Research Article Open Access
A Study on Germination Rate, Dry Matter Weight and Amylase Activity of Medicago sativa L. (alfalfa) under Induced NaCl Stress
| Nadiya A Al-Saady1*, Akhtar J Khan2 and Lakshmi R2 | ||
| 1The Research Council, P.O. Box 1422, Muscat, Oman | ||
| 2Department of Crop Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University, P.O. Box-34, Al-Khod 123, Oman | ||
| Corresponding Author : | Nadiya A Al-Saady The Research Council P.O. Box 1422, Muscat, Oman E-mail: nadiya.alsaady@trc.gov.om |
|
| Received April 15, 2013; Accepted August 24, 2013; Published August 27, 2013 | ||
| Citation: Al-Saady NA, Khan AJ, Lakshmi R (2013) A Study on Germination Rate, Dry Matter Weight and Amylase Activity of Medicago sativa L. (Alfalfa) under Induced NaCl Stress. Adv Crop Sci Tech 1:108. doi:10.4172/2329-8863.1000108 | ||
| Copyright: © 2013 Al-Saady NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Different Omani alfalfa cultivars were exposed to NaCl concentration in the range of 4, 6, 8 and 10 dSm-1 to measure germination, vegetative growth and amylase activity. The results showed moderate tolerance of alfalfa against salt stress. Alfalfa genotypes A215 and A29 appeared to be more tolerant than all other 8 genotypes. Dry matter stress index (DSMI) were calculated which varied from 0.2 to 1.2 with a high index value for 8 dSm-1 than the other NaCl dilutions. The amylase activity was found to be directly proportional to the salt stress. Thus the results showed that alfalfa is moderately tolerant to salinity especially A29 and A215 genotypes, which can be recommended for cultivation in semi arid regions.
| Keywords | |
| Salinity; Alfalfa; Dry matter index; Germination; Amylase. | |
| Abbreviations | |
| NaCl: Sodium Chloride; Fw: Fresh weight; Dw: Dry weight; Tw: Turgor weight; RWC: Relative water content; DM: Dry matter; DMSI: Dry matter stress index | |
| Introduction | |
| Salinity is known to be major environmental factor that contributes to limited plant growth and productivity [1]. For decades, salinity stress biology and plant responses to high salinity have been discussed [2-6]. Salt stress is known to affect all major process such as photosynthesis, protein synthesis and lipid metabolism. This results from an array of biochemical reactions that are going on in plant cells exposed to stress which includes (i) change in photosynthetic pathway, (ii) selective accumulation or exclusion of ions, (iii) control of ion uptake by roots and transport into leaves, (iv) compartmentalization of ions at the cellular and whole-plant levels,(v) synthesis of compatible solutes, (vi) induction of plant hormones,(vii)alteration in membrane structure and (viii) induction of antioxidative enzymes [7]. | |
| Salinity appears to affect plant processes likeionic and water relations. In the initial exposure to salinity, plants experience water stress, leading to the reduction in leaf expansion. With long-term exposure to salinity, plants experience ionic stress, which can lead to reduction in the photosynthesis [8] and its components such as chlorophylls, enzymes, and carotenoids. Changes in these parameters depend on the severity and duration of stress and on plant species, which in turn affects the overall plant mass [9]. | |
| Seed germination, one of the most important phases in the life cycle of plant, is highly responsive to the existing environment [10]. The study of salt tolerance during seed germination in early and late growth stages of plants is important for determining saline limits at each developmental phase [11]. Botia et al. [12] have reported delayed seed germination in melons. Similar results were reported by Ghoulam and Fares [13]. Younis et al. [14] mentioned that low moisture content under salt stress reduces metabolic sequences of germination. Thus, during germination, the intake of toxic ions increases which alter certain enzymatic or hormonal activities of seeds [15]. Studies shown that saline stress limited hydrolysis of food reserves from storage tissues as well as it impaired their translocation from storage tissues to developing embryo axes [16,17]. | |
| Alfalfa (Medicago sativa L.), also known as lucerne is a perennial, clover-like, leguminous plant of the pea family (Fabaceae). Alfalfa is known for its tolerance of heat, cold, and drought. It is widely grown primarily for pasturage, hay, and silage. The study area, Oman has diverse local cultivars as well as land races of many crop plants in which alfalfa is one among them [18]. Among the 83 local germplasm accessions in alfalfa we selected 10 commonly cultivated accessions to investigate the physiological basis of salt tolerance in alfalfa (Medicago sativa L.), to evaluate the dry mater index and to detect the variation in quantity of stored food as amylase in germinating embryos and cotyledons during salt stress this is a major problem in Sultanate of Oman. | |
| Materials and Methods | |
| Plant material and growth conditions | |
| Seeds of 10 commonly cultivated germplasm accessions of alfalfa were selected for this study, which was obtained from the Ministry of Agriculture and Fisheries (MAF) Al-Rumais, Sultanate of Oman. Seeds having uniform size and similar weight were selected, rinsed thoroughly and incubated in distilled water at room temperature. 50 seeds of each accession were germinated on petri dish lined with sterilized Whatman #2 filter paper. Five replicates of each set were maintained for the experiment. | |
| Salinity stress treatment | |
| The seeds were germinated in 90 mm Petri dish lined with 2 layers of Whatman #2 (Whatman, UK) filter paper moistened with different concentration of NaCl (4, 6, 8 and 10 dSm-1) and distilled water as control. The filter paper was moistened periodically with NaCl dilutions and distilled water in control plates. The Petri dishes were kept in the growth chamber for 8 days with day/night temperature of 27°C/23°C. The number of germinated seeds was counted on alternate days starting with day two from the beginning of the test. | |
| After eight days total number of germinated seeds were recorded and the Promptness Index (PI) and the Germination Stress Index (GSI) was calculated according to the formula described previously [19]. | |
| PI=nd2 (1.00)+nd4 (0.75)+nd6 (0.50)+nd8 (0.25), | |
| where nd2, nd4, nd6, nd8 represent percentages of seeds germinated after 2, 4, 6 and 8 days after sowing, respectively. | |
| GSI (%) = (PIS/PINS)×100 | |
| Where, PIS is PI under stress and PINS is PI under normal condition | |
| Determination of Amylase content in sprouted seeds | |
| Amylase content was determined according to the procedure of Caraway [20].Germinated seeds were homogenized in 20ml phosphate benzoate buffer using chilled mortar and pestle. The homogenate was then filtered and centrifuged at 12,000 rpm for 30 min at 4°C.The reaction mixture consisted of 1ml of enzymatic extract and 5ml of phosphate benzoate buffer of pH7.0 which were incubated at 37°C in a boiling water bath for 30 min. A volume of 1ml iodine solution was added to each test tube after incubation and the absorbance reading were made at 660nm against 1% starch solution as blank. Amylase activity was expressed as milligram digested starch per minute per milligram protein. | |
| The quantity of protein in enzyme extract used for amylase assay was measured using the method of Lowry et al. [21]. The quantification was made at 660nm against bovine serum albumin (BSA) as blank in a Lambda EZ 150 UV/Vis spectrophotometer (Perkin Elmer, USA). | |
| Calculation of RWC and DMSI | |
| For calculating the Relative Water Content (RWC) and Dry Matter Stress Index (DMSI) in alfalfa, seeds were germinated in Petri dishes lined with filter paper moistened with distilled water. The germinated seedlings were transferred to sterile plastic pots (16cm diameter) contains sterile sand. Before applying salinity treatment, one week of pre-conditioning period were given to the seedling. During this period the seedlings were supplies with Hoagland’s solution. On alternate weeks for a period of 6 weeks, the salinity treatments were imposed on the seedlings. Hoagland’s solution was also supplied during this treatment. | |
| Plants were uprooted for calculating RWC and DSMI. | |
| The RWC, stated by Slatyer [22], express as percentage of water content at a given time and tissue related to the water content at full turgor, which was calculated using the equation: | |
| RWC %=(FW-DW)/ (TW-DW)×100 | |
| After taking the fresh weight (FW in g), plants were immersed in tap water for 4 hours for making the cells turgid. Then turgor weights (TW in g) of the plants were recorded. Dry weight (DW in g) was recorded after placing the plants in oven at 70°C for 6 hours. Dry mass stress index (DMSI) was calculated using the formula: | |
| DMSI=(DM of stresses seedlings/DM of non-stressed seedlings)×100. | |
| Results and Discussion | |
| Effect of salinity on alfalfa seed germination | |
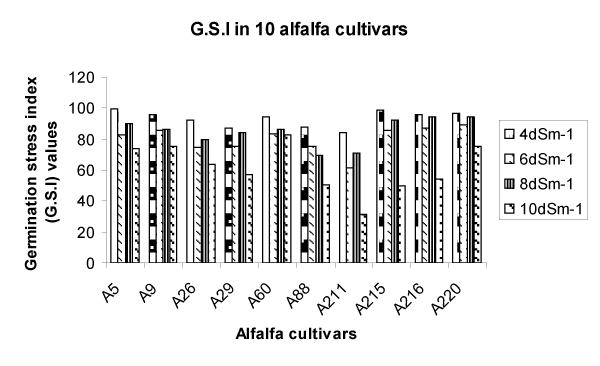
| The results revealed that there occurred not a strong effect of NaCl on alfalfa seeds. Increased salt concentration decreased the germination rate but not in all salt concentrations. Among all the 5 dilution of NaCl, 6 dSm-1 showed very good germination rate, which was greater than in 4 dSm-1. Germination at 10 dSm-1 showed least germination but then again it was above 50%. The data in presented in Figure 1 showed the increase in germination rate in 6 dSm-1that was nearly equal to that of control (A 215, A 216, A 220). | |
| The results partially agree with that in Plantago where salt treatment did not affect Plantago crassifolia L. seed germination, at all salt concentration [23].The seeds abilityto germinate at increased levels of salinity was reported to be partly dependent on the test temperature [24]. However, our results differed from those of Musa [25] who found out that salinity showed deleterious effect on growth and nitrogen fixation in alfalfa in both pot and field experiments. | |
| A study in Oman to evaluate the effect of salinity and temperature on the germination of alfalfa found that the percentage and rate of germination were limited at salinity levels above 12.2 dSm-1 [26].Thus we can conclude that the cultivars, which we selected from Oman are highly tolerant to NaCl stress which can with stand salinity level up to 10 dS-1 m. | |
| Effect of salinity on amylase content | |
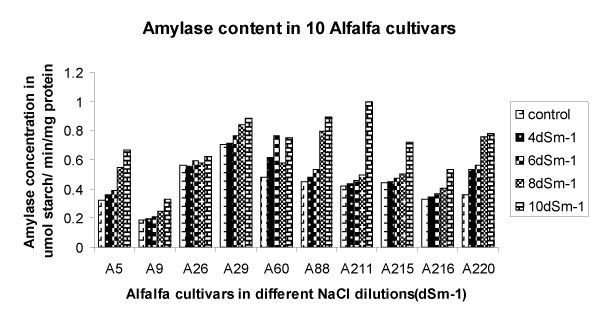
| The amount of amylase enzyme detected in germinated alfalfa seeds increased with the increase in NaCl even at higher concentration (Figure 2). Amylase activity increased particularly in 10 dSm-1 of NaCl. The results are in disagreement with that in sugar where starch content decreased with increased NaCl [27]. | |
| In enzymatic action, the presence of specific metallic ions can inhibit or enhance amylase activity, and therefore the rate of digestion. Mayzaud [28] also reported inactivation of amylase caused by Mg ions and enhancement in amylase activity in the presence of Cu ions. In our results, amylase activity was affected positively by high salt concentration. This may due to the presence of Na ions. Thus the increase in starch in alfalfa can be considered as an adaptive response to NaCl in stressed condition, which again proves that alfalfa plants in Oman can with stand high salt concentration in adverse environment. | |
| Effect of salinity on dry matter weight of alfalfa seedlings | |
| The seedlings drymass (DM) decreased with the increasing salinity levels. But plants grown in 6 dSm-1 and 8 dSm-1 showed greater DM and RWC that in 10 dSm-1. At 4, 6, 8 and10 dSm-1 NaCl levels mean values of the RWC (percentage to the control) were 6.1, 3.51, 7.26 and 1.9, respectively (Table 1 and 2). Cultivar differences were pronounced in this character. At high level of salinity (10 dSm-1) dry matter reduction in all the cultivars were serious. For a better appreciation of the salt effect on growth, we calculated the relative water content percentage (RWC), which showed a decrease from 8.84% to 1.9% in correspondence to the dilutions. | |
| Acknowledgements | |
| This work was supported by HM Fund research grant # SR/AGR/CROP/07/01. | |
| References | |
References
|
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 15236
- [From(publication date):
September-2013 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 10545
- PDF downloads : 4691
