A Review on Enzymatic Treatment of Phenols in Wastewater
Received: 24-Sep-2016 / Accepted Date: 20-Oct-2016 / Published Date: 27-Oct-2016 DOI: 10.4172/2155-952X.1000249
Abstract
Tyrosinase is a natural enzyme and mainly catalyses the o-hydroxylation of monophenols into their corresponding o-diphenols. The synthesis of o-diphenols is a potentially valuable catalytic ability and thus tyrosinase has attracted a lot of attention with respect to industrial applications. In environmental technology, it is used for the detoxification of phenol-containing wastewaters and contaminated soils and also used in cosmetic and food industries as important catalytic enzyme. Phenols are present in effluents of a number of industries such as coal conversion, resins, plastic, petroleum refining, textiles, dyes and organic chemicals. Conventional processes for removal of phenols have drawbacks of incomplete removal of phenols. Hence, an alternative based on enzymes has been investigated. This review summarizes the current research based on removal of phenol from waste streams by enzyme polyphenoloxidase (tyrosinase).
Keywords: Wastewaters; Phenols; Tyrosinase; Polyphenoloxidase
249689Introduction
Phenolic compounds are mainly found in wastewater of many industries such as coal conversion, resin, plastic and petroleum refineries. These compounds are toxic pollutants in industrial waste imposing risk to human health and some of them are suspected carcinogens. Phenols and cresols are highly corrosive and toxic, cause, damage to the respiratory, scarring of the skin damage to gastrointestinal tracts, kidney failure, hematological changes and nervous system depression [1,2]. Therefore, some steps should be taken to treat wastewaters [3- 12] and also to degrade such environmental pollutants [13-21]. Some commonly used conventional treatment [22-29] often fails to generate final effluents at affordable cost with the required discharge quality. To overcome conventional biological treatment drawbacks, alternative technologies are sought for the treatment of phenolic wastewater such as treatment of narrow range of contaminant concentrations, generation of high sludge volumes and delays related to biological acclimatization.
Many researchers have proposed the use of peroxidase for removal of phenol and aromatic amines as a potential alternative due to following three reasons [30-35]:
a. Enzymes are highly selective and can effectively treat even dilute wastes.
b. They are less likely to be inhibited by substances which may be toxic to living organisms and their costs could eventually be less than that of other methods if commercially available enzymes are produced in bulk quantities.
c. Enzymes operate over a broad concentration range and require low retention times with respect to other methods [36].
Peroxidases and laccases show a wide substrate range, especially with regards to phenols and amines. This suggests that these oxidative enzymes may not have specific substrate binding sites. Another problem is the susceptibility peroxidase to inactivation as it got absorbed on the final product of reaction. To remove phenols by peroxidase and laccase is expensive in comparison to tyrosinase as in case of peroxidase, hydrogen peroxide is used as an electron acceptor which is costly in comparison to oxygen which acts as an electron acceptor in case of laccase and tyrosinase. Moreover, cost of laccase isolation is a hurdle in its use to decontaminate wastewater. Therefore, use of tyrosinase is a cheaper alternative for enzymatic wastewater treatment.
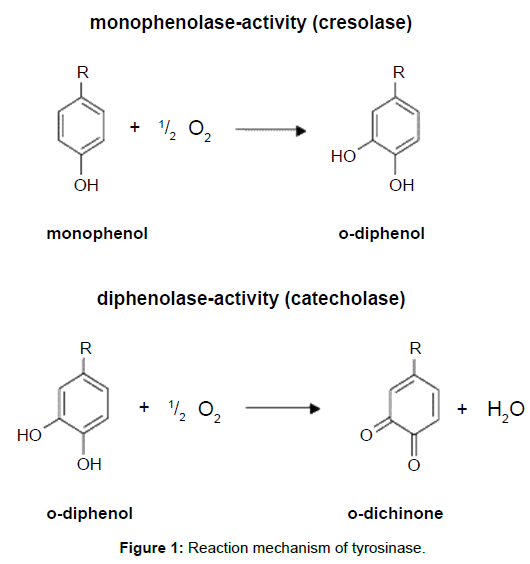
Tyrosinase (EC 1.14.18.1), known as poly-phenol oxidase, is a coppercontaining enzyme that catalyses an analogous phenol oxidation reaction to peroxidase [37-40]. This enzyme is widely distributed in vegetable products and fruits such as potato, mushroom, pear, tobacco, papaya, apple, avocado, banana, Florida spiny lobster, brown shrimp and others [41-43]. It is mainly responsible for browning in these food products [44]. It catalyses two different reactions. The primary reaction is the hydroxylation of monophenols resulting in o-diphenols, commonly known as mono-phenolase or cresolase [45]. The second reaction is the oxidation of o- diphenols to o-quinones, often known as o-phenolase or catecholase (Figure 1). Oxygen is used as an oxidant in the both of the oxidation reactions. This enzyme could also be used for the oxidation of many types of phenolic compounds such as chlorophenols, diphenols, methylphenols and naphtanols [46]. Chlorinated anilines and anilines can be also oxidised to some extent and co-polymerisation with un-substituted phenol can result in good removals for these difficult-to-oxidise compounds [47].
General characteristics and structure
Tyrosinase has been isolated and purified from a variety of plant and animal sources [48]. However, few of those are well defined, and preparations show a significant degree of heterogeneity [49]. The molecular weight of tyrosinase ranges from 13.4 KDa to 128 KDa depending on the sources [45,49] (Figure 2).
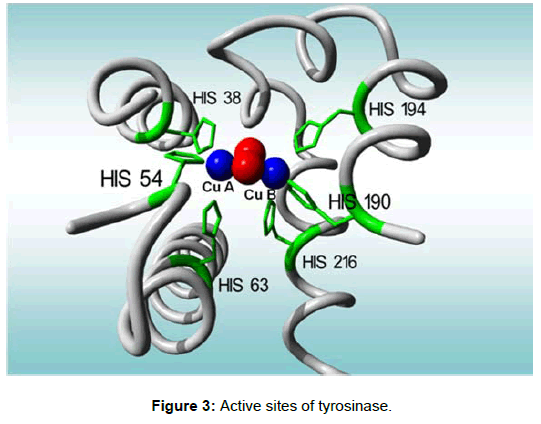
The enzymatic structure of tyrosinases has been studied by both biological and chemical approaches with respect to domain structure, primary, secondary, and tertiary structure, Cu binding sites, and activation mechanism [50-53]. It is widely accepted that the active site of tyrosinase is quite similar to that of hemocyanin, which is a respiratory copper protein, and contains coupled binuclear coppers (Figure 3).
| Source | Reference(s) |
|---|---|
| Apple | [55] |
| Avocado | [56] |
| Florida Spiny Lobster | [57] |
| Dog-rose -Fruit | [58] |
| Mushroom Agaricusbisporus | [59] |
| Neurospora | [60] |
| Papaya | [61] |
| Pear | [42] |
| Potato Leaf | [62] |
| Tobacco | [63] |
Table 1: Sources of tyrosinase and literature sources describing methods of enzyme preparation.
Mushroom Agaricus bisporus tyrosinase is the only commercially available tyrosinase and is considered to be one of the most studied tyrosinases [44,45,54]. Although tyrosinase was isolated and purified from various bacteria, fungi, plants and animals, the objectives were mostly aimed at preventing food browning. The sources of tyrosinase and the authors who described preparation methods of enzymes are summarised in Table 1.
Catalytic activity and its inhibition
Catalytic cycle: Tyrosinase catalytic mechanism has been studied for a long time [45,64]. This mechanism is complex and was believed to be an allosteric mechanism which involves two distinct binding sites for oxygen and aromatic compounds [45,65]. The oxidation state of copper in resting tyrosinase is mostly met derivative, which has two cupric centres [66,67]. In order to initiate the catalytic cycle, a reducing agent reacts with the two copper (II) atoms of met-tyrosinase and reduces them to de oxy-tyrosinase. However, if mono-phenol was used as a substrate, the enzyme activation could not occur without an aid of reducer [68]. Molecular oxygen binds with the deoxy tyrosinase and oxidises it to oxy-tyrosinase.
Tyrosinase catalysed removal of phenolic compounds from wastewaters: Atlow et al. first reported use of tyrosinase for the treatment of phenolic wastewaters [69-78] in 1984. Several monophenols and o-diphenols such as phenol [79], cresol, chlorophenol and catechol were removed very effectively with both commercially obtained and laboratory extracted tyrosinases. Tyrosinase was effective in removing phenol with initial concentrations ranging from 0.01 g/L to 1 g/L. The optimum enzyme dose to treat 50 mg/L (approximately 0.53 mM) phenol was determined to be 60 units/mL. Wada and his colleagues followed up with the tyrosinase catalysed phenol removal from wastewaters according to the procedures of Atlow et al.; however, no precipitate was formed but the reaction solution changed from colourless to dark-brown. They assumed that the enzyme purity might have an effect on the formation of precipitate: i.e., treatment with lower purity enzyme resulted in the precipitation.
Factors affecting transformation of phenols catalysed by tyrosinase
pH and temperature: The optimum pH of tyrosinase activity depends on the source of enzyme and on the substrates [42]. However, only one study has been reported involving the treatment of phenol so far. Literature dealing with tyrosinase-catalysed phenolic wastewater treatment [80-84], commercially obtained or crude extract mushroom tyrosinase was used. 50 mg/L phenol was treated with 30 units/mL tyrosinase in different pH buffers and the best removal efficiency was achieved when 50 mM sodium phosphate buffer at pH 8 was used [39].
No such temperature effect has been examined for phenol treatment with tyrosinase. Probably the reason could be the indication of its instability upon exposure to high temperature conditions according to some thermal inactivation studies of tyrosinase [85].
Enzyme concentrations: Atlow et al. demonstrated the relationship between concentrations of phenol and tyrosinase dose required to achieve over 98% removal [46]. The required tyrosinase dose was directly proportional to initial phenol concentration over the range of 50 mg/ L to 1 g/L, and the ratio was 1.2 units/ml of tyrosinase for each 1 mg/L of phenol. Wada and his colleagues however found that the optimum tyrosinase dose for 0.5 mM was 20 units/ml, which was about three times as small as that reported by Atlow et al. after following up with experiments involving the same procedures (including the use of the same tyrosinase activity assay) [86].
Substrates: Tyrosinase can transform many phenolic and other aromatic compounds such as phenol, 2 methyl phenol, 3-methylphenol, 2-chioropheno1, 3-chlorophenol, 2- methoxyphenol, catechol, resorcinol, 2,3-dimethylphenol, 1-naphthol [46], 4-chlorophenol, 3-methoxy-phenol, 4 methoxyphenol, 4-methylphenol, hydroquinone, 3,4-dichloro-aniline [47], 2-hydroxy-acetophenone and 4-hydroxylacetophenone [87].
Aniline was quite difficult to oxidise when treated alone by tyrosinase while in the presence of two molar equivalents of phenol, this compound was transformed to high levels [47]. 0.5 mM aniline was completely removed from the solution in the presence of 1 mM phenol, whereas only 28% of aniline was removed when aniline was the only substrate. It is suggested that the removal of aniline was caused by the co-polymerisation reaction of aniline with o-quinones, which was derived from phenol oxidation with tyrosinase. In the case of aromatic compounds which are not substrate of tyrosinase such as anisole and benzyl alcohol, there was no oxidation and removal observed when in the presence of phenolic substrates [88].
The removal efficiency of substituted phenol was dependent on the type of substituent group and its positions [87,89]. Usually, parasubstituted phenols were more easily oxidised then meta-substituted phenols.
Chemical additives: Unlike the studies involving other phenol oxidase, chemical additives were examined mostly for the purpose of removal of coloured soluble matter when tyrosinase was studied. Since precipitate has rarely been observed in phenol solutions treated with tyrosinase, the colour product remaining in the solution should be removed. Chitosan [90-98] and other natural or synthetic cationic polymers were investigated to accomplish the removal of colour from solutions [47,86,88,99-103].
Although the mechanism of the reaction between chitosan [104- 112] and oxidised phenols has not yet been clearly established, the 2-amino groups of chitosan are likely to perform a nucleophilic attack on o-quinones to form covalent bonds [113,114].The result strongly suggested that the adsorption of the quinones onto the chitosan was presumably the result of covalent interactions, which is referred to as chemisorptions. On the other hand, the interaction between phenol, pyrocatechol, or p-quinone and activated charcoal were weak and considered the result of low-energy physical forces such as hydrophobic interaction.
Wada and his colleagues investigated the use of cellulose, chitin, chitosan [115-121], hexamethylenediamine-epichlorohidrin polycondensate and polyethylenemine to remove coloured products [86]. The first three were natural polymers and the last two were synthetic cationic polymers that had amino groups. Chitin also removed colour effectively, but cellulose had no effect on the colour removal.
Immobilisation of tyrosinase: The attachment of o-quinone on the amino acid residue in proximity to the active site of enzyme is associated with the inactivation of tyrosinase [122]. Therefore, in order to improve the catalytic lifetime, stability for storage and reusability of the enzyme, tyrosinase was immobilised on several support materials. Wada et al. immobilised tyrosinase on magnetite (Fe3O4) activated by aminopropyltriethoxy-silane and glutaraldehyde [88]. When 1 mg of tyrosinase was used for immobilisation, immobilisation yield was about 80% and retained activity 70-80% with 500 mg of the support material. After 15 days of storage, the loss of activity of immobilised tyrosinase was about 5%. Three types of methylphenols, chlorophenols and methoxyphenols were treated with this immobilised tyrosinase. The soluble tyrosinase was inactivated rapidly, whereas immobilised tyrosinase could be used 5 times without significant reduction of activity.
Payen et al. immobilised tyrosinase into chitosan gel in many ways [87,123,124]. They prepared chitosan gel [125], by dissolving chitosan in 8% (v/v) acetic acid solution and stirred overnight. After centrifugation of the solution-undissolved chitosan was removed, then the viscous chitosan solution was added through a syringe needle into an 8% NaOH solution. The chitosan gel formed in the NaOH solution was spread on square glass slides. Highly concentrated tyrosinase was added to the central region of the square and then two gel films were combined so as to containing tyrosinase between them and were then sealed using rubber cement. Chitosan was used for the support of the enzyme as well as for the sorbent, in this way, this immobilised tyrosinase could not be reused.
Discussion
Characterisation of tyrosinase activity was done using L-tyrosine as a substrate. At pH 7 the maximum catalytic activity was observed. Tyrosinase appeared to be unstable at low pH and at elevated temperature. According to the 5 min Microtox assay, most chlorinated phenol solutions and phenol treated with tyrosinase enzyme had lower toxicities when compared to their corresponding untreated solutions. However, toxic products and coloured products were formed during wastewaters treatment [126-131]. Chitosan was found to be effective in inducing their precipitation, resulting in detoxified and colourless solutions. The addition of chitosan was very effective in inducing the precipitation of the coloured products generated by the transformation of phenol with tyrosinase. It was found that the toxicities of the phenol solutions when treated with tyrosinase were substantially lower than the solutions treated with peroxidase enzymes. This represents a very strong advantage when considering this enzyme for applications in wastewater treatment [132-134]. In addition, tyrosinase requires oxygen as an oxidant, which is comparatively much less expensive than the hydrogen peroxide, required by peroxidise enzymes. These might be critical points of interest while considering tyrosinase for applications in wastewater treatment.
References
- Tallur PN, Megadi VB, Kamanavalli CM, Ninnekar HZ (2006) Biodegradation of p-cresol by Bacillussp. Strain PHN 1. Current Microbial 53: 529-533.
- Dabhade MA, Saidutta MB, Murthy DVR (2009) Adsorption of phenol on granular activated carbon from nutrient medium: Equilibrium and kinetic study. Int J Environ Res 3: 557-568.
- Arone Soul Raj GP, Elumalai S, Sangeetha T, Roop Singh D (2015) Botryococcusbraunii as a phytoremediation tool for the domestic waste water recycling from Cooumriver, Chennai, India. J BioremedBiodeg 6: 294.
- Kehinde FO, Aziz HA (2014) Textile waste water and the advanced oxidative treatment process, an overview. IJIRSET.
- Dhanwani AP, Meka S, Patel SB (2014) Selection of technology to treat waste water generated from dye intermediate manufacturing industry: A case study. IJIRSET.
- AmteGK, MhaskarTV (2013) Assessment of the toxicity of waste water from a textile dyeing industry to fresh water teleostOreochromismossambicus. Journal of Industrial Pollution Control.Â
- Sharma AK, Sharma S, Hinge M, Khan A (2013) Performance evaluation and design of uasb reactor for treatment of dairy waste water with the help of multiple seeds. Journal of Industrial Pollution Control.
- Varatharajan B, Kanmani S (2007) Treatability study of pharmaceutical waste water by combined solar photo fenton and activated sludge process. Journal of Industrial Pollution Control.
- Sahu S (2011) Synthesis of some higher membered macro cyclic complexes of heavy metal ions present in industrial waste water. Journal of Industrial Pollution Control.Â
- Shrivastava PV, Soni AB (2010) Decolorization of dye waste water using waste material of sugar cane industry. Journal of Industrial Pollution Control.
- Rani DFG, Arunkumar K, Sivakumar SR (2005) Physico-chemical analysis of waste water from cement units. Journal of Industrial Pollution Control.Â
- Parikh P, Rao KS (2005) Theresponse of chara and oscillatoria to remove ni (ii) ions from industrial waste water. Journal of Industrial Pollution Control.
- Lange JH, Heymann WC,Cegolon L (2013) Environmental pollution, public health and environmental medicine-oil spills. Occup Med Health Aff 1: 110.
- Akintunde JK, Oboh G (2012) In vitro oxidative damage induced in livers, hearts and kidneys of rat treated with water extract (leachate) from municipal battery recycling site: evidence as environmental pollution and tissue damage. J ClinExpPathol 2: 129.
- Abdul-Aziz KK (2012) Thehealth status and genetic variations of the bivalve,Pinctalaradiata affected by environmental pollution. J Environ Anal Toxicol 2: 138.
- Tosti E, Gallo A (2012) Best biomarker and bioindicator for marine environmental pollution. J Marine Sci Res Development 2: e101.
- Achudume AC (2012) Analysis of the impacts of environmental pollution of pesticides on oxidative stress profile in liver and kidney: A case of raid? Inwistar rat. J Environment Analytic Toxicol 2: 124.
- (2012) Teeth as indicators of environmental pollution with lead. J Environment Analytic Toxicol 2: 118.
- Goyal MK, Chauhan A (2015) Environmental pollution remediation through solidification/fixation of heavy metal ions in Portland cement. J Environ Anal Toxicol 5: 323.
- Udoh FD, Akpanika OI (2009) Anapproach towards effective monitoring of environmental pollution resulting from petroleum industry activities. Journal of Industrial Pollution Control.
- Sharma CM (2015) Can bio-manipulation be related to fisheries and aquaculture through environmental pollution perspective? Fish Aquac J 6: e116.
- Bryant IM, Tetteh-Narh R (2015) Using slow sand filtration system with activated charcoal layer to treat salon waste water in a selected community in cape coast, Ghana. J AdvChemEng 5: 135.
- Khorshid AF, Issa YM, Ami RR (2015) A modified carbon paste sensor for determination of Zn in vitamin and waste water using thiosemicarbazide and acetaldehyde thiosemicarbazone complexes. J BiosensBioelectron 6: 176.
- Rossi T, Bassani B, Gallo C, Maramotti S, Noonan DM, et al. (2015) Effect of a purified extract of olive mill waste water on endothelial cell proliferation, apoptosis, migration and capillary-like structure in vitro and in vivo. J Bioanal Biomed.
- Hafeez A (2015) Arsenic distribution in green bean yield irrigated by waste water. Adv Crop Sci Tech 3: 165.
- Venkatesan K, Saseetharan K, Arutchelvan V (2004) Determination of bio-kinetic coefficients for dairy waste water. Journal of Industrial Pollution Control.
- Mamatha M, Aravinda HB, Puttaiah ET, Manjappa S (2013) Factors and kinetics involved in adsorption of copper from aqueous and waste water onto Pongamiapinnata. IJIRSET.
- Taj L, Hussain S, Ali S, Farid M, Anwar-ul-Haq M, et al. (2013) Physio-chemical analysis of ground water contamination caused by industrial waste water in Faisalabad, Pakistan. IJPAES.
- Pinkal C, Punita P, Krupa U, Payal V (2010) Application of the aquatic indigenous plant material coagulants for waste water treatment. Journal of Industrial Pollution Control.
- Klibanov AM, Tu TM, Scott KP (1983) Peroxidase catalyzed removal of phenolsfrom coal conversion waste waters. Sci221: 259-261.
- KlibanovAM, Morris ED (1981) Horseradish peroxidase for the removal of carcinogenic aromatic amines from water. Enz Micro Tech 3: 119-122.
- Atlow SC, Aparo LB, Klibanov AM (1984) Dephenolization of industrial wastewaters catalyzed by polyphenol oxidase. Biotech Bioengineer26: 599-603.
- Dordick JS, Marletta MA, Klibanov AM (1987) Polymerization of phenols catalyzedby peroxidase in non-aqueous media. Biotech Bioengineer30: 31-36.
- Klibanov AM, Zina B, Alberti BN (1981) Preparative hydroxylation of aromaticcompounds catalyzed by peroxidase. J Am ChemSoc103: 6263-6264.
- Singh BK, Dubey M (2008) Sorption dynamics for removal of phenol from waterand wastewater onto bituminous coal. J Environ Res Develop 2: 545-552.
- Karam J, Nicell JA (1997) Potential applications of enzymes in waste treatment. JChem Tech Biotechnol69: 141-153.
- Zamani NP, Gazali M, Batubara I (2015) The Study of tyrosinase and antioxidant activity of Xylocarpusgranatumkoenig seed kernel extract toward evidence based indigenous knowledge from Togeanarchipelago, Indonesia. J Marine Sci Res Dev 5: 168.
- Liu SH, Ma LJ (2015) Effects of Chinese herbal extracts on tyrosinase activity and melanogenesis. Nat Prod Chem Res 3: 183.
- Coutinho EC, Mishra NB, Bhattacharya D, Srivastava S, Joshi M, et al (2015) Conformation of the antigenic peptide tyrosinase (192-200) in water and dmso-d6 by NMR spectroscopy and md simulations. Pharm Anal Acta 6: 392.
- Chang TMS (2012) PEG-PLA Nanocapsulescontaining a nanobiotechnological complex of polyhemoglobin-tyrosinase for the depletion of tyrosine in melanoma: Preparation and in vitrocharacterisation. J NanomedineBiotherapeuticDiscov 2: 103.
- Janovitz-Klapp AH, Richard FC, Goupy PM, Nicolas JJ (1990) Kinetic studies on apple polyphenol oxidase. J Agric Food Chem 38: 1437-1441.
- Espin JC, Morales M, Varon R, Tudela J, Garcia-Canovas F (1997a) Monophenolase activity of polyphenol oxidase from Blnquilla pear. Phytochemistry 44: 17-22.
- Chen JS, Balaban MO, Wei C, Gleeson RA, Marshall MR (1993) Effect of carbon dioxide on the inactivation of Florida spiny lobster polyphenol oxidase. Journal of the Science of Food Agriculture 61: 253-259.
- Kahn V (1985) Effect of proteins, protein hydrolyzates and amino acids on o-dihydroxyphenolaseactivity of polyphenol oxidase of mushroom, avocado and banana. Journal of Food Science 50: 111-115.
- Duckworth KW, Coleman JE (1970) Physiochemical and kinetic properties of mushroom tyrosinase. The Journal of Biological Chemistry 245: 1613-1625.
- AtlowST, Bonadonna-AparoL, Klibanov AM (1984) Dephenolization of industrial waste water catalyzedby polyphenol oxidase. Biotechnology and Bioengineering 26: 599-603
- Wada S, Ichikawa EL, Tatsumi K (1995) Removal of phenols and aromatic amines from wastewater by combination treatment with tyrosinase and a coagulant. Biotechnology and Bioengineering 45: 304-309.
- Sharma S, Agarwal P, Sharma M, Takshak A (2014) Isolation and characterization of tyrosinase(a carbon trapping enzyme) producing microorganisms, in the agricultural soil of western Uttar Pradesh and the study of enzymatic activity of tyrosinase produced. Accounts of Biotechnology Research 1: 51-55.
- Solomon EL, Sundaram UM, Macho- TE (1996) Multicopper oxidase and oxygenases. Chemical Reviews 96: 2563-2605.
- Casella L, Carugo O, Gullotti M, Garofani S, Zanello P (1993) Hemocyanin and tyrosinase models. Synthesis, azide binding and electrochemistry of dinuclearcopper(II) complexes with poly (benzimidazole) ligands modeling the met forms of the proteins. Inorganic Chemistry 32: 2056-2067.
- Getlicherman H, Giessner-Prettre C, Maddaluno J (1996) Theoretical evidence of electrophilic superoxides in models of oxyhemocyanin/oxytyrosinase active sites. Influence of the ligand's arrangement. J PhysChem 100: 6819-6824.
- Van Gelder CWG, Flurkey WH, Wichers HJ (1997) Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 45: 1309-1323.
- Zhang X, Flurkey WK (1997) Phenoloxidases in Portabella mushrooms. Journal of Food Science 62: 97-100.
- Murata M, Tsurutani M, Hagiwara S, Homma S (1997) Sub cellular location of polyphenol oxidase in apples. Bioscience Biotechnology und Biochemistry 61: 1495-1499.
- Espin JC, Trujano Mf, Tudela J, Garcia-Canovas F (1997a) Monophenolase activity of polyphenol oxidase from Hass avocado. J Agric Food Chem 45: 1091-1096.
- Chen JS, Balaban MO, Wei C, Marshall MR, Hsu WY (1992) Inactivation of polyphenol oxidase by hi&-pressure carbon dioxide. JO& of Agriculture and Food Chemistry 40: 2345-2349.
- Sakiroglu H, Kufrevioglu OI, Kocacaliskan I, Oktay M, Onganer Y (1996) Purification and characterization of Dog-rose (Rosa dumalisRechst.) polyphenol oxidase. J Agric Food Chem 44: 2982-2986.
- Albisu L, King RD, Kozlov A (1989) Inhibition of the catecholase activity of mushroom tyrosinase by carbon monoxide. Journal of Agriculture and Food Chemistry 37: 775-776.
- Lerch K (1976) Netaosporatyrosinase: Molecular weight, copper content and spectral properties. FEBS Lefters 61: 157-160.
- Cano MP, Lob MG, De Ancos B (1998) Peroxidase and polyphenol oxidase in long-term frozen stored papaya slices. Differences among hermaphrodite and female papaya fruits. Journal of the Science of Food Agriculture 76: 135-141.
- Sanchez-Ferrer A, Levada F, Garcia-Carmona F (1993a) Substrate-dependent activation of latent potato leaf polyphenol oxidase by anionic surfactants. J Agric Food Chem 41: 1583-1586.
- Richardson A, McDougail GJ (1997) A laccase-type polyphenol oxidase from lignifying xylem of tobacco. Phytochemistry 44: 229-235.
- Makino N, Mason HS (1973) Reactivity of oxy-tyrosinase toward substrates. The Journal of Biological Chemistry 248: 5731-5735.
- Jolley RL Jr., Evans LH, Makino N, Mason HS (1974) Oxytyrosinase. J BiolChem 249: 335-345.
- KerteszD, RotilioG, Brunori M, ZitoR, AntoniniE (1972) Kinetics of reconstitution of polyphenoloxidase from apoenzymeand copper. Biochemical and Biophysical Research Communication 49: 1208-1215.
- Makino N, McMahill P, Mason HS (1974) The oxidation state of copper in resting tyrosinase. The Journal of Biological Chemistry 249: 6062-6066.
- Naish-Byfield S, Riley PA (1992) Oxidation of monohydric phenol substrates by tyrosinase: An oximetric study. Biochemical Journal 288: 6347.
- Paphane BD, Ramirez LLZ (2013) Chemical pre-treatment of anionic surfactants contaminated waste water at Enaspol A. S. using H2O2/UV light waste water pre-treatment method. J Environ Anal Toxicol 3: 181.
- Singh A, Kumar V, Srivastava JN (2013) Assessment of bioremediation of oil and phenol contents in refinery waste water via bacterial consortium. J Pet Environ Biotechnol 4: 145.
- Thaiyalnayaki D, Sowmeyan R (2012) Effect of carrier materials in inverse anaerobic fluidized bed reactor for treating high strength organic waste water. J Environment Analytic Toxicol 2: 134.
- Singh N, Gadi R (2012) Removal of Ni(II) and Cu(II) from their solutions and waste water by nonliving biomass of Pseudomonas oleovorans. Hydrol Current Res 3: 126.
- Shastry V, Shashidhar S (2011) Waste water treatment using eco-friendly oxidising agent Fe (VI). Hydrol Current Res.
- Akbari A, Homayoonfal M, Jabbari V (2010) Fabrication of new photografted charged thin film composite (tfc) nanofiltration membrane applied to waste water treatment: Effect of filtration parameters on the rejection of salts and dyes. Hydrol Current Res 1: 106.
- Mubarak NM, Shapnathayammal S, Suchithra TG, Sahu JN, Abdullah EC, et al. (2016) Adsorption and kinetic study on Mg2+removal from waste water using rice husk based magnetic biochar. Pure and Applied Physics.
- Khan I, Ghani A, Abd-Ur-Rehman, Awan SA, Noreen A (2016) Comparative analysis of heavy metal profile of Brassica campestris (l.) and Raphanussativus (l.) irrigated with municipal waste water of Sargodha city. J ClinToxicol 6: 307.
- Damayanti A, Sari TK, Afifah AS, Sutikno, Sunarno LT (2016) Theperformance operation of zeolite as membrane with using laundry waste water. J MembraSciTechnol 6: 148.
- Orata F, Birgen F (2016) Fish tissue bio-concentration and interspecies uptake of heavy metals from waste water lagoons. J PollutEffCont 4: 157.
- El-Shahawi MS, Hamza A, Alwael H, Bashammakh AS, Al-Sibaai AA et al. (2014) Retention profile and selective separation of trace concentrations of phenols from water onto iron(iii) physically loaded polyurethane foam solid sorbent: Kinetics and thermodynamic study. J Chromatogr Sep Tech 5: 241.
- Shah MP, Reddy GV (2016) Microbial diversity of ammonia oxidizing bacteria through waste water genomics. Appli Micro Open Access.
- Sonia V, Rajagopalsamy CBT, Ahilan B, Francis T (2015) Influence of bioremediation on the growth and survival of Cyprinuscarpiovarkoi using aquaculture waste water. Journal of Industrial Pollution Control.
- Kumar S, Reddy Ch. V, Mandla VR, ChaithanyaSudha M, Shanawaz SM (2015) Integrated water and waste water management for a township. Journal of Industrial Pollution Control.Â
- Ahmed M (2015) Use of aquifers as storage for treated waste water. Journal of Industrial Pollution Control.
- Odeh LH, Musameh S, Abdelraziq IR (2016) Influence of waste water used in irrigation on the physical properties of olive oil in Palestine. J Material SciEng 5: 209.
- Robert C, Spatz A, Faivre S, Armand JP, Raymond E (2003) Tyrosine kinase inhibition and grey hair. Lancet 361(9362): 1056.
- Wada S, Ichikawa K, Tatsumi K (1993) Removal of phenols from wastewater by soluble and immobilized tyrosinase. Biotechnology and Bioengineering 42: 854-858.
- Lenhart JL, Sun WQ, Payne GF (1997) Coupling enzymatic reaction with chemisorption for the selective removal of substituted phenolic isomers. Chemical Engineering Science 52: 645-648.
- Payne GF, Sun WQ,Sohrabi A (1992)Tyrosinase reaction/chitosan adsorption for selectively removing phenols from aqueous mixtures.Biotechnology and Bioengineering 40: 1011-1018.
- Wada S, Ichikawa H, Tatsumi K (1992) Removal of phenols with tyrosinase immobilized on magnetite. Water Science and Technology 26: 2057-2059.
- Muthulakshmi AN, Anuradha J (2015) Removal of cadmium ions from water/waste water using chitosan - A review. JEES.
- Jana S, Trivedi MK, Tallapragada RM, Branton A, Trivedi D, et al. (2015) Characterization of physicochemical and thermal properties of chitosan and sodium alginate after biofield treatment. Pharm Anal Acta 6: 430.
- Chiu HT, Hsu XY, Yang HM, Ciou YS (2015) Synthesis and characteristics of m-tmxdi-based waterborne polyurethane modified by aqueous chitosan. J Textile SciEng 5: 218.
- Freitas JHES, Mahnke LC, Estevam-Alves MHM, Santana KV, Campos-Takaki GM, et al. (2015) Evaluation of the potential of cadmium and dyes removal by chitosan obtained from zygomycetes. J Mol Genet Med S4: 003.Â
- Amouzgar P, Salamatinia BÂ (2015)Â A short review on presence of pharmaceuticals in water bodies and the potential of chitosan and chitosan derivatives for elimination of pharmaceuticals. J Mol Genet Med S4: 001.
- Thirumavalavan M, Lee JF (2015) Ashort review on chitosan membrane for biomolecules immobilization. J Mol Genet Med 9: 178.
- Aminabhavi TM (2015) Chitosan-based hydrogels in biomedicine. J Pharma Care Health Sys 2: e133.
- Arafat A, Samad SA, Huq D, Moniruzzaman M, Shah Md. M (2015) Textile dye removal from wastewater effluents using chitosan-ZnOnanocomposite. J Textile SciEng 5: 200.
- Deshmukh YK, Sao S (2014) Chitin and chitosan? Most convenient natural matter that use in different useful ways. Journal of Industrial Pollution Control.
- Sun WQ, Payne GF, Moas MSGL, Chu JH, Wallance KK (1992) Tyrosinase reaction/chitosan adsorption for removing phenols from wastewater. Biotechnology Progress 8: 179-186.
- Youwei Y, Yinzhe R (2013) Effect of chitosan coating on preserving character of post-harvest fruit and vegetable: A review. J Food Process Technol 4: 254.
- Ganguly S (2013) Chitosan from shellfishes having promising biomedical importance: An editorial. AdvPharmacoepidemiol Drug Saf2: e122.
- Jianglian D, Shaoying Z (2013) Application of chitosan based coating in fruit and vegetable preservation: A review. J Food Process Technol 4: 227.
- Sarkar K, Debnath M, Kundu PP (2012) Recyclable cross-linked o-carboxymethyl chitosan for removal of cationic dye from aqueous solutions. Hydrol Current Res.
- Sarkar K, Banerjee SL, Kundu PP (2012) Removal of anionic dye in acid solution by self cross-linked insoluble dendronized chitosan. Hydrol Current Res 3: 133.
- ThiYeu Ly NVSH (2012) Adsorption of U(VI) from aqueous solution by chitosan grafted with citric acid via cross-linking with glutraldehyde. J ChemEng Process Technol 3: 128.
- Bhattarai N (2012) Nanofibrousstructure of chitosan for biomedical applications. J NanomedineBiotherapeuticDiscov 2: 102.
- Moussa SA, Farouk AF, Opwis K, Schollmeyer E (2011) Production, characterization and antibacterial activity of Mucorrouxii DSM-119 chitosan. J Textile SciEngg.
- Patnaik S , Mishra PC, Nayak RN, Giri AK (2016) Removal of fluoride from aqueous solution using chitosan-iron complex. J Anal Bioanal Tech 7: 326.
- Bhavani K, RoshanAra Begum E, Selvakumar S, Shenbagarathai R (2016) Chitosan: A low cost adsorbent for electroplating waste water treatment. J BioremediatBiodegrad 7:346.
- Muniyappan Gandhi R (2016) Recentadvances in chitosan based biosorbent for environmental clean-up. J BioremediatBiodegrad 7: e173.
- Thien DT, An NT and Hoa NT (2015) Preparation of fully deacetylated chitosan for adsorption of Hg(ii) ion from aqueous solution. ChemSci J 6: 95.
- Zueva SB, Ostrikov AN, Ilyina NM, De Michelis I, Velio F (2013) Coagulation processes for treatment of waste water from meat industry. Int J Waste Resources.
- AlbisuL, King RD, KozlovA (1989) Inhibition of the catecholase activity of mushroom tyrosinase by carbon monoxide. Journal of Agriculture and Food Chemistry 37: 775-776.
- Nithanandam VS, Erhan (1991) Qurnoneamine polymers: 10. Use of calcium hypochlorite in the synthesis of polyaminequinone (PAQ) polymers. Polymer 32: 1146-1149.
- Rashid K, Saddiqa A, Nawaz A (2015) Parametricstudy for adsorption of sodium rhodizonate on chitosan. J Environ Anal Chem 2: 136.
- Abbaas AAF (2015) Dynamic assessment for biocidal properties of silicon-chitosancontaining hydrogels. IJIRSET.
- Tutar Y (2015) Biomimetic marine material chitosan and its applications. Single Cell Biol 4: 110.
- Raval K, Sunil D, Singhania S, Deshpande P (2013) Enhanced bioavailability of 6-[3-(4- chlorophenyl)-1h-pyrazol-4-yl]-3-[(2-naphthyloxy) methyl][1,2,4] triazolo[3,4- b][1,3,4] thiadiazoleon c6 cell lines using chitosan encapsulation at nano scale. IJIRSET.
- Adarsh KJ, Madhu G (2014) Acomparative study on metal adsorption properties of different forms of chitosan. IJIRSET.Â
- Patil MK, Nayak PL (2011) Graft copolymerization of vinyl monomers onto chitosan:iii: graft copolymerization of acrylamide onto chitosan for antibacterial activity. IJPAES.Â
- Perchyonok VT, Zhang S, Basson N, Grobler S, Oberholzer T, et al. (2014) Insights into functional erythromycin/antioxidant containing chitosan hydrogels as potential bio-active restorative materials: structure, function and antimicrobial activity. Adv Tech Biol Med 2: 116.
- Dietler C, Lerch K (1982) Reaction inactivation of tyrosinase. Oxidases and related redox system (King, T. E., Editor) Pergamon Press, Oxford 305-317.
- Patel AR, Sun WQ, Payne Gf (1994) Tyrosinase reaction/chitosan adsorption:Potential for removing polymeriation storage inhibition. Industrial and Engineering Chemistry Research 33: 2168-2173.
- Sun WQ, Payne GF (1996) Tyrosinase-containing chitosan gels: A combined catalyst and sorbent for selective phenol removal. BiotechnolBioeng 51: 79-86.
- Thien DT, An NT, Hoa NT (2015) Preparation of fully deacetylated chitosan for adsorption of hg(ii) ion from aqueous solution. ChemSci J 6: 95.
- Solanki M, DwivediAk (2011) Studies on physico-chemical parameters during the treatment of waste water from viscose fiber unit by chemical coagulation. Journal of Industrial Pollution Control.Â
- Nirmalkumar K, Sivakumar V (2008) Astudy on the durability impact of concrete by using recycled waste water. Journal of Industrial Pollution Control.Â
- Pandey B, Baghel PS, Sharma B (2014) Impact of domestic waste water of Bhilai Steel PlantTownship on macrophytes like Calocaciasp. around the flow passage. Journal of Industrial Pollution Control.
- Trivedi P, Khandelwal M, Srivastava P (2014) Statisticallyoptimized synthesis of silver nanocubes from peel extracts of Citrus limetta and potential application in waste water treatment. J Microbial BiochemTechnol S4-004.
- Mehta SK, Panchal PA, Butala BN, Sane SA (2014) Bacillus cereusmediated e-caprolactam degradation: An initiative for waste water treatment of nylon-6 production plant. J BioremedBiodeg 5: 230.
- Kumar R, Yadav N, Rawat L, Goyal MK (2014) Effect of two waves of ultrasonic on waste water treatment. J ChemEng Process Technol 5: 193.
- Olawale AM (2014) Bioremediation of waste water from an industrial effluent system in Nigeria usingPseudomonas aeruginosa: Effectiveness tested on albino rats. J Pet Environ Biotechnol 5: 167.
- Awaleh MO, Soubaneh YD (2014) Waste water treatment in chemical industries: The concept and current technologies. Hydrol Current Res 5: 164.
- Tuncan A, Onur MI, Sarikavakli A, Tuncan M (2016) Design of landfill liner for boron mine waste water. Int J Waste Resour.
Citation: Agarwal P, Gupta R, Agarwal N (2016) A Review on Enzymatic Treatment of Phenols in Wastewater. J Biotechnol Biomater 6:249. DOI: 10.4172/2155-952X.1000249
Copyright: © 2016 Agarwal P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17106
- [From(publication date): 12-2016 - Sep 02, 2025]
- Breakdown by view type
- HTML page views: 15842
- PDF downloads: 1264