Research Article Open Access
A Laboratory Study for the Treatment of Turbidity and Total Hardness Bearing Synthetic Wastewater/Ground Water Using Moringa oleifera
Sasikala S and Muthuraman G*
Department of Chemistry, Presidency College, University of Madras, Chennai, Tamil Nadu, India
- *Corresponding Author:
- G. Muthuraman
Department of Chemistry, Presidency College
University of Madras, Chennai-600 005, Tamil Nadu, India
Tel: +9104428544894
Fax: +9104428510732
E-mail: raman.gm@gmail.com
Received date: December 22, 2015; Accepted date: January 12, 2016; Published date: January 17, 2016
Citation: Sasikala S, Muthuraman G (2016) A Laboratory Study for the Treatment of Turbidity and Total Hardness Bearing Synthetic Wastewater/Ground Water Using Moringa oleifera. Ind Chem 2:112. doi:10.4172/2469-9764.1000112
Copyright: © 2016 Sasikala S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Industrial Chemistry
Abstract
The coagulation performance of Moringa oleifera act as natural macromolecular coagulant was studied by the jar test. The Moringa oleifera coagulation attained comparatively high turbidity removal efficiency and water with turbidity less than 5 NTU could be obtained from 100 to 500 NTU. The ground water sample was collected from Chennai, Chepauk. The water sample containing bicarbonate (HCO3), Calcium (Ca2+) and Magnesium (Mg2+) hardness. The Moringa oleifera seed powder and Moringa oleifera (MO) that was extracted with 0.5M Sodium chloride solution (MO - NaCl) was used to reduce the turbidity and hardness of the ground water sample. Maximum reduction of hardness was attained at basic pH 8-11. The adsorption equilibrium was attained at short contact time of 2 hrs maximum uptake of hardness was 76 mg/l. The flocs formed using Moringa oleifera were observed to be bigger and to sediment faster when compared with flocs formed using alum. Moringa oleifera sludge volume produced was less than other methods. The comparison to the surface morphology structure of dried seed powder and total suspended solids were also studied.
Keywords
Moringa oleifera; Hardness; Turbidity removal; Surface morphology; Sludge volume
Introduction
About one billion people do not have healthy drinking water. More than six million people (about two million people) die because of diarrhea which is caused by the polluted water. Developing countries pay a high cost to import chemicals including Polyaluminium chloride and alum [1-3]. This is the reason why these countries need low cost methods requiring low maintenance and skill. Nowadays, Inorganic coagulants (e.g., aluminium sulphate, ferric chloride and calcium carbonate) and synthetic organic polymers (e.g., polyaluminium chloride (PACl) and polyethylene mine) are common coagulants used in this treatment [4,5]. Among all the available coagulants, including other inorganic and organic chemicals, aluminium salts are the most widely used worldwide because of their effectiveness and competitive cost. However the sludge obtained from treatments using aluminum salts leads to disposal, problems such as aluminium accumulation in the environment. Moreover some studies have reported that residual aluminium sulphate (alum) and polyaluminium chloride may induce Alzheimer’s disease. Whereas the synthetic organic polymers such as acrylamide have neurotoxic and carcinogenic effects [6,7].
Moringa oleifera is a tree of Moringaceae family with 14 species. This is the species belonging to the south of India which is the most famous one among all species. The advantages of Moringa oleifera usage in water treatment will be mentioned later in the paper. Antimicrobial factor (rhamnosyloxy benzyl-Isothiocyanate) has been found in 4 log coli forms from water. The extracted part of Moringa seeds prevents the growth of coli forms, sodomonas aeroginoras which reduces the requirement for disinfection [8-10]. The extract of oleifera seed removes the 50% to 60% of hardness as well as 99% turbidity. Coagulating active element in extracts is a cationic dimeric protein [11] with molecular weights of 6-14 kDa and isoelectric point 10-11. Extract efficiency of Moringa oleifera seed for turbidity removal equals that of alum. Proteinase active element extracted from Moringa oleifera seeds by dialysis and Ion exchange is 34 times more effective than that extracted by distilled water. This active Proteinase material has a molecular weight of 3000 Daltons and removes 99.9% and 10 NTU turbidity with a dosage of 20 mg/l [12]. The use of this protein doesn’t increase dissolved organic carbon in water [13,14]. The volume of sludge produced by extract of Moringa oleifera seed is 5 times less than that produced by alum. The mechanism of coagulation with MO that extracted with distilled appears to consist of adsorption and neutralization of the colloidal charges. Some studies reported that coagulation efficiency of MO can be improved by extraction of its active agents with sodium chloride solution. The natural coagulants are readily available, affordability, Ecofriendly and low cost [15]. Chemical coagulants produce secondary contaminants, and Natural coagulants are nontoxic and food grade nature. This improvement was apparently due to the salting in mechanism proteins solubility as the salt ionic strength increases. However the active agents in their extraction methods were predominantly caused by the lower molecular weight compound.
The present study was therefore carried out to explore further the potential of this multipurpose tropical plant as a new method for use in the softening of hard groundwater, effect of pH in hardness removal, Turbidity removal from synthetic wastewater, effect of pH, sedimentation time and surface Morphological structure of dried Moringa oleifera seed and suspended particles were studied.
Materials and Methods
Materials
Sulphuric acid (98%), Hydrochloric acid (35.4%), Methyl orange and phenolphthalein indicator, EDTA, Eriochrome black T and Murexide indicator, Sodium hydroxide, Sodium chloride were obtained from Merck (India). Seeds were purchased from local markets.
Instrumentation and procedure
Turbidity was measured using a turbidity meter (ELICO CL 52D NEPHELOMETER) and it was expressed in nephelometric turbidity units (NTU), pH is measured using a pH meter (ELICO LI 120 pH meter). Analytical instrument (ELICO PE 135 DO Analyzer) was used to determine the Dissolved oxygen. Conductivity meter (ELICO CM 180) was to measure the water conductivity, Total hardness were measured by using EDTA titrimetric method. Chloride was measured by using Silver nitrate method [16]. Morphologies of the air-dried flocks were examined and measured spectroscopic ally using a SEM (S-3500N, Hitachi) under a 20 kV voltage.
Preparation of synthetic turbid water
In this study, synthetic turbid water was prepared by adding kaolin, in distilled water for all coagulation experiments. The kaolin suspension was prepared by dissolving 10 g of kaolin powder in 1 L of distilled water. The suspension was stirred slowly at 20 rpm for 1 h to achieve uniform dispersion of the kaolin particles. The suspension was then permitted to stand for 24 h to allow for complete hydration of the kaolin. This suspension was used as a stock solution for the preparation of water samples of varying turbidity for the coagulations tests. The initial pH was adjusted with 0.1M NaOH (or) 0.1M HCl to obtain desired values of turbidity and pH of the synthetic turbid water.
Preparation of Moringa oleifera seed suspension
Dry Moringa oleifera seeds were from Tami Nadu State, Tindivanam. The seed wings and coat from selected good quality. Moringa oleifera seed kernals were removed and the seed ground to a fine powder using the coffee mill attachment of a domestic food blender [17].
Two grams of the powder were put in a high speed mixer (ATO MIX MSE) 200 ml 0.5M NaCl and 200 ml distilled water blended for 30 s to extract the active ingredient respectively. The resulting suspension was filtered through a filter paper. The solution had a pH of 6.5.
Ground water
Ground water samples were collected from tube wells at Chepauk, Chennai, Tamil Nadu.
Equilibrium experiments
Equilibrium studies at different hardness concentrations were investigated using the batch adsorption process as described earlier. This water sample was agitated at a speed at 500 rpm in a thermo shaker until the equilibrium was attained. After equilibrium supernatant was filtered and the equilibrium concentration of hardness was analyzed. The percentage of hardness removal was calculated using the following equations.
 (1)
(1)
where Ci and Ce are the initial and equilibrium concentrations of the hardness removal.
Results and Discussion
Physicochemical characteristics of raw water
The physicochemical characteristics such as turbidity, alkalinity, hardness, TDS, Chloride and other ions concentration was measured for ground water and given in Table 1.
| S No | Parameters | Values |
|---|---|---|
| 1 | Turbidity | 50 |
| 2 | pH | 7.1 |
| 3 | Conductivity | 1309μscm-1 |
| 4 | Dissolved oxygen | 8 |
| 5 | Chloride | 203mg/l |
| 6 | Alkalinity | 236mg/l |
| 7 | Calcium Hardness as CaCO3 | 450mg/l |
| 8 | Magnesium Hardness as CaCO3 | mg/l |
| 9 | Total Hardness as CaCO3 | 150mg/l |
| 10 | Total Solids | 940mg/l |
| 11 | Total Dissolved solids | 840mg/L |
| 12 | Total Suspended Solids | 100mg/l |
Table 1: Characteristics of Ground water at Chennai.
Removal of turbidity in synthetic wastewater
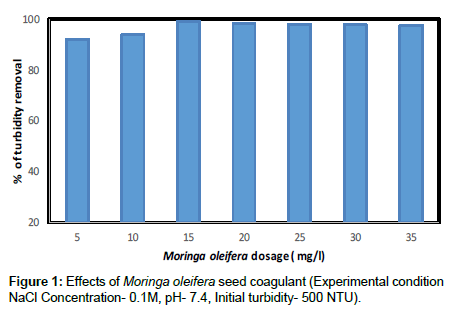
The removal of turbidity in synthetic turbid water by using MO that extracted by sodium chloride is shown in Figure 1. The initial concentration of turbidity in synthetic water has been set based at 500 NTU. Initially, the turbidity concentration for water samples tend to be decreased with low MO dosage until the optimum turbidity removal efficiencies approaching 99%. The Moringa oleifera NaCl extracts exhibits the highest removal of turbidity at lower dosage. The similar results were found by other researchers [14,17].
Effect of pH
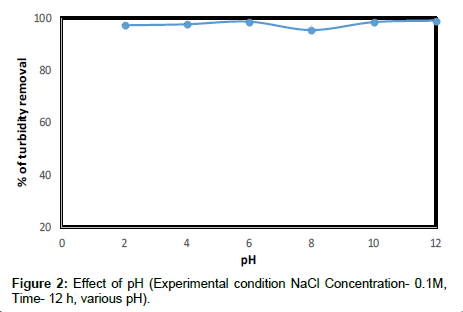
The pH of each beaker was adjusted to the desired pH before the coagulation treatment took place using Moringa oleifera seed extract. During the experiments, big flocks were observed, especially for condition at pH 3. Smaller and fewer flocks were seen for pH 5 to 9. As can be seen in Figure 2 the percentage of turbidity removal for pH 1 to 3 is about 99%. On the other hand, at pH 11, the percentage of turbidity removal rose again to 97%. It seemed that the coagulation is good at acidic pH less than 6 and also at pH greater than 11.
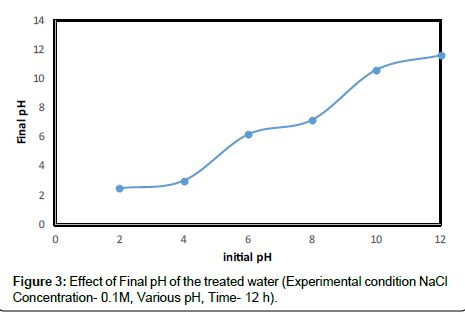
This may be because, at pH less than 3, the positive charges on the amino acids that make up the protein molecule dominate. Most the amino acids will have the positive charges at pH less than 3, and hence the protein can work very well as a cationic coagulant agent. As an amphoteric molecule, the charge on the protein is very much dependent on the pH values. Moringa oleifera contains the dimeric cationic protein it is believed that adsorption and neutralization of charges are the main mechanisms of coagulation. Further investigation on the final pH of the treated kaolin samples with Moringa oleifera revealed that not much alteration was recorded (Figure 3) compared with the initial pH.
Effect of sedimentation time
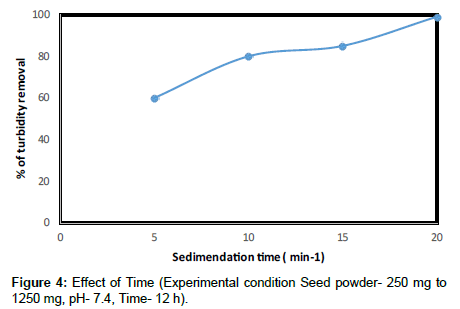
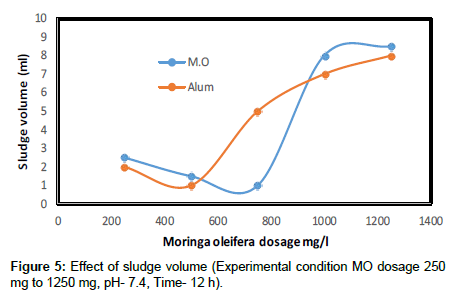
Figure 4 shows the residual turbidity observed as a function of contact time for the range of contact time for the range of natural coagulants used in this experiment. At a coagulant dose of 500 mg/l the residual turbidity is observed to decrease as the contact time increased. The highest residual turbidity 500 NTU for the 500 mg coagulant dose occurred at a contact time of 12 hrs. The percentage of turbidity removal in Moringa oleifera seeds is 98.2%. Moringa oleifera coagulant to remove the turbidity of synthetic wastewater at short time. Furthermore when the sludge volume was measured, Moringa oleifera produced a smaller amount compared with alum, for dosages lower than 1 g/l (Figure 5). For this comparison the sludge volume (ml sludge 200 ml of kaolin sample) at the bottom of the beakers was used. For dosage 1 g/l Moringa oleifera produced slightly more sludge compared with alum. Since Moringa oleifera comes from plant material, its byproducts are of an organic and biodegradable nature and hence offer a more environmentally friendly alternative to the other treatment process.
Removal of hardness in ground water
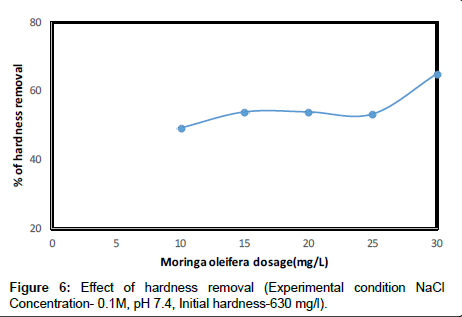
The removal of residual hardness of Groundwater is shown in Figure 6. The removal efficiency of hardness was observed to increase with increasing dosage of MO. The highest MO dosage in this experiment indicates the highest removal of hardness to less than 20 ml l-1. This pattern is suggested to decline with the increase in MO dosage. At MO dosage between 10 to 30 mg/l, the removal of hardness in samples ranging between CaCO3. The same dosage was found to be the most efficient for hardness at these concentrations is considered as moderate classed. While at high hardness concentrations up to more than 600 mg l-1 CaCO3 as a relatively higher MO dosage at approximately. This indicates the potential advantage in the use of MO seeds extract with sodium Chloride solution is softening the hard water.
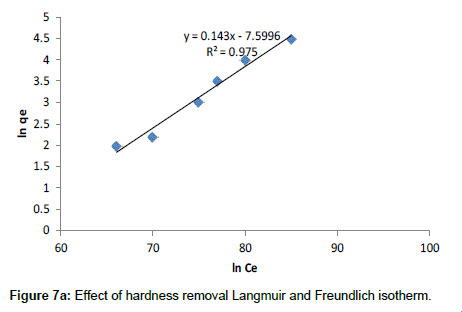
Langmuir model: Langmuir isotherm model mainly is based on the assumption that maximum adsorption corresponds to a saturated monolayer of adsorbate molecules on the adsorbent surface. The Langmuir adsorption isotherm has been greatly used to many ground water effluent treatment processes and it has been used to explain the adsorption of hardness by Moringa oleifera adsorbents. Langmuir theory tells that adsorption takes place at specific homogeneous sites within the adsorbent. It is then assumed that once a metal ion molecule occupies a site after that no adsorption takes place adsorbent sites and adsorbed layer was unimolecular. The theory can be represented by the following linear form:
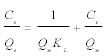 (2)
(2)
Where Ce is the equilibrium concentration (mg L-1), Qe is the amount adsorbed at equilibrium (mg g-1), Qm (mg g-1) and KL (L mg-1) are Langmuir constants related to adsorption capacity and energy of adsorption respectively. Figure 7a shows the linear plot of Ce/Qe vs. Ce for Moringa oleifera. The values of Qm and KL were determined from slop and intercept of the linear plot of Ce/Qe vs. Ce. The experimental data and the correlation coefficients (R2) values of Moringa oleifera indicate the applicability of the Langmuir isotherm model. The essential feature of Langmuir isotherm can be expressed by means of dimensionless constant referred to as the separation factor or equilibrium parameter, RL which is defined by the following equation:
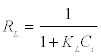 (3)
(3)
Where Ci is the initial metal ion concentration (mg L-1). The value of separation factor RL, indicates the nature of the adsorption process as given below (Table 2).
| RL value | Nature of adsorption process |
|---|---|
| RL>1 | Unfavorable |
| RL=1 | Linear |
| 0<RL<1 | Favorable |
| RL=0 | Irreversible |
Table 2: Nature of the adsorption process.
The adsorption process will be favorable if the RL values lie between 0 and 1. The RL values very well lie in this range and hence the adsorption process is favorable.
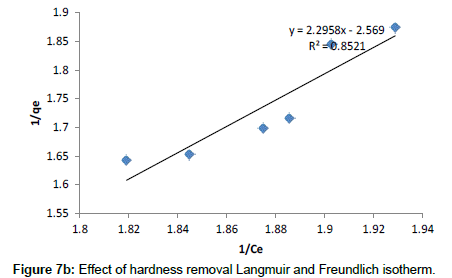
Freundlich model: The Freundlich adsorption model is the most widely used isotherm for the explanation of adsorption of hardness on a wide variety of biosorbents. It is an empirical equation that can be used for non-ideal sorption that involves heterogeneous sorption. The empirical model was shown to be consistent with an exponential distribution of active centers, characteristic of heterogeneous surfaces. The amount of solute adsorbed, Qe is related to the equilibrium concentration of solute in solution, Ce as following:
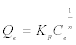 (4)
(4)
This expression can be linearized to give the following equation:
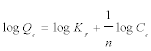 (5)
(5)
Where, KF is a constant for the system, related to the bonding energy. KF can be defined as the adsorption or distribution coefficient and respects the quantity of metal ion adsorbed onto adsorbent for a unit equilibrium concentration (a measure of adsorption capacity, mg g-1). The slope 1/n, ranging between 0 and 1, is a measure of adsorption intensity or surface heterogeneity (becoming more heterogeneous as its value gets closer to zero). A value for 1/n below one indicates a normal Freundlich isotherm while 1/n above one is an indicative of cooperative adsorption. A plot of log (Qe) vs. log (Ce) is shown in Figure 7b, where the values of KF and 1/n are determined from the intercept and slope of the linear regressions.
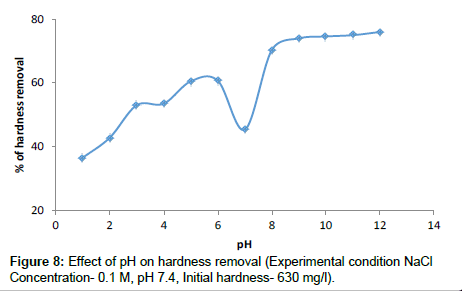
Effect of pH in hardness removal
For the Groundwater sample, the pH of each beaker was adjusted to the desired pH before the coagulation treatment. As can be seen in Figure 8 the percentage of hardness removal for pH 1 to 7 is about 50%. In general the pH of the product water for the water samples was within the recommended standard (WHO, 1984). In a present study observed that in a completely randomized factorial experiment (five factors: the dosage of Moringa oleifera, pH, rate and time of rapid mix, initial hardness) pH did have a significant effect on the rate of hardness. Moringa oleifera is known to be a natural cationic polyelectrolyte and flocculent, with a chemical composition of basic polypeptides with molecular weights ranging from 6000 to 16,000 daltons, containing up to six amino acids of mainly glutamic acid, methionine and arginine. As a polyelectrolyte it may therefore be postulated that Moringa oleifera removes hardness in water through adsorption and inter particle bridging.
Secondly, with the observation that light, slow setting solids/flocks were formed, precipitation reaction lead to the conversion of soluble hardness-causing ions to insoluble compounds would also be a good prediction of the reaction. Mechanism the highest MO dosage in this experiment indicates the highest removal of hardness at basic pH. For the ground water the alkalinity decreased slightly with increasing dosage from 430 mg/l as HCO3- to a constant value of 310 mg/l as HCO3-, an average of 30% reduction
The slight decrease in alkalinity and pH of water samples may be due to precipitation of insoluble products of the reaction between the Moringa oleifera and the hardness-causing ions similar to precipitation softening using lime/soda water. The Moringa oleifera seed extract appears to have natural buffering capacity. The precipitates (solids/ flocks) were light and did not settle easily. The chemical constituent of the precipitate is however not known.
Scanning electron microscopy
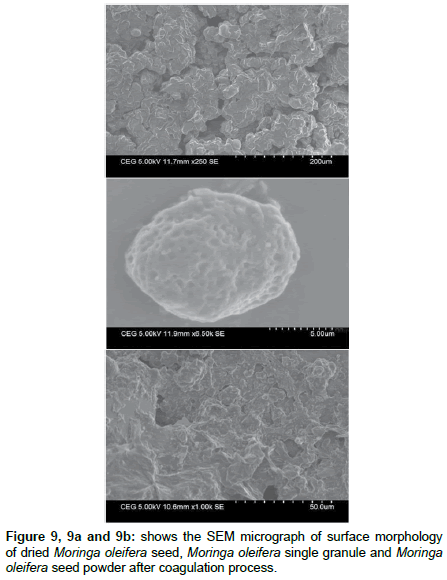
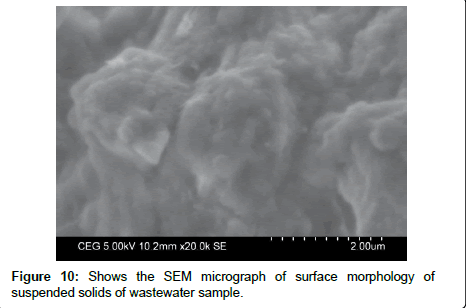
The SEM micrograph shows that Figure 9 dried Moringa oleifera seed powder surface texture and porosity. This photomicrograph shows fibrous structure of Moringa oleifera seed powder. It shows that very fine particles size to the order of a millimeter or less and that there are pores within the particles of varying size. Using single Moringa oleifera as a coagulant, the flocks formed with porous structure, where particles and colloids exist as chain-like or spherical structure and some porous structures can be seen Figure 9a. The SEM micrographs show that dried Moringa oleifera granules prior to being dispersed in water are approximately spherical in shape. Each granule is composed of large number of smaller Moringa oleifera particles surface morphology texture shown in Figure 9b loosely agglomerate and the granules are readily disaggregated when dispersed in water. The resultant suspensions consist of disaggregated primary particles. It is believed that the chain –like and spherical structures contributed to lowering the turbidity in the settled water. The mechanism of the enhanced coagulation using a combined coagulant is probably that the chainlike and spherical structures can absorb the turbidity and pathogens from the wastewater. The difference in the coagulation performance using different coagulants can also be reflected in the flock structures observed by Scanning Electron Microscope. Figure 10 shows that SEM micrograph of suspended solids in the wastewater sample.
Conclusion
From this study, it can be concluded that Moringa oleifera seeds have a good potential as a natural coagulant and are suitable for treatment of wastewater with a wide range of initial turbidity values. Moringa oleifera improvement in removing turbidity from synthetic raw water was found. Alter dosing NaCl soluble extract of Moringa oleifera reduced turbidity to 5 NTU respectively from 500 NTU.
Hardness removal efficiency of Moringa oleifera was found to increase with increasing dosage. The more species causing hardness that are present in the water sample, the higher the dosage required to achieve equivalent residual hardness for water with the same initial hardness. Moringa oleifera seed is environmentally friendly material the sludge can be used as a bio fertilizer/ bio compost. The efficiency of Moringa oleifera seed extract decreases the turbidity reduction.
References
- Flaten TP (2001) Aluminium as a risk factor in Alzheimer's disease, with emphasis on drinking water. Brain Res Bull 55: 187-196.
- Martyn CN, Barker DJ, Osmond C, Harris EC, Edwardson JA, et al. (1989) Geographical relation between Alzheimer's disease and aluminum in drinking water. Lancet 1: 59-62.
- Mallevialle J, Brichet A, Fiessinger F (1987) How safe are organic polymers in water treatment. J Am Waterworks Associ 76: 87-93.
- Vickers JC, Thompson MA, Kelkar UG (1995) The use of membrane filtration with coagulation processes for improved NOM removal. Desalination 102:57-61.
- Childress AE, Deshmuch SS (1998) Effect of humic substances and anionic surfactants on the surface charge and performance of reverse osmosis membranes. Desalination 118: 167-174.
- Lee EK, ChenV, Fane AG (2009) Natural organic matter (NOM) fouling in low pressure membrane filtration effect of membrane and operation modes. Desalination 218: 257-280.
- Hong S, Kim S, Bae C (2009) Efficiency of enhanced coagulation for removal of NOM on GAC. Des wat Treat 2: 90-95.
- Ndbigengesere A, Narasiah KS (1998) Quality of water treated by coagulation using Moringa oleifera seeds. Water Res 32: 781-791.
- Ndabigengesere A, Narasiah KS, Talbat BG (1995) Active agents and mechanism of coagulation of turbid water using Moringa oleifera. Water Res 9: 703-710.
- Muyibi SA, Okufu CA (2007) Coagulation of low turbidity surface water with Moringa oleifera seeds. Int J Environ Stud 48: 263-273.
- Jahn SA(1988) Using Moringa seeds as coagulants in developing countries.J Am Wat Works Assoc 80: 43-50.
- Jahn SAA (1986) Proper use of African natural coagulants for rural water supplies-New projects. GTZ Manual No 191.
- Abidin ZZ, Ismail N, Yunus R, Ahamad IS, Idris A (2011) A preliminary study on Jatropha curcas as coagulant in wastewater treatment. Environ Technol 32: 971-977.
- Suleyman AMM, Megat MN, Leong TK (2002) Effects of oil extraction from Moringa oleifera seeds on coagulation of Turbid water.Water 59: 261-269.
- Tzouponas ND, Zoubolis AI (2010) Novel inorganic-organic composite coagulant based on aluminium. Des Wat Treat13: 340-347.
- Hu N, Zheng JF, Ding DX, Liu J, Yang LQ, et al. (2009) Metal pollution in Huayuan River in Hunan Province in China by manganese sulphate waste residue. Bull Environ Contam Toxicol 83: 583-590.
- Sasikala S, Muthuraman G (2015) Chromium (VI) removal using biosorbents derived from Moringa oleifera.Ind Chem 1:1.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 19128
- [From(publication date):
March-2016 - Aug 19, 2025] - Breakdown by view type
- HTML page views : 17442
- PDF downloads : 1686