A Role for Mast Cells in Alcohol-Induced Tissue Damage and Remodeling
Received: 09-Dec-2014 / Accepted Date: 11-Mar-2015 / Published Date: 15-Mar-2015 DOI: 10.4172/2161-0681.1000218
Abstract
Introduction: Alcohol continues to be one of the most frequently abused drugs in the world. While low levels of alcohol consumption may have health benefits, chronic abuse of alcohol deleteriously impacts most body systems and contributes to or exacerbates over sixty disease conditions. The mechanisms of organ and tissue damage in response to alcohol abuse include altered metabolic pathways, accumulation of reactive oxygen species and depressed immune function. Mast cells are multi-functional cells that have been classically described for their role in hypersensitivity reactions. More recently, roles for these cells have been elucidated in innate immunity and tissue remodeling. Mast cells perform these functions primarily through the secretion of a plethora of mediators that include histamine, heparin, serine proteases, cytokines and others. The specific factors that are produced and secreted at any time by mast cells depend in part on the tissue microenvironment providing the basis for extensive plasticity of these cells. Recent studies are beginning to define the role of mast cells in mediating the deleterious effects of chronic alcohol abuse. For instance, alcohol-induced damage to the gastrointestinal mucosa is at least in part mediated by activation of mast cells. Pharmacological inhibition of mast cell degranulation attenuates the increased permeability of the gastrointestinal epithelium associated with alcohol abuse. Conclusion: Mast cells and their secretory products have been implicated in promoting a number of disease conditions. Recent studies have suggested an important role for these cells in alcohol-induced tissue remodeling. These cells and their specific secretory mediators may provide novel therapeutic targets in prevention or reversal of alcohol-induced tissue damage.
Keywords: Mast cells; Remodelling; Alcohol abuse
312510Introduction
Despite the clear health ramifications of chronic alcohol abuse, this continues to be one of the most frequently abused chemicals in the world. Approximately fourteen million Americans chronically abuse alcohol (National Institute of Alcohol Abuse and Alcoholism) and excessive alcohol use is the third leading cause of preventable death in the United States [1]. Alcohol abuse affects most organ systems and causes or exacerbates over sixty diseases including heart disease, Alzheimer’s, stroke, liver disease, diabetes mellitus and others [2-6]. The pathological effects of chronic alcohol abuse involve a number of mechanisms including altered metabolic, immunological, signaling and inflammatory pathways. Studies in several organ systems have illustrated that chronic consumption of high levels of alcohol alters immune and inflammatory responses including impairment of antimicrobial and antiviral immunity. Excessive consumption of alcohol is associated with increased incidence of pneumonia, tuberculosis, hepatitis C infection, enhanced susceptibility to HIV and increased propensity for some tumors [7-9]. These effects have been attributed to suppression of immune function by alcohol abuse. Many questions remain regarding the cellular and molecular mechanisms of these effects; however, substantial advances have recently been made in this area. Exposure to ethanol affects cytokine production by various immune cells [10-12]. Among the cells shown to be affected by chronic alcohol exposure, mast cells fill a particularly important role in innate immunity and deleterious tissue remodeling.
Mast cells
Mast cells are bone marrow-derived effector cells found abundantly in connective tissues situated at the interface of the body and the external environment including the integument and mucous membranes [13,14]. They are also found in connective tissue components of many organs, particularly in association with blood vessels, nerves and mucous glands [15]. Mast cells were originally described based upon their unique histological staining of large cytoplasmic granules [16]. The initial view that these cells were involved in nutrition of surrounding tissue gradually evolved as it was discovered that mast cell granules contain histamine and a functional relationship between histamine and anaphylaxis was developed [17,18]. These and other discoveries set the stage for the classical view of mast cells functioning in the early phases of immediate hypersensitivity reactions. In this process, antigens react with immunoglobulin E bound to the surface of mast cells promoting degranulation and release of mast cell mediators that are responsible for a number of the features typically associated with allergies including mucous secretion, bronchoconstriction and increased vascular permeability [19,20].
As research into mast cell function has continued, it has become increasingly clear that these cells are multifunctional and their specific roles are likely regulated by the tissue microenvironment. Mast cells are involved in a variety of physiological processes including tissue repair, wound healing, angiogenesis and likely adaptive immunity [21]. Mast cells also play roles in defense against parasitic and bacterial infections [22]. More recently, dysregulation of mast cells and their secretory mediators has been implicated in playing a causal role in deleterious tissue remodeling, autoimmune disorders and cancers [23].
Mast cells produce and secrete a variety of biochemical mediators. Indeed, the large array of mediators produced by mast cells contributes to their functional plasticity [24]. These mediators are subdivided into three general groups: 1) preformed mediators, 2) neoformed or lipid mediators and 3) newly synthesized mediators. Preformed mediators are stored in cytoplasmic granules which are released upon mast cell activation (degranulation). Mast cells store a wide variety of preformed mediators including histamine, heparin, proteases and select cytokines such as tumor necrosis factor-α [21]. The release of preformed mediators typically occurs within seconds to minutes of stimulation providing an advantage for the involvement of these cells in immune surveillance [25]. One of the first and most extensively studied mast cell preformed mediators is histamine [18]. Histamine is a biogenic amine with diverse functions associated with allergic reactions including vasodilation, increased capillary permeability and bronchoconstriction [26-29]. More recent studies have described novel roles for histamine including involvement in autoimmune diseases and regulation of dendritic cell function [30]. Mast cell granules also contain active enzymes of the mast cell protease family, which includes chymases, tryptases and carboxypeptidease A [31]. Mast cells can be stimulated to degranulate by cross-linking of immunoglobulin E receptors, mechanical force, electrical activity and by various chemical activators [32]. The neoformed mediators are of the eicosanoid family and are synthesized as required from the fatty acids of membrane phospholipids [33,34]. Two families of enzymes catalyze fatty acid oxygenation to produce eicosanoids from arachidonic acid - cyclooxygenases and lipooxygenases. The mast cell-produced eicosinoids, prostaglandins and leukotrienes, have a variety of functions including stimulation of leukocyte migration, smooth muscle contraction, mucus production and others [15,35,36]. Members of the final group of mediators are newly synthesized when the mast cell is activated and are regulated in a stimulus-specific manner. These include diverse cytokines, chemokines and growth factors. Unlike release of preformed mediators during degranulation, production of newly synthesized mediators is a slower process more in line with the responses seen in other inflammatory and immune cells.
Mast cells exhibit extensive plasticity and heterogeneity in the production and release of mediators that directly reflect their microenvironment and the stimulus encountered. Multiple phenotypes of mast cells have been described based upon their localization and array of biochemical mediators produced. In rodents, two subtypes have been described that vary in the proteases produced. In mice, mucosal mast cells typically reside in the mucosa of the lung and gastrointestinal tract and produce mouse mast cell proteases 1 and 2. In contrast, connective tissue mast cells are located in intestinal submucosa, peritoneum and skin and are characterized by the production of mouse mast cell proteases 4,5 and 6 [37,38]. In humans, mature mast cells have also been divided into two subsets based upon their protease content. The mast cell tryptase (MCT) subset store tryptases in their granules while the mast cell tryptase/chymase (MCTC) subset store tryptases, chymases and carboxypeptidases in their granules [38-40]. While these characterizations provide a simple scheme for classifying mast cells, it is clear that these phenotypes can vary between tissues or even between cells in the same tissue. This is likely modulated by the biochemical milieu of the local environment as particular cytokines have been shown to promote the differentiation of specific mast cell phenotypes [15,41,42].
Roles for mast cells in the response to alcohol
While mast cells have begun to receive more attention regarding their role in normal tissue physiology and in diverse disease conditions, very few studies have focused on the contribution of these cells to alcohol-induced tissue damage. However, accumulating evidence suggests a role for these cells in pathogenesis associated with alcohol abuse. These studies will be summarized below.
Ethanol, mast cells and asthma
While controversial for some time, there is now compelling evidence that mast cells play a substantial causative role in the consequences of asthma [43]. Mast cells secrete mediators that can stimulate mucus secretion, bronchoconstriction and mucosal edema, which are all features of asthma. Bronchial mast cells exhibit features of ongoing activation in patients with asthma [44]. This is associated with alterations in the microlocalization of mast cells within respiratory tissues [45]. Most of the mast cells in the airway smooth muscle are characterized by expression of both tryptase and chymase. Experimental studies have illustrated that delivery of exogenous tryptase induces bronchoconstriction in dog and sheep models [46]. This response is blocked by anti-histamine suggesting that the tryptase effect on bronchoconstriction is mediated by histamine. Thus, mast cell activation appears to be an important mediator of asthma. Indeed, prostaglandin 2 has a protective effect against asthma and this may be in part due to prevention of mast cell activation [47]. Despite these advances, the relationship of mast cells to alcohol-induced respiratory conditions has not been extensively investigated.
The incidences of asthma and binge drinking are on the rise, particularly in young adults [48]; however, their interactions have remained relatively unexplored. A number of clinical studies have illustrated that alcohol use exacerbates asthmatic symptoms [49,50]. A recent study in allergen-sensitized mice illustrated that ethanol treatment induced mast cell degranulation and exacerbated the symptoms of asthma including airway constriction and mucous production [48]. Similarly, acetaldehyde, the first metabolite of ethanol, induces degranulation and histamine release from airway mast cells resulting in constriction of bronchi [51,52]. To date, perturbation studies have not been carried out to conclusively define the functional role of mast cells and their mediators in the effects of alcohol on asthmatic conditions.
Ethanol-induced gastric damage
Exposure of the gastrointestinal tract to ethanol results in vascular leakage in mucosal capillaries and postcapillary venules [53]. This contributes to hemorrhage and the development of necrotic lesions in the gastric wall [54]. Several studies have illustrated a correlation between the number of mast cells and the appearance of gastric lesions [55,56]. Compounds that promote mast cell degranulation (compound 48/80, for instance), induce gastric damage that appears very similar to the damage elicited by ethanol exposure [57]. A hallmark of the mast cell product histamine is its effects on the vasculature and induction of vascular leakage. The mechanism of this effect is the production of small gaps between endothelial cells in capillaries and small venules [58,59]. Exposure to ethanol elicits similar effects on mucosal blood vessels as histamine itself, suggesting a functional role for mast cells in the response to ethanol [60]. Administration of mast cell stabilizers such as sodium chromoglycate and ketotifen prevent ethanol-induced lesion formation in vivo and in tissue culture models [53,61-63]. Furthermore, application of ketotifen prevents ethanol-induced alterations in vascular structure and function [53]. More recent studies have illustrated that epidermal growth factor has protective effects from alcohol-induced gastric damage [64]. Treatment with epidermal growth factor prior to ethanol exposure results in decreased gastric ulcer content and diminished release of histamine. The effects of epidermal growth factor appear to emanate in part from its ability to stabilize mast cells in the gastrointestinal tract.
Exposure of the gastrointestinal tract to alcohol also increases permeability of the mucosal epithelium [65,66]. This appears to be due to the metabolism of ethanol to acetaldehyde as ethanol itself has little direct effect on the epithelium. Acetaldehyde has been demonstrated to disrupt epithelial tight junctions [67]. Disruption of the epithelial barrier results in increased blood endotoxin levels, which in turn has substantial effects on other organs, particularly the liver. Endotoxin is a potent activator of liver Kupffer cells and appears to be a prerequisite for cirrhosis [68,69]. Mast cells themselves can induce disruption of the epithelial barrier suggesting that they may be involved in ethanol-induced alterations in this barrier [70,71]. Studies have illustrated that gastrointestinal bacteria are able to metabolize ethanol into acetaldehyde and inhibition of this with antibiotics reduces the disruption of the epithelial barrier by alcohol [72]. In addition, stabilization of mast cells with doxantrazole reduces the disruption of epithelial barrier function by ethanol exposure. These studies have established a paradigm whereby ethanol is metabolized by endogenous gut bacteria and epithelial function is reduced in part by activation of mucosal mast cells by acetaldehyde.
Alcohol, mast cells and cancer
While the impact of alcohol abuse on a number of diseases has been investigated, its impact on cancer has not been as widely appreciated. Chronic alcohol use, in fact, promotes several epithelial cancers including esophageal, liver, breast and colorectal cancers [73-75] and results in poor prognosis of these. The underlying mechanisms mediating the effects of ethanol on cancer susceptibility and progression are not well understood but may include the formation of DNA adducts, lipid peroxidation, oxidative stress, inflammation or other processes. In the colon, mast cells participate in innate immunity to protect against microbial pathogens and parasites and to modulate the permeability of the gut epithelium. However, mast cells also respond to oncogenic signals in ways that appear to contribute to cancer progression [76-78]. Recent studies illustrated that chronic alcohol consumption induces an increase in intestinal polyps in the APC min/+ mouse model [79]. There was a corresponding increase in the number of mast cells in the stroma of polyps in the ethanol-treated animals including both tryptase-positive and chymase-positive cells. The functional significance of these alterations remain to be determined.
Mast cells and alcoholic cardiomyopathy
Mast cells and their secretory products have been implicated in mediating cardiac remodeling associated with a number of pathological conditions including hypertension, myocarditis, myocardial infarction and heart transplantation [80-84]. Increased cardiovascular load as occurs in hypertension results in myocardial hypertrophy and fibrosis. Concurrent with these changes is an increase in mast cell density in the myocardium [85]. Studies in which mast cells were stabilized with nedocromil illustrated a causal role for mast cells in myocardial remodeling and fibrosis of spontaneously hypertensive rats [84]. In these studies, mast cell stabilization also normalized the cytokine expression profiles associated with chronic hypertension. Mast cells have also been implicated in damage resulting from myocardial ischemia/reperfusion. In response to myocardial ischemia/reperfusion, mast cells release renin, which activates the local renin-angiotensin system [86,87]. This culminates in the local formation of angiotensin II, increased sympathetic activity and arrhythmias [87,88]. Interestingly, ischemic preconditioning inhibits subsequent ischemia/reperfusion-induced mast cell release of renin and activation of the local renin-angiotensin system [89]. Collectively these studies illustrate an important role for mast cells in diverse cardiovascular diseases and suggest these cells may be an important therapeutic target.
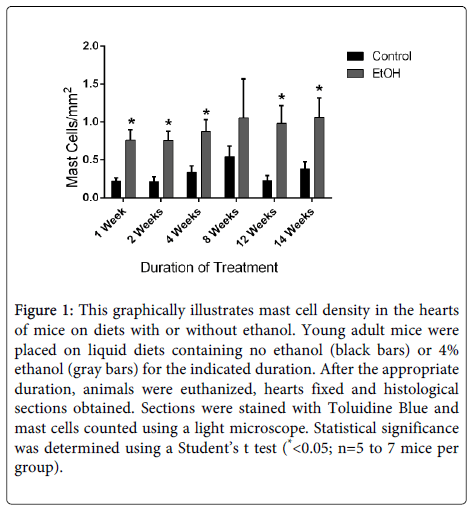
Chronic alcohol exposure results in a form of heart disease termed alcoholic cardiomyopathy. Approximately thirty percent of alcoholics will develop this disorder, making it one of the predominant forms of nonischemic heart disease [90-92]. This disorder is characterized by myocyte damage, contractile dysfunction, myocardial fibrosis and eventually heart failure [93]. The role of mast cells has not been investigated in this disease. We conducted studies in a mouse model to evaluate potential alterations in myocardial mast cells in response to chronic alcohol consumption. In these studies, mice were placed on a liquid diet containing 4% ethanol [94]. Control mice were placed on an isocaloric liquid diet without ethanol. Previous studies have illustrated that animals on the ethanol-containing diet develop myocardial hypertrophy and fibrosis within two weeks [93]. Tissue sections of hearts were obtained from mice after specific durations on the control or ethanol-containing diets and stained with toluidine blue to identify mast cells. These studies illustrated that mast cell density is rapidly increased in the hearts of mice on the alcohol-containing diet (Figure 1). Further studies will be required to determine the functional role of mast cell activation in the progression of alcohol-induced myocardial remodeling; however, it is tempting to speculate that activation of these cells contributes to the progression of alcoholic cardiomyopathy similar to other cardiovascular conditions.
Figure 1: This graphically illustrates mast cell density in the hearts of mice on diets with or without ethanol. Young adult mice were placed on liquid diets containing no ethanol (black bars) or 4% ethanol (gray bars) for the indicated duration. After the appropriate duration, animals were euthanized, hearts fixed and histological sections obtained. Sections were stained with Toluidine Blue and mast cells counted using a light microscope. Statistical significance was determined using a Student’s t test (*<0.05; n=5 to 7 mice per group).
Cellular and molecular mechanisms of the alcohol effects
While it is becoming increasingly clear that mast cells contribute to the progression of alcohol-induced pathology; many questions remain regarding the molecular mechanisms of their effects. Alcohol exposure may impact a number of parameters of mast cell physiology including differentiation, activation/degranulation, gene expression, proliferation, migration and other processes. The response of mast cells and other inflammatory cells to alcohol will likely depend upon the local microenvironment. To date, few studies have focused on the direct effects of alcohol on mast cells.
As mentioned previously, chronic alcohol abuse impairs the immune response resulting in increased susceptibility of alcoholics to infectious diseases. Several studies have illustrated that alcohol treatment of animals inhibits expression of proinflammatory cytokines by macrophages and other cell types [7,95]. Pretreatment of isolated bone marrow-derived mast cells with ethanol in vitro results in attenuated IgE-mediated degranulation [12]. Similarly, long-term exposure to ethanol in an inhalation model, results in diminished mast cell degranulation following treatment with compound 48/80 [96]. These studies also illustrated that treatment of the HMC-1 human mast cell line with ethanol, inhibits tumor necrosis-alpha (TNF-α) and interleukin-8 production. In contrast to this, other studies have illustrated that treatment of mast cells with low doses of ethanol or acetaldehyde results in increased expression of cytokines including TNF-α, transforming growth factor-beta and interleukin-6 [97]. These effects were attenuated by pretreatment of the cells with mitogen-activated protein kinase (MAPK) inhibitors. The differences in these results may reflect a dose-dependent response of mast cells to ethanol and contribute to a potential explanation for the beneficial effects of low doses of alcohol consumption versus harmful effects of alcohol abuse.
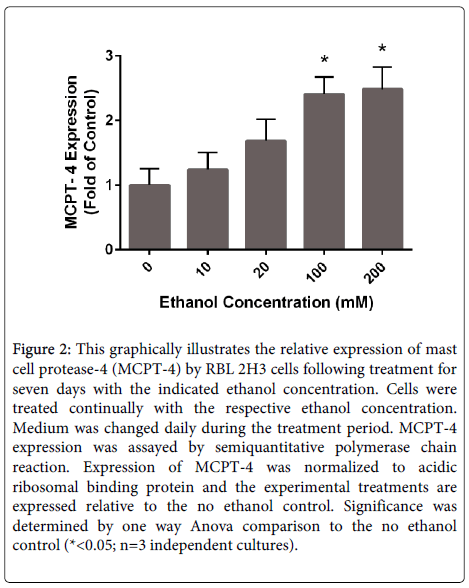
Mast cells produce and store exceptionally high levels of serine proteases collectively termed mast cell proteases. These proteases include tryptase, chymase and carboxypeptidase A. Mast cell proteases may account for up to 25% of the total protein content in mast cells [98]. A diverse array of functions has been attributed to these enzymes including roles in the clearance of parasites and bacteria, cleavage of extracellular matrix components, activation of matrix metalloproteases and others. Studies have illustrated that relatively high doses of acetaldehyde stimulates the production of chymase by mast cells [99]. We have performed experiments to evaluate the effects of long-term (1 week) exposure of the rat basophil/ mast cell line (RBL 2H3 cells) on mast cell protease expression. Following culture for 1 week in ethanol, the expression of mRNAs for mast cell proteases was evaluated. Mast cell protease 4, also known as β chymase, mRNA levels were significantly increased in a dose-dependent manner in these experiments (Figure 2). The mechanisms of this response and the functional significance of enhanced chymase production in alcohol-induced tissue remodeling remain to be determined.
Figure 2: This graphically illustrates the relative expression of mast cell protease-4 (MCPT-4) by RBL 2H3 cells following treatment for seven days with the indicated ethanol concentration. Cells were treated continually with the respective ethanol concentration. Medium was changed daily during the treatment period. MCPT-4 expression was assayed by semiquantitative polymerase chain reaction. Expression of MCPT-4 was normalized to acidic ribosomal binding protein and the experimental treatments are expressed relative to the no ethanol control. Significance was determined by one way Anova comparison to the no ethanol control (*<0.05; n=3 independent cultures).
Mast cells are generated from bone marrow-derived precursors and mature in response to c-kit ligand, stem cell factor and the cytokine/growth factor milieu of the tissue. Increased numbers of mast cells in tissues can arise by proliferation of resident mast cells or recruitment of additional cells. Few studies have examined the effects of alcohol on either of these aspects of mast cell biology. Treatment of bone marrow-derived mast cells and the human mast cell line, HMC-1, with ethanol inhibits proliferation [12,100]. This was not accomplished by treatment of mast cells with acetaldehyde suggesting that this is a direct effect of ethanol on these cells [100]. Due to the effects of alcohol abuse on neurological development, a number of studies have evaluated the modulation of proliferation in the developing nervous system by ethanol. Exposure to high doses of ethanol inhibits proliferation of multiple cell types including glial cells [101,102], dermal fibroblasts [103] and neural crest cells [104]. Studies with a binge alcohol exposure model in adolescent rats illustrated an alteration of cell cycle kinetics in hippocampal progenitor cells [105]. Mechanistic studies evaluating inhibition of hepatic cell proliferation by ethanol have suggested dysfunction of iron metabolism as a contributing factor [106]. In neural stem cells, inhibition of proliferation appears to be dependent on alteration of extracellular signal-regulated kinase and phopholipase D by ethanol exposure [107]. In contrast to other cell types, ethanol exposure results in enhanced cell growth and proliferation of human embryonic stem cell lines [108]. Further studies are required to conclusively determine the effects of alcohol on proliferation of mast cells and their progenitors and to elucidate the mechanisms of these effects.
Ethanol exposure induces apoptosis of various cells including liver Hep G2 cells, macrophages, embryonic neural crest cells and cardiac myocytes [90,109,110]. Recent studies have also illustrated that treatment of human mast cells in vitro with ethanol decreases viability of the cells [100]. This was true of both the HMC-1 cell line and primary bone marrow-derived mast cells. Ethanol treatment promoted apoptosis of mast cells, as determined by TUNEL staining and activation of caspase-3. The fact that exposure to ethanol is thought to decrease proliferation of mast cells and promote apoptosis appears contradictory to the increase numbers of mast cells in tissues following alcohol consumption. Other processes that would increase mast cell number in tissues include enhanced migration of precursor cells or the promotion of mast cell differentiation by alcohol.
As mentioned previously, chronic alcohol consumption results in impaired immune function. Long-term ethanol exposure in an inhalation model results in reduced neutrophil and eosinophil migration [96]. Similarly, ethanol exposure reduces migratory capacity of cutaneous dendritic cells [111] and natural killer cells [112]. In the former studies, exposure to ethanol prevented the transition of the dendritic cells to a migratory phenotype with concomitant changes in gene expression that promote migration [113]. Other studies have illustrated that ethanol exposure alters microtubule assembly, which may impact migration, proliferation and other cellular processes [114]. In contrast to this, ethanol exposure appears to enhance the migratory ability of some cancer cells. Recent studies have illustrated that exposure to alcohol results in enhanced epithelial to mesenchymal transition including increased expression of matrix metalloproteases required for migration and metastasis [115]. Alcohol also has effects on the tumor microenvironment including alterations in the extracellular matrix that may enhance migration through the matrix [116]. The effect of alcohol on migration of mast cells has not been evaluated, but is an important aspect of mast cell-mediated responses that needs to be explored.
Conclusions
Mast cells play substantial roles in a variety of disease processes. Several recent studies have indicated a role for these cells in mediating the deleterious effects of chronic alcohol abuse making mast cells an intriguing therapeutic target. Ethanol or acetaldehyde treatment in vivo and in vitro has been shown to impact a variety of parameters important to mast cell function including gene expression, differentiation, degranulation/secretion, migration, proliferation and even survival. However, the molecular and cellular mechanisms whereby mast cells affect alcohol-induced disease are not well established. Further research in this area will be critical to development of specific therapeutic approaches with few side effects. Global inhibition of mast cell degranulation has been shown to prevent or slow the progression of several pathological conditions; however, this is likely to have significant detrimental effects as these cells play important physiological roles. Identification of individual factors involved in mast cell activation or secretory products of mast cells involved in specific processes will provide more specific targets for therapeutic purposes.
Acknowledgements
This research was supported by funding through the University of South Carolina School of Medicine RDF program.
References
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL (2004) Actual causes of death in the United States, 2000. JAMA 291: 1238-1245.
- Marinho V, Laks J, Engelhardt E, Conn D (2006) Alcohol abuse in an elderly woman taking donepezil for Alzheimer disease. J ClinPsychopharmacol 26: 683-685.
- Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, et al. (2009) Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 32: 2123-2132.
- Ohkubo T, Metoki H, Imai Y (2009) Alcohol intake, circadian blood pressure variation, and stroke. Hypertension 53: 4-5.
- Cederbaum AI, Lu Y, Wu D (2009) Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 83: 519-548.
- George A, Figueredo VM (2010) Alcohol and arrhythmias: a comprehensive review. J Cardiovasc Med (Hagerstown) 11: 221-228.
- Szabo G (1997) Alcohol's contribution to compromised immunity. Alcohol Health Res World 21: 30-41.
- Naveau S, Raynard B, Ratziu V, Abella A, Imbert-Bismut F, et al. (2005) Biomarkers for the prediction of liver fibrosis in patients with chronic alcoholic liver disease. ClinGastroenterolHepatol 3: 167-174.
- de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, et al. (2006) Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest 129: 1219-1225.
- Spolarics Z, Spitzer JJ, Wang JF, Xie J, Kolls J, et al. (1993) Alcohol administration attenuates LPS-induced expression of inducible nitric oxide synthase in Kupffer and hepatic endothelial cells. BiochemBiophys Res Commun 197: 606-611.
- Szabo G, Mandrekar P, Girouard L, Catalano D (1996) Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol ClinExp Res 20: 900-907.
- Toivari M, Mäki T, Suutarla S, Eklund KK (2000) Ethanol inhibits IgE-induced degranulation and cytokine production in cultured mouse and human mast cells. Life Sci 67: 2795-2806.
- Metcalfe DD, Baram D, Mekori YA (1997) Mast cells. Physiol Rev 77: 1033-1079.
- Jamur MC, Grodzki AC, Berenstein EH, Hamawy MM, Siraganian RP, et al. (2005) Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood 105: 4282-4289.
- Galli SJ, Nakae S, Tsai M (2005) Mast cells in the development of adaptive immune responses. Nat Immunol 6: 135-142.
- Ehrlich P (1878) BeitragezurTheorie und Praxis der histologichenFarbung.Dissertation Thesis, Leipzig University, Germany.
- Holmgren H, Willander O (1937) BeitragzurKenntnis der Chemie und Funktion der EhrlichschenMastzellen. Z MikroskAnatForsch 42: 242-278.
- RILEY JF, WEST GB (1952) Histamine in tissue mast cells. J Physiol 117: 72P-73P.
- Metzger H (1992) The receptor with high affinity for IgE. Immunol Rev 125: 37-48.
- Kinet JP (1999) The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol 17: 931-972.
- da Silva EZ, Jamur MC, Oliver C (2014) Mast cell function: a new vision of an old cell. J HistochemCytochem 62: 698-738.
- Echtenacher B, Männel DN, Hültner L (1996) Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381: 75-77.
- Rao KN, Brown MA (2008) Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y AcadSci 1143: 83-104.
- Oskeritzian CA (2015) Mast cell plasticity and sphingosine-1-phosphate in immunity, inflammation and cancer. MolImmunol 63: 104-112.
- Choi HW, Abraham SN (2015) Mast cell mediator responses and their suppression by pathogenic and commensal microorganisms. MolImmunol 63: 74-79.
- Schayer RW (1974) Histamine and microcirculation. Life Sci 15: 391-401.
- BOVET D, KOHN R, MAROTTA M, SILVESTRINI B (1958) Some effects of histamine in the normal and Haemophilus pertussis vaccinated rat. Br J PharmacolChemother 13: 74-83.
- Castells M (2006) Mast cell mediators in allergic inflammation and mastocytosis. Immunol Allergy Clin North Am 26: 465-485.
- Lundequist A, Pejler G (2011) Biological implications of preformed mast cell mediators. Cell Mol Life Sci 68: 965-975.
- Simon T, László V, Falus A (2011) Impact of histamine on dendritic cell functions. Cell BiolInt 35: 997-1000.
- Schwartz LB, Bradford TR (1986) Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J BiolChem 261: 7372-7379.
- Fowlkes V, Wilson CG, Carver W, Goldsmith EC (2013) Mechanical loading promotes mast cell degranulation via RGD-integrin dependent pathways. J Biomech 46: 788-795.
- Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, et al. (1991) A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 65: 1043-1051.
- Berenbaum F, Humbert L, Bereziat G, Thirion S (2003) Concomitant recruitment of ERK1/2 and p38 MAPK signalling pathway is required for activation of cytoplasmic phospholipase A2 via ATP in articular chondrocytes. J BiolChem 278: 13680-13687.
- Weller CL, Collington SJ, Brown JK, Miller HR, Al-Kashi A, et al. (2005) Leukotriene B, an activation product of mast cells, is a chemoattractant for their progenitors. J Exp Med 201: 1961-1971.
- Carlos D, Machado ER, De Paula L, Sá-Nunes A, Sorgi CA, et al. (2011) Evidence for eosinophil recruitment, leukotriene B4 production and mast cell hyperplasia following Toxocaracanis infection in rats. Braz J Med Biol Res 44: 319-326.
- Welle M (1997) Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J LeukocBiol 61: 233-245.
- Pejler G, Rönnberg E, Waern I, Wernersson S (2010) Mast cell proteases: multifaceted regulators of inflammatory disease. Blood 115: 4981-4990.
- Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB (1986) Two types of human mast cells that have distinct neutral protease compositions. ProcNatlAcadSci U S A 83: 4464-4468.
- Schwartz LB (2006) Analysis of MC(T) and MC(TC) mast cells in tissue. Methods MolBiol 315: 53-62.
- Karimi K, Redegeld FA, Heijdra B, Nijkamp FP (1999) Stem cell factor and interleukin-4 induce murine bone marrow cells to develop into mast cells with connective tissue type characteristics in vitro. ExpHematol 27: 654-662.
- Oskeritzian CA, Wang Z, Kochan JP, Grimes M, Du Z, et al. (1999) Recombinant human (rh)IL-4-mediated apoptosis and recombinant human IL-6-mediated protection of recombinant human stem cell factor-dependent human mast cells derived from cord blood mononuclear cell progenitors. J Immunol 163: 5105-5115.
- Bradding P, Walls AF, Holgate ST (2006) The role of the mast cell in the pathophysiology of asthma. J Allergy ClinImmunol 117: 1277-1284.
- Bradding P (2000) Mast cells in asthma. In: Busse WW, Holgate ST (eds.). Asthma and Rhinitis. Blackwell Scientific Publications, Inc, Boston, pp. 319-338.
- Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, et al. (2002) Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 346: 1699-1705.
- Sekizawa K, Caughey GH, Lazarus SC, Gold WM, Nadel JA (1989) Mast cell tryptase causes airway smooth muscle hyperresponsiveness in dogs. J Clin Invest 83: 175-179.
- Torres R, Picado C, de Mora F (2015) The PGE2-EP2-mast cell axis: an antiasthma mechanism. MolImmunol 63: 61-68.
- Bouchard JC, Kim J, Beal DR, Vaickus LJ, Craciun FL, et al. (2012) Acute oral ethanol exposure triggers asthma in cockroach allergen-sensitized mice. Am J Pathol 181: 845-857.
- Ayres JG (1997) Alcohol-induced bronchial asthma. J Allergy ClinImmunol 99: 860.
- Vally H, Thompson PJ (2002) Alcoholic drinks and asthma. ClinExp Allergy 32: 186-191.
- Kawano T, Matsuse H, Kondo Y, Machida I, Saeki S, et al. (2004) Acetaldehyde induces histamine release from human airway mast cells to cause bronchoconstriction. Int Arch Allergy Immunol 134: 233-239.
- Matsuse H, Fukushima C, Shimoda T, Sadahiro A, Kohno S (2007) Effects of acetaldehyde on human airway constriction and inflammation. Novartis Found Symp 285: 97-106.
- Kalia N, Bardhan KD, Reed MW, Jacob S, Brown NJ (2000) Mast cell stabilization prevents ethanol-induced rat gastric mucosal injury: mechanisms of protection. J GastroenterolHepatol 15: 133-141.
- Natale G, Lazzeri G, Blandizzi C, Gherardi G, Lenzi P, et al. (2001) Seriatehistomorphometry of whole rat stomach: an accurate and reliable method for quantitative analysis of mucosal damage. ToxicolApplPharmacol 174: 17-26.
- Kahraman A, Erkasap N, Köken T, Serteser M, Aktepe F, et al. (2003) The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology 183: 133-142.
- Kanter M, Demir H, Karakaya C, Ozbek H (2005) Gastroprotective activity of Nigella sativa L oil and its constituent, thymoquinone against acute alcohol-induced gastric mucosal injury in rats. World J Gastroenterol 11: 6662-6666.
- Ohta Y, Kobayashi T, Inui K, Yoshino J, Nakazawa S (2009) Repeated recurrence of gastric mucosal lesions in rats after a single treatment with compound 48/80, a mast cell degranulator. J PhysiolPharmacol 60 Suppl 7: 139-148.
- MAJNO G, PALADE GE (1961) Studies on inflammation. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J BiophysBiochemCytol 11: 571-605.
- MAJNO G, PALADE GE, SCHOEFL GI (1961) Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J BiophysBiochemCytol 11: 607-626.
- Kalia N, Brown NJ, Jacob S, Reed MW, Bardhan KD (1997) Studies on gastric mucosal microcirculation. 1. The nature of regional variations induced by ethanol injury. Gut 40: 31-35.
- Takeuchi K, Nishiwaki H, Okabe S (1986) Cytoprotective action of mast cell stabilizers against ethanol-induced gastric lesions in rats. Jpn J Pharmacol 42: 297-307.
- Beck PL, Morris GP, Wallace JL (1989) Reduction of ethanol-induced gastric damage by sodium cromoglycate and FPL-52694. Role of leukotrienes, prostaglandins, and mast cells in the protective mechanism. Can J PhysiolPharmacol 67: 287-293.
- Tariq M, Moutaery MA, Elfaki I, Arshaduddin M, Khan HA (2006) Protective effects of nedocromil sodium and sodium cromoglycate on gastroduodenal ulcers: a comparative study in rats. Inflammopharmacology 14: 163-169.
- Erkasap S, Erkasap N, Aral E, Koken T, Kahraman A, et al. (2005) Mast cell stabilizator and antioxidant effects of epidermal growth factor (EGF) on gastric mucosal injury induced by ethanol in rats. Chin J Physiol 48: 1-6.
- Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C (2000) Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32: 742-747.
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, et al. (2001) Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J PharmacolExpTher 299: 442-448.
- Atkinson KJ, Rao RK (2001) Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J PhysiolGastrointest Liver Physiol 280: G1280-1288.
- Arteel GE (2003) Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 124: 778-790.
- Rao RK, Seth A, Sheth P (2004) Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J PhysiolGastrointest Liver Physiol 286: G881-884.
- Santos J, Yang PC, Söderholm JD, Benjamin M, Perdue MH (2001) Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut 48: 630-636.
- McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, et al. (2003) Mast cells disrupt epithelial barrier function during enteric nematode infection. ProcNatlAcadSci U S A 100: 7761-7766.
- Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, et al. (2006) Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol 168: 1148-1154.
- Kune GA, Vitetta L (1992) Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer 18: 97-111.
- Boffetta P, Hashibe M (2006) Alcohol and cancer. Lancet Oncol 7: 149-156.
- Schütze M, Boeing H, Pischon T, Rehm J, Kehoe T, et al. (2011) Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort studies. BMJ 342: d1584.
- Cheon EC, Khazaie K, Khan MW, Strouch MJ, Krantz SB, et al. (2011) Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice. Cancer Res 71: 1627-1636.
- Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, et al. (2007) Mast cells are an essential hematopoietic component for polyp development. ProcNatlAcadSci U S A 104: 19977-19982.
- Maltby S, Khazaie K, McNagny KM (2009) Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. BiochimBiophysActa 1796: 19-26.
- Wimberly AL, Forsyth CB, Khan MW, Pemberton A, Khazaie K, et al. (2013) Ethanol-induced mast cell-mediated inflammation leads to increased susceptibility of intestinal tumorigenesis in the APC Δ468 min mouse model of colon cancer. Alcohol ClinExp Res 37 Suppl 1: E199-208.
- Olivetti G, Lagrasta C, Ricci R, Sonnenblick EH, Capasso JM, et al. (1989) Long-term pressure-induced cardiac hypertrophy: capillary and mast cell proliferation. Am J Physiol 257: H1766-1772.
- Li QY, Raza-Ahmad A, MacAulay MA, Lalonde LD, Rowden G, et al. (1992) The relationship of mast cells and their secreted products to the volume of fibrosis in posttransplant hearts. Transplantation 53: 1047-1051.
- Hara M, Ono K, Hwang MW, Iwasaki A, Okada M, et al. (2002) Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med 195: 375-381.
- Jaggi AS, Singh M, Sharma A, Singh D, Singh N (2007) Cardioprotective effects of mast cell modulators in ischemia-reperfusion-induced injury in rats. Methods Find ExpClinPharmacol 29: 593-600.
- Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, et al. (2009) Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension 53: 1041-1047.
- Panizo A, Mindán FJ, Galindo MF, Cenarruzabeitia E, Hernández M, et al. (1995) Are mast cells involved in hypertensive heart disease? J Hypertens 13: 1201-1208.
- Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, et al. (2004) Mast cells: a unique source of renin. ProcNatlAcadSci U S A 101: 13607-13612.
- Mackins CJ, Kano S, Seyedi N, Schäfer U, Reid AC, et al. (2006) Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest 116: 1063-1070.
- Aldi S, Takano K, Tomita K, Koda K, Chan NY, et al. (2014) Histamine H4-receptors inhibit mast cell renin release in ischemia/reperfusion via protein kinase C ε-dependent aldehyde dehydrogenase type-2 activation. J PharmacolExpTher 349: 508-517.
- Koda K, Salazar-Rodriguez M, Corti F, Chan NY, Estephan R, et al. (2010) Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation 122: 771-781.
- Ren J, Wold LE (2008) Mechanisms of alcoholic heart disease. TherAdvCardiovasc Dis 2: 497-506.
- George A, Figueredo VM (2011) Alcoholic cardiomyopathy: a review. J Card Fail 17: 844-849.
- Walker RK, Cousins VM, Umoh NA, Jeffress MA, Taghipour D, et al. (2013) The good, the bad, and the ugly with alcohol use and abuse on the heart. Alcohol ClinExp Res 37: 1253-1260.
- Law BA, Levick SP, Carver WE (2012) Alterations in cardiac structure and function in a murine model of chronic alcohol consumption. MicroscMicroanal 18: 453-461.
- DeCarli LM, Lieber CS (1967) Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr 91: 331-336.
- Standiford TJ, Danforth JM (1997) Ethanol feeding inhibits proinflammatory cytokine expression from murine alveolar macrophages ex vivo. Alcohol ClinExp Res 21: 1212-1217.
- Carvalho EM, Brito GA, Pessoa BB, Ribeiro RA, Capaz FR (2005) Long-term ethanol intoxication reduces inflammatory responses in rats. Braz J Med Biol Res 38: 81-89.
- Jeong HJ, Hong SH, Park RK, An NH, Kim HM (2005) Ethanol induces the production of cytokines via the Ca2+, MAP kinase, HIF-1alpha, and NF-kappaB pathway. Life Sci 77: 2179-2192.
- Schwartz LB, Bradford TR, Irani AM, Deblois G, Craig SS (1987) The major enzymes of human mast cell secretory granules. Am Rev Respir Dis 135: 1186-1189.
- Brecher AS, Dubord R (2008) Effect of acetaldehyde upon cathepsin G and chymase. NRAS implications. Dig Dis Sci 53: 1311-1315.
- Nurmi K, Methuen T, Mäki T, Lindstedt KA, Kovanen PT, et al. (2009) Ethanol induces apoptosis in human mast cells. Life Sci 85: 678-684.
- Snyder AK, Singh SP, Ehmann S (1992) Effects of ethanol on DNA, RNA, and protein synthesis in rat astrocyte cultures. Alcohol ClinExp Res 16: 295-300.
- Burkhardt U, Wojcik B, Zimmermann M, Klein J2 (2014) Phospholipase D is a target for inhibition of astroglial proliferation by ethanol. Neuropharmacology 79: 1-9.
- Ranzer MJ, Chen L, DiPietro LA (2011) Fibroblast function and wound breaking strength is impaired by acute ethanol intoxication. Alcohol ClinExp Res 35: 83-90.
- Jaurena MB, Carri NG, Battiato NL, Rovasio RA (2011) Trophic and proliferative perturbations of in vivo/in vitro cephalic neural crest cells after ethanol exposure are prevented by neurotrophin 3. NeurotoxicolTeratol 33: 422-430.
- McClain JA, Hayes DM, Morris SA, Nixon K (2011) Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. J Comp Neurol 519: 2697-2710.
- Tuoi Do TH, Gaboriau F, Ropert M, Moirand R, Cannie I, et al. (2011) Ethanol effect on cell proliferation in the human hepatomaHepaRG cell line: relationship with iron metabolism. Alcohol ClinExp Res 35: 408-419.
- Fujita Y, Hiroyama M, Sanbe A, Yamauchi J, Murase S, et al. (2008) ETOH inhibits embryonic neural stem/precursor cell proliferation via PLD signaling. BiochemBiophys Res Commun 370: 169-173.
- Krishnamoorthy M, Gerwe BA, Scharer CD, Sahasranaman V, Eilertson CD, et al. (2013) Ethanol alters proliferation and differentiation of normal and chromosomally abnormal human embryonic stem cell-derived neurospheres. Birth Defects Res B DevReprodToxicol 98: 283-295.
- Katz GG, Shear NH, Malkiewicz IM, Valentino K, Neuman MG (2001) Signaling for ethanol-induced apoptosis and repair in vitro. ClinBiochem 34: 219-227.
- Debelak-Kragtorp KA, Armant DR, Smith SM (2003) Ethanol-induced cephalic apoptosis requires phospholipase C-dependent intracellular calcium signaling. Alcohol ClinExp Res 27: 515-523.
- Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, et al. (2008) Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol ClinExp Res 32: 657-668.
- Zhang H, Zhu Z, Meadows GG (2011) Chronic alcohol consumption decreases the percentage and number of NK cells in the peripheral lymph nodes and exacerbates B16BL6 melanoma metastasis into the draining lymph nodes. Cell Immunol 266: 172-179.
- Parlet CP, Schlueter AJ (2013) Mechanisms by which chronic ethanol feeding impairs the migratory capacity of cutaneous dendritic cells. Alcohol ClinExp Res 37: 2098-2107.
- Smith KJ, Butler TR, Prendergast MA (2013) Ethanol impairs microtubule formation via interactions at a microtubule associated protein-sensitive site. Alcohol 47: 539-543.
- Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A (2010) Alcohol stimulates activation of Snail, epidermal growth factor receptor signaling, and biomarkers of epithelial-mesenchymal transition in colon and breast cancer cells. Alcohol ClinExp Res 34: 19-31.
- Huang CS, Ho CT, Tu SH, Pan MH, Chuang CH, et al. (2013) Long-term ethanol exposure-induced hepatocellular carcinoma cell migration and invasion through lysyl oxidase activation are attenuated by combined treatment with pterostilbene and curcumin analogues. J Agric Food Chem 61: 4326-4335.
Citation: Law B, Fix C, Barton B, Carver W (2015) A Role for Mast Cells in Alcohol-Induced Tissue Damage and Remodeling. J Clin Exp Pathol 5:218. DOI: 10.4172/2161-0681.1000218
Copyright: © 2015 Law B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 21460
- [From(publication date): 4-2015 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 16713
- PDF downloads: 4747