Research Article Open Access
Activation of Neutrophils by the Extracellular Polymeric Substance of S.Epidermidis Biofilms is Mediated by The Bacterial Heat Shock Protein Groel
Susanne Maurer1, Philippe Fouchard1, Eva Meyle1, Birgit Prior1, Gertrud M Hänsch1 and Ulrike Dapunt2*1Institute of Immunology, University of Heidelberg, 69120 Heidelberg, Germany
2Clinic for Orthopaedics and Trauma Surgery, Heidelberg University Hospital, Schlierbacher Landstr, Heidelberg, Germany
- Corresponding Author:
- Ulrike Dapunt
Clinic for Orthopaedics and Trauma Surgery
Heidelberg University Hospital, Schlierbacher Landstr. 200a
69118 Heidelberg, Germany
Tel: 0049/ 6221 56-35561
Fax: 0049/6221 56-5536
E-mail: Ulrike.Dapunt@med.uni-heidelberg.de
Received date: February 25, 2016; Accepted date: April 11, 2015; Published date: April 23, 2015
Citation: Maurer S, Fouchard P, Meyle E, Prior B, Hänsch GM, et al. (2015) Activation of Neutrophils by the Extracellular Polymeric Substance of S. Epidermidis Biofilms is Mediated by The Bacterial Heat Shock Protein Groel. J Biotechnol Biomater 5:176. doi:10.4172/2155-952X.1000176
Copyright: © 2015 Maurer S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
In patients with implant-associated osteomyelitis, formation of bacterial biofilms on osteosynthesis materials or endoprosthetic devices is considered the “common cause of persistent infection”. As we showed previously for Staphylococcus aureus or S. epidermidis implant infection, activation of the local host response with infiltration of phagocytic cells and release of bactericidal and potentially cytotoxic entities, as well as production of proinflammatory and osteolysis-inducing cytokines contributes greatly to the persistence of the infection and the ensuing tissue damage with bone degradation. In this study we addressed the question, how phagocytic cells, particularly neutrophils, recognise bacterial biofilms. We found that the protein fraction of the biofilm extracellular substance (EPS) activated neutrophils: up-regulation of defence-relevant functions, among others increased surface expression of the adhesion proteins CD11b and CD66b was seen, as was production of oxygen-radicals. Subsequently, we identified the bacterial heat-shock protein GroEL as a likely candidate: GroEL is present in the EPS; depletion of GroEL from the EPS reduced neutrophil activation, culture of neutrophils with recombinant GroEL up-regulated CD11b and CD66b surface expression, and induced oxygen radical production. According to the literature GroEL and its human homologue heat shock protein (HSP)60 may bind to different surface receptors, including toll-like receptor (TLR)4 and scavenger receptors. Under our experimental conditions, the TLR4 pathway appeared to be crucial for the EPS-induced up-regulation of CD11b and CD66b, but not for induction of oxygen-radical production; suggesting involvement of additional receptors. In conclusion, we identified within the bacterial biofilm the bacterial heat-shock protein GroEL as an activator of the local innate immune response.
Keywords
GroEL; Biofilms; Neutrophil activation; Extracellular polymeric substance; Staphylococcus epidermidis
Introduction
Host-defence against bacteria is well studied and there is abundant literature on the activation and the activity of neutrophils as “first line defence” [1,2]. In most studies, free-swimming, so-called “planktonic” bacteria were used. The seminal work of Costerton and co-workers, however, revealed that this is not the only, and may not even be the preferred life-style of bacteria [3]. Rather, bacteria form communities, the so-called bacterial biofilms. Biofilm formation contributes to the pathogenicity of bacterial infection, especially by opportunists, and is considered as the “common cause of persistent infection [3-5].
A characteristic feature of biofilms is the production of an extracellular matrix, also known as Extracellular Polymeric Substance (EPS), a hydrated polymer, which is apparent as ”slime” or “film”. EPS serves as a scaffold for the three-dimensional structure of the biofilm; it protects the bacteria against environmental stress, facilitates horizontal gene transfers, and sequesters nutrients from the surroundings [6]. In addition, EPS might protect bacteria also against predators – protozoan grazers – or in the case of infection, against cells of the host defence [7,8].
The EPS varies widely among bacteria species, but also within one species; different environmental or culture conditions affect the biofilm. The major constituents – aside from water – are complex carbohydrates, proteins, and DNA. In staphylococci specifically, poly N-acetyl (1-6) β-glucosamine (a.k.a. PIA or PNAG) has been identified a crucial component, as has extracellular DNA [9-11]. Moreover, proteins have been identified in the EPS, some differentially expressed in biofilms compared to the planktonic counterpart [12-14].
In our previous work, we analysed the host response to staphylococcus biofilms. The prototype for biofilm-infection, the implant-associated osteomyelitis, was found to be associated with a profound local host response, characterised by infiltration of neutrophils, monocytes and T lymphocytes, and by the generation of proinflammatory cytokines [15-18]. Moreover, the pro-inflammatory environment induced the generation of bone-degrading osteoclasts [19].
In vitro studies with biofilms from staphylococci species revealed activation of neutrophils by the biofilm, particularly with regard to phagocytosis [20,21]. Of note, activation occurred in the absence of opsonising antibodies, quite in contrast to phagocytosis of planktonic bacteria, which requires opsonisation with IgG [22]. Apparently, neutrophils recognise constituents of the extracellular polymeric substance (EPS), a notion that was supported by data by others, describing various effects of the bacteria-surrounding “slime” [23,24]. There is the notion that these substances interfere with the host response, thus rendering the bacteria insensitive to host defence mechanisms [25]. However, in a previous study, we demonstrated that neutrophils were activated by EPS extracted from Staphylococcus epidermidis biofilms, seen as release of lactoferrin and up-regulation of the activation-associated adherence proteins CD11b/CD18 and of CD66 [26]. These data suggested the presence of activating entities within the EPS. The aim of the present study was to identify activating entities in the EPS of S. epidermidis. We focussed on S. epidermidis because it is a major pathogen in implant-associated osteomyelitis and because it is known to induce massive neutrophil activation. Furthermore, we chose a strain that was known to form biofilms [27] [26]. We found that the EPS contained the bacterial heat shock protein GroEL; moreover, we found evidence that GroEL modulated neutrophil functions in a Tolllike receptor (TLR)4-dependent manner.
Materials and Methods
Generation of S. epidermidis biofilms and extraction of EPS S. epidermidis (strain RP64A; purchased from ATCC, No.35984, Manassas, VA, USA) was added to 1.5 L of pre-warmed Trypticase Soy Broth (TSB) to reach a final concentration of 3 x106 CFU/mL, then transferred to 30 polysterol dishes (Nunc 150 x 20, Thermo Fisher Scientific, Roskilde, Denmark) with a final volume of 50 mL per dish. After incubation for 2 days at 37°C without shaking, the medium was removed and the remaining biofilm was scrapped off. The following treatment was adapted from Liu and Fang [28]: per 10 mL of slime, 60 μL of 37 % formaldehyde was added and mixed for 1h at 4°C, followed by the addition of 4 mL 1 M NaCl and mixing for another 3 hours at 4°C. The resulting suspension was then centrifuged (Sorvall 5B Plus) for 15 min (18000 rpm at 4°C). The pellet was discarded, the supernatant filtrated (Millex Syringe-driven Filter Unit 0.22 um, Merck Milipore Ltd, Tullagreen, Ireland) and then dialysed overnight against Milipore water at 4°C (membrane cut off 3600 Da; Spectrum Labs, Rancho Dominguez, CA, USA). The water was replaced and the isolated EPS was again dialysed for another 3 hours, then concentrated using Vivaspin 20 (Sartorius Stedim Biotech, Göttingen, Germany) to a final volume of 4 mL and frozen at -20°C until use.
Limulus assay and adsorption of lipopolysaccharides (LPS)
The assay was performed using a Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, Bonn, Germany) following the instructions provided by the manufacturer. The adsorption of LPS was accomplished using Pierce High Capacity Endotoxin Removal Spin Column following the instructions provided but adjusting the incubation time to 2 hours in order to maximize LPSremoval.
Digestion of EPS with trypsin and precipitation with trichloroacetic acid (TCA)
Trypsin (40 μL of 2.5% w/v, Gibco, Life technologies, Carlsbad, California, USA) was added to 100 μL isolated EPS and incubated for 1 hour at 37°C. To stop the digestion, 300 μL aprotinin (5-10 TIU /mL; from bovine lung, Sigma-Aldrich (Taufkirchen, Germany) were added. The result of trypsin digestion was assessed by SDS-PAGE (see below). For protein precipitation, 30% TCA was added to 8 mL isolated EPS to reach a final concentration of 5% TCA. The mixture was incubated on ice for 10 min, followed by centrifugation for 15 min (15000 rpm at 4°C). The pellet was resuspended in water, and dialysed against phosphate-buffered saline.
Separation of EPS extracts by gelfiltration on Sephadex-G-75
For size exclusion chromatography a glass column (Besta Technik, Wilhelmsfeld, Germany; 90 cm with 0.9 cm internal diameter) was filled with Sephadex 75 (Sigma-Aldrich), equilibrated with TRIS-buffer (100 mM TRIS; pH=7.5, 50 mM KCl). Of the extracted EPS, 2.5 mL were applied; the flow-rate was set to 2.5 mL/min and fractions of 2.5 mL volume were collected. Exclusion volume and size distribution were estimated using a Gel Filtration Molecular Weight Markers Kit (Sigma- Aldrich).
Detection of GroEL by Laser scan microscopy
Biofilms were prepared as described above on glass cover slips (Labtech Chamber slides, Nunc) and fixated with paraformaldehyde (4%, 15 min, 37°C). Bacteria were stained with SytoBC green (Molecular Probes, Eugene, Or, USA)(1:10000 in phosphate buffered saline (PBS), 1 h, room temperature), and GroEL was visualised using anti-GroEL (Enzo Life Science Loerrach, Germany)(2 μg in PBS, supplemented with bovine serum albumin (BSA), 2 %, over night at 4°C) followed by anti-mouse IgG-Cy3 (Dianova, Hamburg, Germany; 1:400 in PBS/2 % BSA,1 h, room temperature). After mounting in Mowiol (Sigma), images were viewed by laser scan microscopy (Nikon). Images were taken at 40x.
SDS-PAGE and Western blotting
A Lämmli system was used with 9 or 12 % bisacrylamid-gels. Of EPS extracts, 25 μL were mixed with 5 μL 5x loading buffer and incubated at 95°C for 10 min. Then 25 μL of the sample were applied. After separation, the samples were blotted to a PVDF membrane (Millipore, Eschborn, Germany). Anti-GroEL was added overnight, as secondary antibody peroxidase-labelled anti-mouse IgG (Jackson Immuno Research, Pennsylvania, USA) was used. The reaction was visualized with ECL Prime Western Blot Detection Reagent (GE Healthcare, Buckinghamshire, UK).
Isolation of neutrophils
Peripheral blood from healthy human volunteers was obtained and neutrophils were isolated by centrifugation on PolymorphPrep™ (Axis-Shield PoC AS, Oslo, Norway) which yielded an 85-95% pure population. Neutrophils were then suspended in Hanks balanced salt solution (HBSS) with 0.5% bovine serum albumin (Sigma-Aldrich). The local ethic committee approved the use of peripheral blood from healthy individuals for the study (S-163/2007/2014); informed consent was obtained from the volunteers, and the institutional guidelines were observed.
Functional assay with neutrophils
Up-regulation of CD11b and CD66b: To heparinized blood 5-10% EPS (v/v), EPS fractions or recombinant GroEL (5μg) was given, followed by incubation for 30 min. CD11b and CD66b expression was measured by cytofluorometry. Alternatively, isolated neutrophils (106 neutrophils/100 μL) were used. Directly labelled antibody to CD11b (FITC) or CD66b (FITC) (both obtained from Beckman Coulter, Krefeld, Germany) were used and for comparison isotypic mouse IgG –FITC (BD Bioscience Pharmingen, San Jose, CA, USA). The samples were analysed using FACSCalibur (Becton Dickinson,Heidelberg, Germany) and Cell Quest Pro software (Version 4.0.2, Becton Dickinson). For the inhibition assays, isolated PMN were incubated with antibodies to either TLR-2 (Biozol,Eching,Germany) or TLR-4 (AbD Serotec,Puchheim,Germany) or mouse IgG. One to two μg of the antibody were added to the PMN 10 min prior to stimulation with EPS. As TLR-inhibitor Pepinh-MYD inhibitory peptide (InvivoGen,Toulouse France) was used in a final concentration of 25μM.
Generation of oxygen radicals
Two methods were used. (1) Isolated neutrophils (2x106/mL) were suspended in 400 μL cytochrome C (1 mg/mL, Sigma-Aldrich) and co-cultivated with EPS (5 % v/v), phorbol 12-myristate 13-acetate (PMA 1 μg/ml, Sigma-Aldrich), lipopolysaccharide (LPS, Salmonella enterica serotype minnesota 1μg/ ml, equivalent to 5 endotoxin units, Sigma-Aldrich) or recombinant GroEL (Enzo Life Sciences). Following incubation for 30 min at 37°C, the cells were removed by centrifugation and reduced cytochrome C was measured at 550 nm. The values are given as difference in extinction (ΔE) between unstimulated cells and stimulated cells; (2) Whole blood was used and the “Phagoburst” test reagent kit from Glyoctope Biotechnology (Heidelberg, Germany) performed following the instructions provided.
Labelling of EPS and binding to neutrophils
EPS was labelled with biotin using EZ-Link Sulfo-NHS-Biotin (Thermo Scientific) following the protocol provided, and the samples were dialysed (membrane cut-off 3600 Dalton, Spectrum Labs, Rancho Dominguez, CA, USA) against phosphate buffered saline (PBS) overnight. Uptake of labelled EPS was determined by cytofluorometry following incubation of isolated neutrophils with EPS for 30 min at 4ºC. Bound EPS-biotin was detected by streptavidin-FITC (BD Bioscience Pharmingen, San Jose, CA, USA).
Depletion of GroEL from EPS
The antibody to GroEL and for comparison IgG were coupled covalently to Protein A-Agarose beads according to the protocol supplied by the manufacturer (Cell Signalling, Danvers, Massachusetts). EPS was incubated over night with the beads, then the supernatant was harvested and used for cell stimulation. The residual beads were washed extensively with PBS, then eluted with 1 M glycin buffer pH 3.0 and the eluate was subjected to Western blotting with an antibody to GroEL.
Results
Extraction of EPS and activation of neutrophils
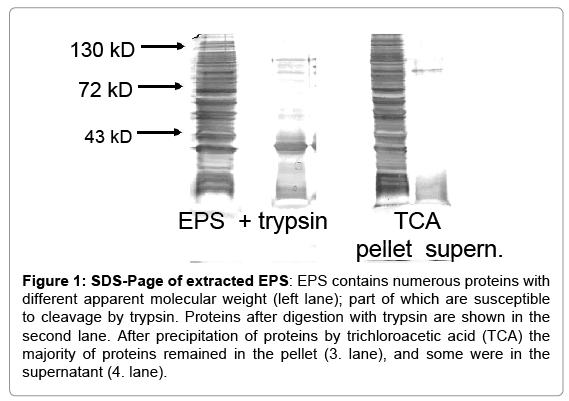
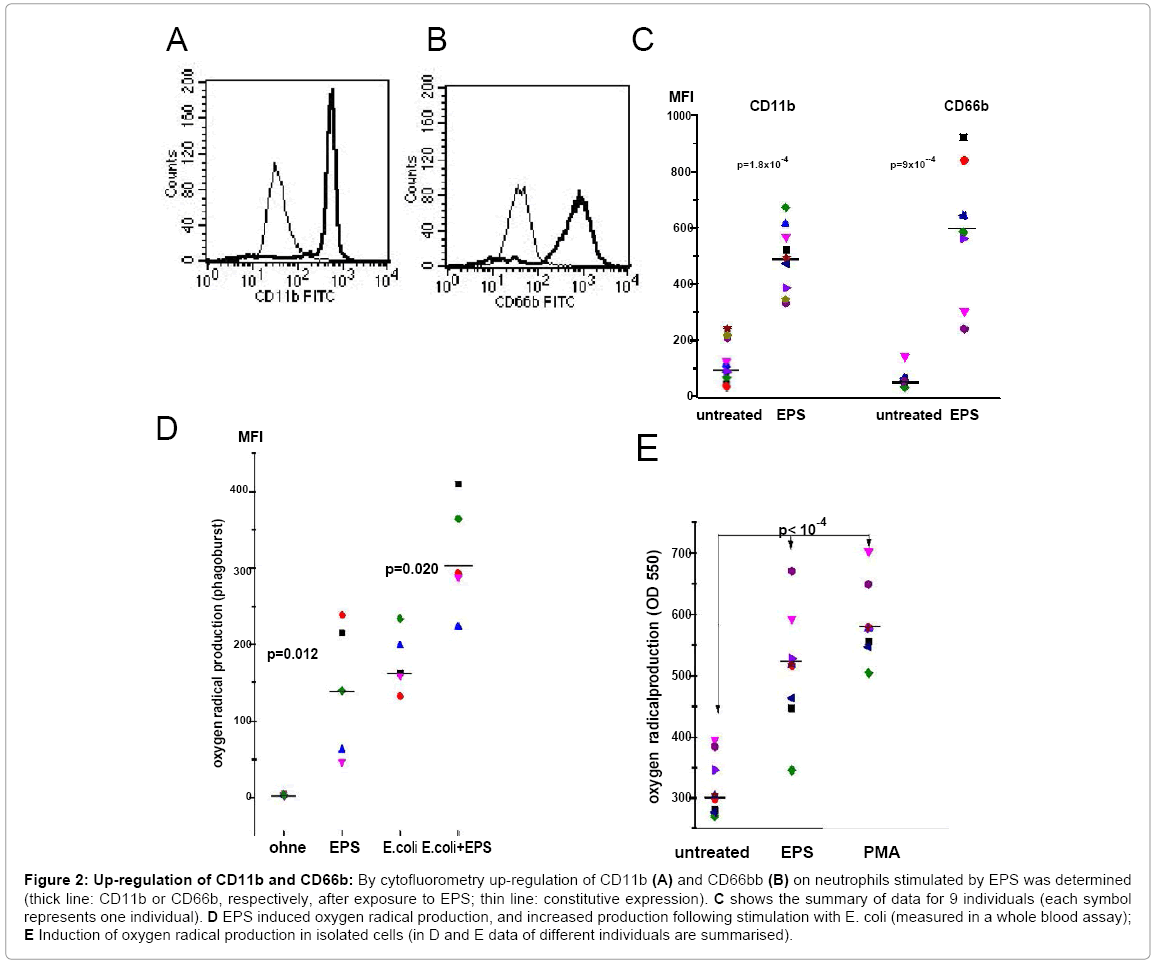
S. epidermidis were cultivated under conditions leading to biofilm formation. Extraction of EPS from 1.79 square meters biofilm yielded on average 44.9 mg of protein. SDS PAGE revealed numerous bands with molecular weights ranging from 10 to 150 kD (Figure 1).Before testing functional activity; extracts were adsorbed to an endotoxin removal spin column to exclude effects of contaminating LPS. When added to whole blood, EPS up-regulated expression of CD11b and CD66b on PMN, and enhanced the reactive oxygen production occurring after phagocytosis of E. coli. Essentially a similar up-regulation was seen with isolated neutrophils (Figures 2A-2E). The response of PMN to EPS varied among individuals, but in all, an up-regulation of was seen. Of note, in concentrations of up to 1 μg, neither LPS nor lipoteichoic acid (LTA) induced up-regulation of CD11b or CD66b, or induced oxygen radical production (data not shown).
Figure 1: SDS-Page of extracted EPS: EPS contains numerous proteins with different apparent molecular weight (left lane); part of which are susceptible to cleavage by trypsin. Proteins after digestion with trypsin are shown in the second lane. After precipitation of proteins by trichloroacetic acid (TCA) the majority of proteins remained in the pellet (3. lane), and some were in the supernatant (4. lane).
Figure 2: Up-regulation of CD11b and CD66b: By cytofluorometry up-regulation of CD11b (A) and CD66bb (B) on neutrophils stimulated by EPS was determined (thick line: CD11b or CD66b, respectively, after exposure to EPS; thin line: constitutive expression). C shows the summary of data for 9 individuals (each symbol represents one individual). D EPS induced oxygen radical production, and increased production following stimulation with E. coli (measured in a whole blood assay); E Induction of oxygen radical production in isolated cells (in D and E data of different individuals are summarised).
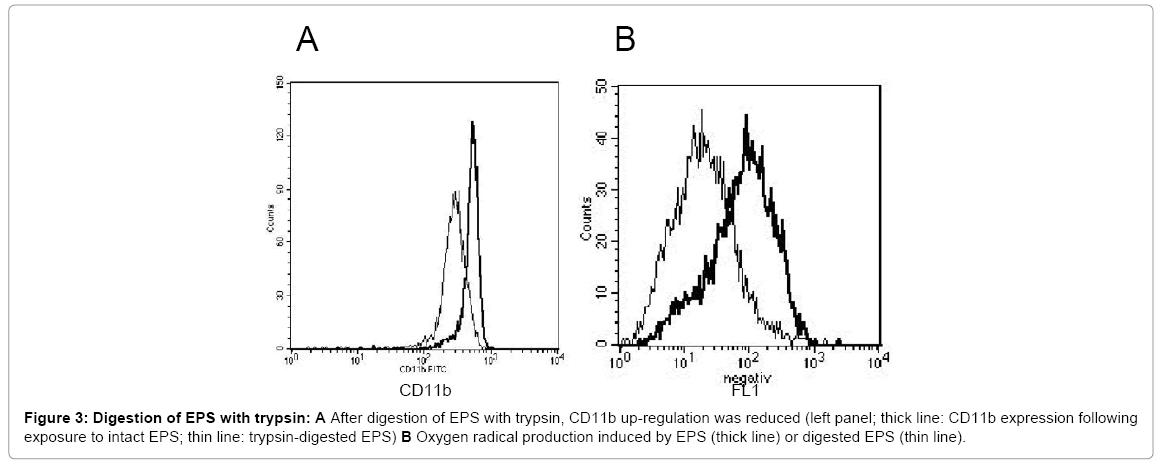
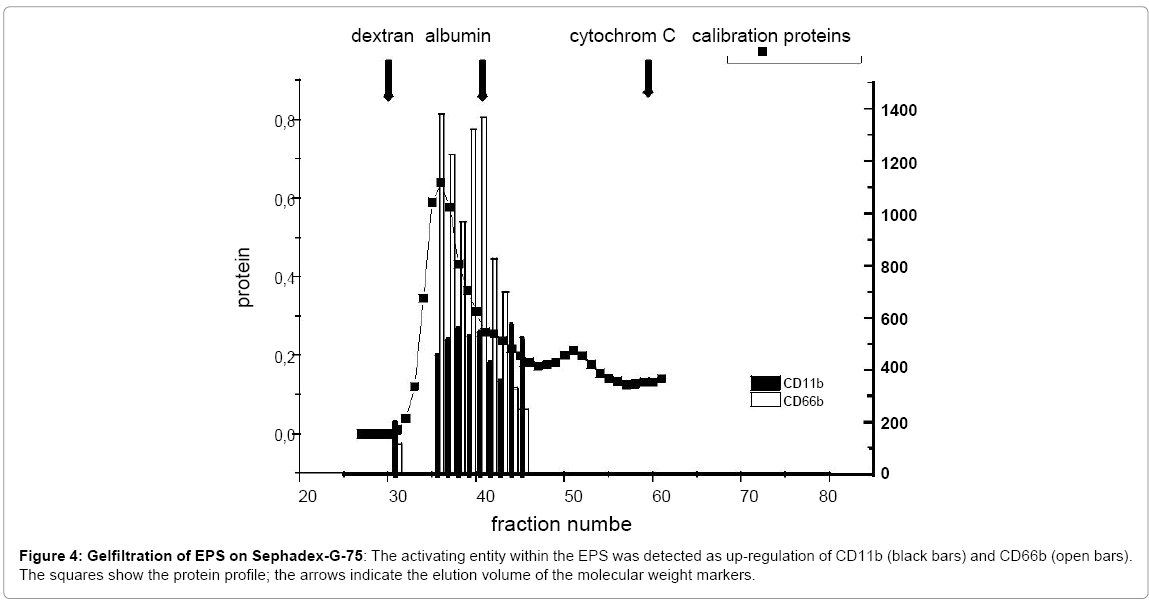
Digestion of the EPS with trypsin resulted in a reduction of high molecular weight proteins, as seen in SDS-PAGE (Figure 1); concomitantly, the capacity to up-regulate CD11b expression and to induce oxygen radical production was reduced (42 % and 71.1 %,respectively; mean of three experiments) (Figure 3). Following precipitation of proteins by TCA, the activating entity was found chiefly in the resuspended pellet, indicating that protein(s) within the EPS activated neutrophils. Gelfiltration revealed the presence of the activating entity/ies in the area of 60 kDa to 80 kDa (Figure 4).
Figure 3: Digestion of EPS with trypsin: A After digestion of EPS with trypsin, CD11b up-regulation was reduced (left panel; thick line: CD11b expression following exposure to intact EPS; thin line: trypsin-digested EPS) B Oxygen radical production induced by EPS (thick line) or digested EPS (thin line).
Antibodies to TLR4 modulate the EPS-mediated stimulation of neutrophils
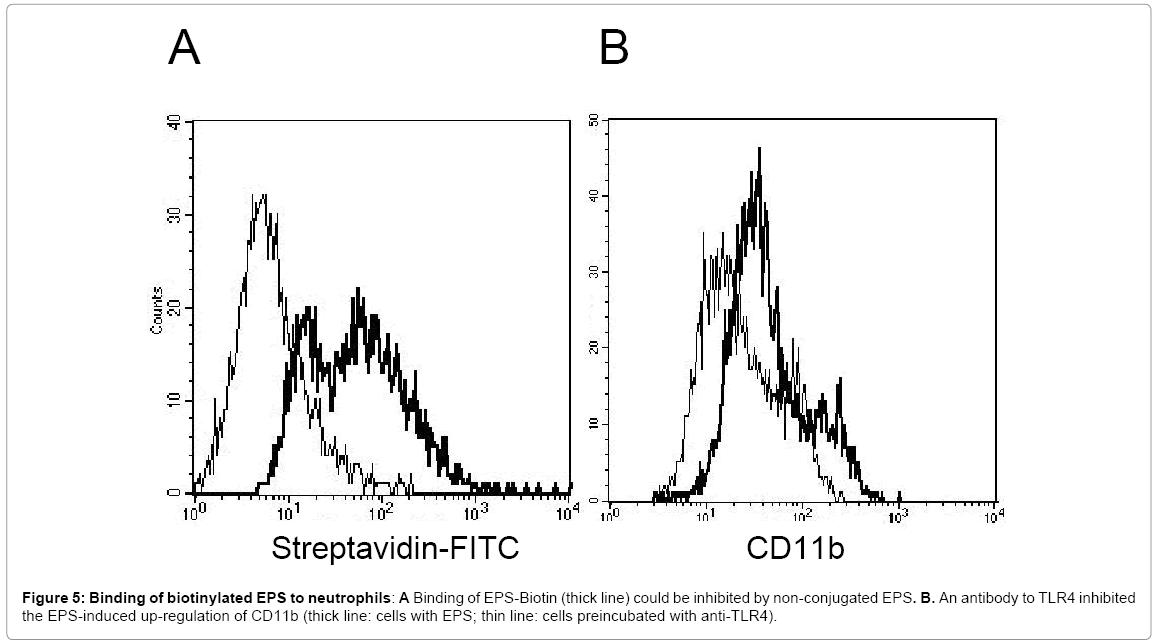
When EPS was labelled with biotin, uptake by neutrophils was seen, which could be inhibited by unlabelled EPS (Figure 5A), suggesting the presence of a surface receptor. To gain information on the putative receptor involved, the effect of antibodies directed to a variety of PMN surface receptors possibly mediating the EPS-induced activation was tested. Among those, an antibody to TLR4 inhibited the EPS-induced up-regulation of CD11b (Figure 5B)(Table 1). Up-regulation of CD66b was also reduced (35% when 1μg of the antibody was used, and 55% with 2μg of the antibody). In line with these data, the TLR-2/4 inhibitor Pepinh-MYD reduced the EPS-mediated up-regulation of CD11b and CD66.
| Inhibition of CD11b up-regulation (mean of 4 experiments) (in %) | |
| anti-TLR2 | 1.8 |
| anti-TLR4 | 52.5 |
| mouse IgG | 5.5 |
| TLR inhibitor | 100 |
Table 1: Antibodies to TLR and TLR inhibit or reduce the EPS-induced up-regulation of CD11b expression.
In contrast, antibodies to either TLR2 or TLR4 did not inhibit the EPS-induced oxygen radical production.
Effect of the heat shock protein GroEL on neutrophils
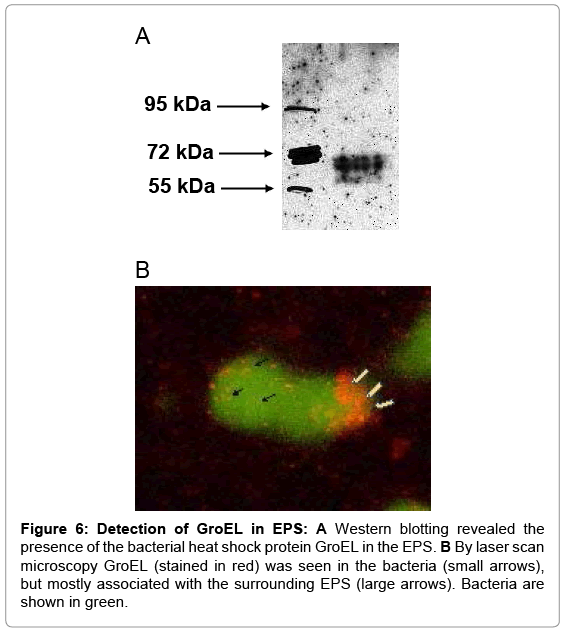
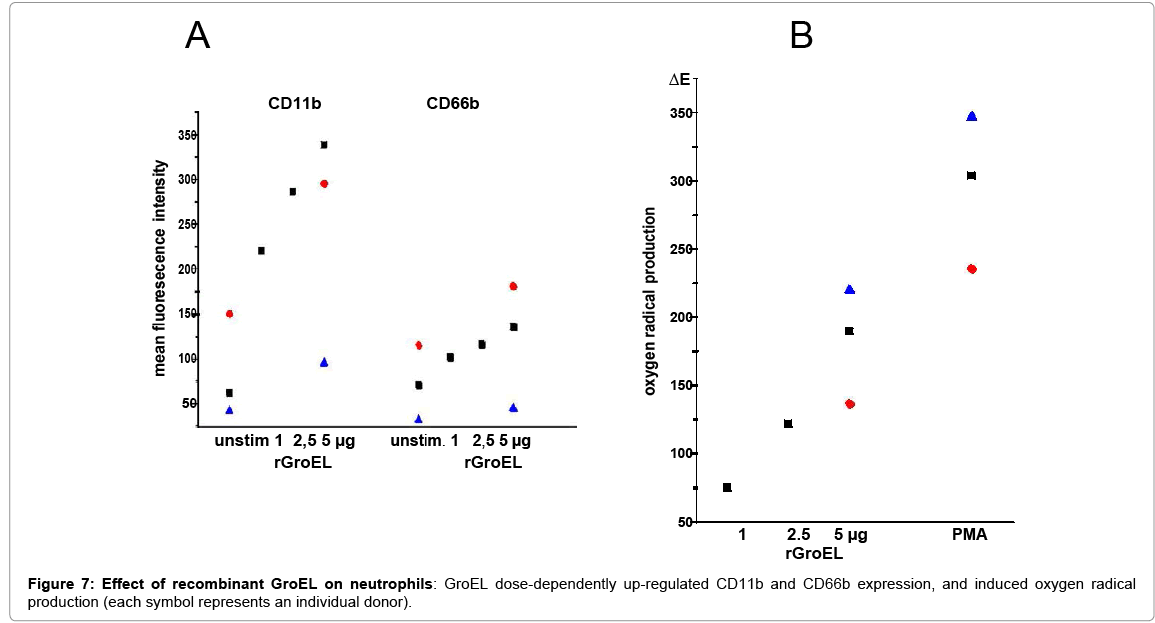
The heat shock protein HSP60 was described as a ligand for TLR4, as was its capacity to activate PMN [29,30]. Because the bacterial heat shock protein GroEL also binds to TLR4 [31], and shares sequence homologies with the human protein its presence in the EPS was tested as well as its effects on PMN. By Western blotting and laser scan microscopy GroEL was detected in the EPS (Figure 6). The latter shows GroEL in association with bacteria, but the majority appears to be deposited at the periphery (Fig.6B). Depletion of GroEL by immunoadsorption reduced the capacity of the residual EPS to induce CD11b; and commercially available recombinant protein GroEL induced both, oxygen radical production and up-regulation of CD11b and CD66b (Figure 7).
Figure 6: Detection of GroEL in EPS: A Western blotting revealed the presence of the bacterial heat shock protein GroEL in the EPS. B By laser scan microscopy GroEL (stained in red) was seen in the bacteria (small arrows), but mostly associated with the surrounding EPS (large arrows). Bacteria are shown in green.
Discussion
Bacteria-host interactions are dependent on and controlled by mutual recognition. In the course of evolution, host cells have developed a wide array of defence mechanisms, including barriers, bactericidal substances, and receptors recognising bacterial entities. Conversely, bacteria generate virulence factors to overcome host defence, among them the formation of biofilms. There are numerous observations indicating that bacteria in biofilms are protected against the host defence, and that biofilm formation is the underlying cause of persistent infections, and chronic inflammatory disease [4,32-35]. Indeed, when studying the prototype of biofilm infection, the implantassociated osteomyelitis, activation of host defence cells was seen with massive infiltration of phagocytic cells into the infected site, and the local generation of proinflammatory cytokines [18,19,36].
In vitro studies with staphylococci biofilms showed that indeed phagocytic cells, particularly neutrophils, are activated after contact with biofilms, and that phagocytosis occurs, also in the absence of opsonising antibodies or complement, the latter quite in contrast to planktonic bacteria [22]. The implication is that bacteria in biofilms are not entirely protected against the attack from neutrophils [26,27] .Moreover, the biofilm apparently contains entities that activate neutrophils. Previous studies described that extracts of S. epidermidis biofilms activated neutrophils, and prompted the present study with the aim to identify the activating entity.
As indicator for neutrophil activation, up-regulation of CD11 was determined, because it participates in adhesion, migration, and phagocytosis, and as such is crucial for the host defence. In addition, generation of reactive oxygen species was determined as a major means of bacteria killing. The latter was measured in two settings: the direct activation of isolated neutrophils or neutrophils in whole blood, and in addition the effect of phagocytosis-induced oxygen radical production. In the two settings, oxygen radical production was increased, as was surface expression of CD11b and CD66b. Digestion by trypsin resulted in loss of the activity, indicative of a protein as activating entity, an interpretation supported by the finding that the activity could be stimulated after precipitation by TCA. These finding ruled out poly N-acetyl (1-6) ß-glucosamine (PIA) as an activating substance in our setting, also LTA or LPS, the latter as a possible contaminant within the EPS preparation.
The identification of the activating entity turned out to be difficult. Although experiments with biotin-labelled EPS suggested the presence of a receptor, the classical pull-down assays were not successful. A major problem was the extensive binding of streptavidin, which we used as a ligand for the biotin-labelled EPS, to PMN-lysates. The occurrence of a multitude of bands in the SDS-PAGE precluded the detection of distinct, specific bands in a reproducible manner (data not shown). We therefore resorted to another approach: the inhibition of the EPSinduced activation by antibodies to a variety of receptors. Among those, antibodies to TLR4 inhibited to some extend EPS-induced activation, measured as up-regulation of CD11b and CD66b expression. TLR4 has multiple ligands [37]. In the context of our study it was of interest that it is also a receptor for exogenous human heat shock protein 60 (HSP60) [38,39], and its bacterial homologue GroEL [29]. Thus, it appeared to be a reasonable candidate, particularly since it is known that HSP60 also activates neutrophils [30]. We found now that GroEL is present in the EPS, that depletion of GroEL by immunoadsorption reduced the capacity of EPS to activate neutrophil, and that recombinant GroEL activated oxygen radical production and up-regulation of CD11b and CD66b as well.
GroEL is a highly conserved molecule and a common bacterial antigen [40], expressed in all bacteria as a chaperone. It participates in the bacterial stress response and in biofilm formation, and thus operates intracellular and extracellular as well [41,42]. Hence, our interpretation that GroEL functions as an activating entity for neutrophils is not limited to S. epidermidis, but might apply to a wide variety of other biofilm-forming bacteria. In that, GroEL can be regarded as a “pathogen-associated molecular pattern” and as “danger signal” for the host response. In line with this interpretation, the pattern-recognition receptor TLR4 was identified as a possible receptor for GroEL. However, as for the human homologue HSP-60 in addition to TLR4 also other receptors have been described, including CD40, CD91 and scavenger receptor [29]; and considering that antibodies to TLR4 did reduce CD11b/CD66b up-regulation, but not the EPS-induced oxygen radical production, it is feasible, that other receptors are involved. This notion could explain that the antibodies to TLR4 inhibited the EPSinduced up-regulation only in part. On the other hand, we cannot exclude other entities within the EPS, which in addition to GroEL may activate neutrophils.
Conclusion
In conclusion, our data provide evidence for neutrophil activation by one or more constituents of the extracellular polymeric substance of S. epidermidis biofilms. Moreover, the bacteria heat shock protein GroEL present in the EPS was identified as a potent activator of neutrophils. Activation of neutrophils – also in the absence of antibodies – provides a first step towards an efficient defence against biofilm infections, and challenge the view that biofilms escape recognition and clearance by phagocytic cells [8]. On the other hand, in cases of insufficient clearance - as it occurs in implant-associated osteomyelitis – activation of neutrophils could promote the local inflammatory response, resulting in tissue damage and eventually in bone degradation [18].
Acknowledgements
U.Dapunt was supported by the Olympia-Morata-Scholarship of the Faculty of Medicine of Heidelberg University
References
- Kumar V, Sharma A (2010) Neutrophils: Cinderella of innate immune system. IntImmuno pharmacol 10: 1325-1334.
- Borregaard N (2010) Neutrophils, from marrow to microbes. Immunity 33: 657-670.
- Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections.Science 284: 1318-1322.
- Hall-Stoodley L, Stoodley P (2009) Evolving concepts in biofilm infections. Cell Microbiol 11: 1034-1043.
- Parsek MR, Singh PK (2003) Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57: 677-701.
- Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the "house of biofilm cells".J Bacteriol 189: 7945-7947.
- Jensen PO, Givskov M, Bjarnsholt T, Moser C (2010) The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol 59: 292-305.
- Hänsch GM (2012) Host Defence against Bacterial Biofilms: “Mission Impossible”? ISRN Immunology 2012: 1-17.
- Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, et al. (1996) The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol 178: 175-183.
- Jabbouri S, Sadovskaya I (2010) Characteristics of the biofilm matrix and its role as a possible target for the detection and eradication of Staphylococcus epidermidis associated with medical implant infections. FEMS Immunol Med Microbiol 59: 280-291.
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295: 1487.
- Karamanos NK, Panagiotopoulou HS, Syrokou A, Frangides C, Hjerpe A, et al. (1995) Identity of macromolecules present in the extracellular slime layer of Staphylococcus epidermidis.Biochimie 77: 217-224.
- Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, et al. (2005) Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol 187: 2426-2438.
- Resch A, Rosenstein R, Nerz C, Götz F (2005) Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol 71: 2663-2676.
- Wagner C, Kondella K, Bernschneider T, Heppert V, Wentzensen A, et al. (2003) Post-traumatic osteomyelitis: analysis of inflammatory cells recruited into the site of infection.Shock 20: 503-510.
- Wagner C, Heck D, Lautenschlager K, Iking-Konert C, Heppert V, et al. (2006) T lymphocytes in implant-associated posttraumatic osteomyelitis: Identification of cytotoxic T effector cells at the site of infection. Shock 25: 241-246.
- Kotsougiani D, Pioch M, Prior B, Heppert V, Hänsch GM, et al. (2010) Activation of T Lymphocytes in Response to Persistent Bacterial Infection: Induction of CD11b and of Toll-Like Receptors on T Cells. Int J Inflam 2010: 526740.
- Gaida MM, Mayer B, Stegmaier S, Schirmacher P, Wagner C, et al. (2012) Polymorphonuclear Neutrophils in Osteomyelitis: Link to Osteoclast Generation and Bone Resorption. European Journal of Inflammation 10: 413-426.
- Dapunt U, Giese T, Lasitschka F, Lehner B, Ewerbeck V, et al. (2014) Osteoclast Generation and Cytokine Profile at Prosthetic Interfaces: A Study on Tissue of Patients with Aseptic Loosening or Implant-Associated Infections. European Journal of Inflammation 12: 147-159.
- Guenther F, Stroh P, Wagner C, Obst U, Hansch GM (2009) Phagocytosis of staphylococci biofilms by polymorphonuclear neutrophils: S. aureus and S. epidermidis differ with regard to their susceptibility towards the host defense. Int J Artif Organs 32: 565-573.
- Gunther F, Wabnitz GH, Stroh P, Prior B, Obst U, et al. (2009) Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). MolImmunol 46: 1805-1813.
- Stroh P, Gunther F, Meyle E, Prior B, Wagner C, et al. (2011) Host defence against Staphylococcus aureus biofilms by polymorphonuclear neutrophils: oxygen radical production but not phagocytosis depends on opsonisation with immunoglobulin G. Immunobiology 216: 351-357.
- Gray ED, Peters G, Verstegen M, Regelmann WE (1984) Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet 1: 365-367.
- Johnson GM, Lee DA, Regelmann WE, Gray ED, Peters G, et al. (1986) Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun 54: 13-20.
- Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, et al. (2011) Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1.Infect Immun 79: 2267-2276.
- Meyle E, Brenner-Weiss G, Obst U, Prior B, Hansch GM (2012) Immune defense against S. epidermidis biofilms: components of the extracellular polymeric substance activate distinct bactericidal mechanisms of phagocytic cells. Int J Artif Organs 35: 700-712.
- Meyle E, Stroh P, Günther F, Hoppy-Tichy T, Wagner C, et al. (2010) Destruction of bacterial biofilms by polymorphonuclear neutrophils: relative contribution of phagocytosis, DNA release, and degranulation. Int J Artif Organs 33: 608-620.
- Liu H, Fang HH (2002) Extraction of extracellular polymeric substances (EPS) of sludges. J Biotechnol 95: 249-256.
- Baranova IN, Vishnyakova TG, Bocharov AV, Leelahavanichkul A, Kurlander R, et al. (2012) Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. J Immunol 188: 1371-1380.
- Osterloh A, Geisinger F, Piédavent M, Fleischer B, Brattig N, et al. (2009) Heat shock protein 60 (HSP60) stimulates neutrophil effector functions. J Leukoc Biol 86: 423-434.
- Argueta JG, Shiota S, Yamaguchi N, Masuhiro Y, Hanazawa S (2006) Induction of PorphyromonasgingivalisGroEL signaling via binding to Toll-like receptors 2 and 4. Oral Microbiol Immunol 21: 245-251.
- Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW (2012) Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33: 5967-5982.
- Donlan RM (2000) Role of biofilms in antimicrobial resistance. ASAIO J 46: S47-52.
- Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms.Clin Microbiol Rev 15: 167-193.
- Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infectious biofilms.Trends Microbiol 13: 34-40.
- Dapunt U, Maurer S, Giese T, Gaida MM, Hansch GM (2014) The macrophage inflammatory proteins MIP1alpha (CCL3) and MIP2alpha (CXCL2) in implant-associated osteomyelitis: linking inflammation to bone degradation. Mediators Inflamm 2014: 728619.
- Kumar H, Kawai T, Akira S (2011) Pathogen recognition by the innate immune system. Int Rev Immunol 30: 16-34.
- deGraaf R, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F (2006) Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect 8: 1859-1865.
- Ohashi K, Burkart V, Flohé S, Kolb H (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164: 558-561.
- Young DB, Ivanyi J, Cox JH, Lamb JR (1987) The 65kDa antigen of mycobacteria-a common bacterial protein? Immunol Today 8: 215-219.
- Lemos JA, Luzardo Y, Burne RA (2007) Physiologic effects of forced down-regulation of dnaK and groEL expression in Streptococcus mutans. J Bacteriol 189: 1582-1588.
- Oscarsson J, Karched M, Thay B, Chen C, Asikainen S (2008) Proinflammatory effect in whole blood by free soluble bacterial components released from planktonic and biofilm cells. BMC Microbiol 8: 206.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15731
- [From(publication date):
April-2015 - Sep 03, 2025] - Breakdown by view type
- HTML page views : 11040
- PDF downloads : 4691