigator and Member of Protocol Committee), International University of Health and Welfare Graduate School of Public Health Medicine, Fukuoka, Japan; T Saikawa (member of Protocol Committee), Oita University, Oita, Japan; T Kuribayashi, Koga General Hospital, Miyazaki; S Biro (member of Protocol Committee), Tsukasa Hospital, Kagoshima, Japan; K Yamamoto (member of the Safety Monitoring Committee), Takagi Hospital, Fukuoka, Japan; S Ikeda, Second Department of Internal Medicine, Nagasaki University, Nagasaki, Japan. The Safety Monitoring Committee consisted of N Okabe, Ayasugi Building Clinic, Fukuoka, Japan. The head of the Registration Center and Data Center was S Kono (member of Protocol Committee), Kyushu University Faculty of Medical Sciences, Fukuoka, Japan. |
| We thank the following physicians who participated in the study: Y Ikeda, T Inou, M Tanaka, K Yamamoto, S Matano, S Nobe, H Ozaki, Y Ohshima, A Yoshimura, T Ooie, H Ono, S Ishida, K Shinozaki, K Yano, M Saito, N Abe, K Okamoto, H Toshimori, S Kariya, M Ageta, T Hashino, H Kaieda, S Suzuki, K Kurobe, M Kojima, R Migita, H Koga, S Ikeda, A Takahashi, and Y Uchida. |
| References |
|
References
- Weir MR (2007) Effects of renin-angiotensin system inhibition on end-organ protection: can we do better? Clin Ther 29: 1803-1824.
- Gupta M, Honos GN, Velazquez EJ, Chung N, Oigman W, et al. (2010) Evidence for the efficacy of ARBs across the cardiovascular continuum. Curr Med Res Opin 26: 1203-1218.
- Verdecchia P, Angeli F, Mazzotta G, Ambrosio G, Reboldi G (2010) Angiotensin receptor blockers in hypertension. New insights from Japan. Hypertens Res 33: 394-397.
- Milan A, Caserta MA, Avenatti E, Abram S, Veglio F (2010) Anti-hypertensive drugs and left ventricular hypertrophy: a clinical update. Intern Emerg Med 5: 469-479.
- Tocci G, Volpe M (2011) End-organ protection in patients with hypertension: focus on the role of angiotensin receptor blockers on renal function. Drugs 71: 1003-1017.
- Saikawa T, Sasaki J, Biro S, Kono S, Otonari T, et al. (2010) Is the reno-protective effect of valsartan dose dependent? A comparative study of 80 and 160 mg day(-1). Hypertens Res 33: 886-891.
- Elliott WJ, Meyer PM (2007) Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 369: 201-207.
- Al-Mallah M, Khawaja O, Sinno M, Alzohaili O, Samra AB (2010) Do angiotensin converting enzyme inhibitors or angiotensin receptor blockers prevent diabetes mellitus? A meta-analysis. Cardiol J 17: 448-456.
- Anan F, Takahashi N, Ooie T, Hara M, Yoshimatsu H, et al. (2004) Candesartan, an angiotensin II receptor blocker, improves left ventricular hypertrophy and insulin resistance. Metabolism 53: 777-781.
- de Vinuesa SG, Goicoechea M, Kanter J, Puerta M, Cachofeiro V, et al. (2006) Insulin resistance, inflammatory biomarkers, and adipokines in patients with chronic kidney disease: effects of angiotensin II blockade. J Am Soc Nephrol 17: S206-212.
- Negro R, Formoso G, Hassan H (2006) The effects of irbesartan and telmisartan on metabolic parameters and blood pressure in obese, insulin resistant, hypertensive patients. J Endocrinol Invest 29: 957-961.
- Satirapoj B, Yingwatanadej P, Chaichayanon S, Patumanond J (2007) Effect of angiotensin II receptor blockers on insulin resistance in maintenance haemodialysis patients. Nephrology (Carlton) 12: 342-347.
- Satoh M, Tabuchi T, Minami Y, Takahashi Y, Itoh T, et al. (2009) Prospective, randomized, single-blind comparison of effects of 6 months of treatment with telmisartan versus enalapril on high-molecular-weight adiponectin concentration in patients with coronary artery disease. Clin Ther 31: 2113-2125.
- Pscherer S, Heemann U, Frank H (2010) Effect of Renin-Angiotensin system blockade on insulin resistance and inflammatory parameters in patients with impaired glucose tolerance. Diabetes Care 33: 914-919.
- Bahadir O, Uzunlulu M, Oguz A, Bahadir MA (2007) Effects of telmisartan and losartan on insulin resistance in hypertensive patients with metabolic syndrome. Hypertens Res 30: 49-53.
- Parhofer KG, Birkeland KI, DeFronzo R, Del Prato S, Bhaumik A, et al. (2010) Irbesartan has no short-term effect on insulin resistance in hypertensive patients with additional cardiometabolic risk factors (i-RESPOND). Int J Clin Pract 64: 160-168.
- Ozaki N, Nomura Y, Sobajima H, Kondo K, Oiso Y (2010) Comparison of the effects of three angiotensin II receptor type 1 blockers on metabolic parameters in hypertensive patients with type 2 diabetes mellitus. Eur J Intern Med 21: 236-239.
- Ohashi K, Ouchi N, Matsuzawa Y (2011) Adiponectin and hypertension. Am J Hypertens 24: 263-269.
- Yilmaz MI, Sonmez A, Caglar K, Celik T, Yenicesu M, et al. (2007) Effect of antihypertensive agents on plasma adiponectin levels in hypertensive patients with metabolic syndrome. Nephrology (Carlton) 12: 147-153.
- Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, et al. (2010) Differential effects of candesartan and olmesartan on adipose tissue activity biomarkers in type II diabetic hypertensive patients. Hypertens Res 33: 790-795.
- Makita S, Abiko A, Naganuma Y, Moriai Y, Nakamura M (2008) Effects of telmisartan on adiponectin levels and body weight in hypertensive patients with glucose intolerance. Metabolism 57: 1473-1478.
- Usui I, Fujisaka S, Yamazaki K, Takano A, Murakami S, et al. (2007) Telmisartan reduced blood pressure and HOMA-IR with increasing plasma leptin level in hypertensive and type 2 diabetic patients. Diabetes Res Clin Pract 77: 210-214.
- de Luis DA, Conde R, González-Sagrado M, Aller R, Izaola O, et al. (2010) Effects of telmisartan vs olmesartan on metabolic parameters, insulin resistance and adipocytokines in hypertensive obese patients. Nutr Hosp 25: 275-279.
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, et al. (2004) Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 363: 2022-2031.
- Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. (2003) Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 362: 759-766.
- Ogihara T, Nakao K, Fukui T, Fukiyama K, Ueshima K, et al. (2008) Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trial. Hypertension 51: 393-398.
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, et al. (2002) Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359: 995-1003.
- Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, et al. (2008) Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 359: 1225-1237.
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators, Yusuf S, Teo K, Anderson C, Pogue J, et al. (2008) Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 372: 1174-1183.
- Schupp M, Janke J, Clasen R, Unger T, Kintscher U (2004) Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation 109: 2054-2057.
- Nakayama S, Watada H, Mita T, Ikeda F, Shimizu T, et al. (2008) Comparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early-stage type-2 diabetics with hypertension. Hypertens Res 31: 7-13.
- van der Zijl NJ, Moors CC, Goossens GH, Hermans MM, Blaak EE, et al. (2011) Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: a randomized controlled trial. Diabetes Care 34: 845-851.
- Lee JM, Kim JH, Son HS, Hong EG, Yu JM, et al. (2010) Valsartan increases circulating adiponectin levels without changing HOMA-IR in patients with type 2 diabetes mellitus and hypertension. J Int Med Res 38: 234-241.
- Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME (2005) Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens 23: 463-473.
- Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, et al. (2006) Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281: 35137-35146.
- Manrique C, Lastra G, Gardner M, Sowers JR (2009) The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am 93: 569-582.
- Derosa G, Cicero AF, Bertone G, Piccinni MN, Fogari E, et al. (2004) Comparison of the effects of telmisartan and nifedipine gastrointestinal therapeutic system on blood pressure control, glucose metabolism, and the lipid profile in patients with type 2 diabetes mellitus and mild hypertension: a 12-month, randomized, double-blind study. Clin Ther 26: 1228-1236.
- Hinoi T, Tomohiro Y, Kajiwara S, Mastuo S, Fujimoto Y, et al. (2008) Telmisartan, an angiotensin II type 1 receptor blocker, improves coronary microcirculation and insulin resistance among essential hypertensive patients without left ventricular hypertrophy. Hypertens Res 31: 615-622.
- Komiya N, Hirose H, Kawabe H, Itoh H, Saito I (2009) Effects of telmisartan therapy on metabolic profiles and serum high molecular weight (HMW)-adiponectin level in Japanese male hypertensive subjects with abdominal obesity. J Atheroscler Thromb 16: 137-142.
- Fogari R, Zoppi A, Ferrari I, Mugellini A, Preti P, et al. (2009) Comparative effects of telmisartan and eprosartan on insulin sensitivity in the treatment of overweight hypertensive patients. Horm Metab Res 41: 893-898.
- Nakamura T, Kawachi K, Saito Y, Saito T, Morishita K, et al. (2009) Effects of ARB or ACE-inhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Heart J 50: 501-512.
- Hsueh W, Davidai G, Henry R, Mudaliar S (2010) Telmisartan effects on insulin resistance in obese or overweight adults without diabetes or hypertension. J Clin Hypertens (Greenwich) 12: 746-752.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 15951
- [From(publication date):
November-2014 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 11106
- PDF downloads : 4845
