Research Article Open Access
Aerobic Degradation of Anthracene, Benzo(a)Anthracene and Dibenzo(a,h) Anthracene by Indigenous Strains of Aerobic Heterotrophic Bacteria and Cyanobacteria Isolates From Crude Oil Contaminated Bodo Creek
Ichor Tersagh*, Aondoakaa EM and Okpa BODepartment of Biological Sciences, University of Agriculture, Makurdi, Nigeria
- *Corresponding Author:
- Tersagh Ichor
Professor, Department of Biological
Sciences, University of Agriculture
Makurdi, Nigeria
Tel.: +234(0)7039256061
E-mail: smartichor@uam.edu.ng
Received Date: December 21, 2016; Accepted Date: December 30, 2016; Published Date: January 04, 2017
Citation: Tersagh I, Aondoakaa EM, Okpa BO (2017) Aerobic Degradation of Anthracene, Benzo(a)Anthracene and Dibenzo(a,h)Anthracene by Indigenous Strains of Aerobic Heterotrophic Bacteria and Cyanobacteria Isolates From Crude Oil Contaminated Bodo Creek. Oil Gas Res 3:125. doi: 10.4172/2472- 0518.1000125
Copyright: © 2017 Tersagh I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Oil & Gas Research
Abstract
Biodegradation of anthracene, benzo(a)anthracene and dibenzo(a,h)anthracene by indigenous strains of aerobic heterotrophic bacteria and cyanobacteria isolated from Bodo creek - a moderate salt aquatic environment polluted with crude oil was monitored for 56 days using GC-MS. Anthracene was mineralized to 0.1, 0.001 and 0.08 mg/l by aerobic heterotrophic bacteria cyanobacteria and consortium of aerobic heterotrophic bacteria and cyanobacteria respectively. Benzo(a)anthracene was reduced to 0.03, 0.01 and 0.02 mg/l in the treatments of aerobic heterotrophic bacteria cyanobacteria and consortium of aerobic heterotrophic bacteria and cyanobacteria respectively where as dibenzo(a,h)anthracene was reduced to 0 mg/l in all the treatment options on the 56th day monitored. Fluctuations in the quantity of PAHs monitored and was observed at some weeks since it did not follow a straight and consistent reduction pattern but did not vary significantly with time when compared with the control at p>0.05 since the monitored PAHs were degraded in both the treatment options and the control.
Keywords
Biodegradation; Anthracene; Aerobic; Heterotrophic bacteria; Cyanobacteria
Introduction
Polycyclic aromatic hydrocarbons are ubiquitous in nature and persist in the environment as a result of their low solubility in water due to their hydrophobicity [1]. They are highly toxic, mutagenic, carcinogenic and genotoxic and also exhibit toxicity to algae, fish and can bio accumulate along the food chain [2]. Anthracene is a tricyclic poly aromatic hydrocarbon which is characterized by a three fused benzene ring. It is used in detecting contamination of PAHs and as a model polycyclic aromatic hydrocarbon for determination of the factors that affect bioavailability, rate of microbial degradation and biodegradation potential of PAHs in the environment [3]. It is among the substances of very high concern by the European chemical agency due to its toxicity [4]. Its utilization by the consortium of the bacteria was reported in four days Arulazhan et al. [5] and has been removed from the environment by E. coli, Mycobacterium Pseudomonas, Rhodococcus, Sphingomonas and Nocardiodes species as reported in previous research findings [6-9]. Mathew and Amund [10,11] reported 91.7% and 51% anthrance degradation by Microcccus luteus and Pseudomonas cetronellolis from crude oil polluted environment respectively. Oladimeji et al. [12] reported degradation of 95.2 percent of anthracene by Corynebacterium sp and 93.5 percent by Pseudomonas putida which is more when compared to degradation by Pseudomonas aeruginosa (312A) at the rate 3.90 mg L-1 day-1 of seventy one percent of anthracene degradation with the addition of the 250 mg L-1 of medium after 48 days as reported by Jacques. Mathew and Amund [10] isolated Micrococcus luteus from a crude oil contaminated environment which degraded about 91.7% of anthracene and Pseudomonas citronellolis (222A) which degraded about fifty one (51%) percent of anthracene as reported by [9,11] observed degradation of 74.8% anthracene which was supplemented in BSM medium at 0.1% with Acridine orange induced plasmid curing by Pseudomonas sp though comparatively less than the degradation done by Corynebacterium sp and Pseudomonas putida. Benzo(a)anthracene a crystalline polycyclic aromatic hydrocarbon has chemical formula C18H12 and consist of four fused benzene rings. It is odourless, colourless to yellow brown flake, plate or powder. Dibenzo(a,h)anthracene is a mutagen, carcinogenic aromatic hydrocarbon which is crystalline in nature and consists of five fused benzene rings and is produced by incomplete combustion of organic matter. It is also found in coal tar, soot, gasoline exhaust, tobacco smoke and certain food products such as smoked foods and barbecued foods. The substance is used for research to induce tumorigenesis. Juhasz et al. [13] reported the degradation of benz(a)anthracene and dibenzene(a,h)anthracene by Burkholderia cepacia as sole carbon and energy source though limited growth was observed on dibenz(a,h) anthracene. The present study aimed at monitoring biodegradation of anthracene, benzo(a)anthracene and dibenzo(a,h)anthracene by indigenous strains of aerobic heterotrophic bacteria and cyanobacteria isolates from petroleum hydrocarbon contaminated Bodo creek characterized bybrackish water – a moderate salt aquatic environment.
Materials and Methods
The study area was Bodo creek; characterized by brackish water which is heavily contaminated with crude oil and is located in Ogoni land in Gokana, LGA of Rivers State, in Niger Delta region.
Media conditions and enumeration of cyanobacteria and aerobic heterotrophic bacteria
BG-11 medium for isolation of cyanobacteria was composed as described by Ichor [14]. Enumeration of cyanobacteria was carried according to the method adopted by Ichor. The aliquot of the cyanobacteria culture comprising of different BG 11 medium – water volume ratio was subjected to 12 hr of sunlight and 12 hr of darkness for 14 days. The mineral salt agar as described in Mills et al. 1978 was used for enumeration of aerobic heterotrophic bacteria. The vapour phase transfer method was adopted for enumeration of hydrocarbon utilizing bacteria, following serial dilutions of water as described by Mills and Ichor [14]. Biodegradation Experiment using Cyanobacteria and Aerobic Heterotrophic Bacteria, a loopful of 24 hours culture aerobic heterotrophic bacteria was transferred into 400 ml starling nutrient broth in 500 Elenmeyer flask this was supplemented with nystatin and CUSO4 to prevent growth of fungi and cynobacteria respectively. It was incubated at 37°C for 24 hours. Treatment options of brackish water and sediment samples labelled A and B are prepared ascetically transferring 200 ml each of the aerobic heterotrophic bacteria and cyanobacteria suspension into the 500 ml sterile distilled water in two separate 1000 ml flask and was calibrated using mac farland standard 0.5 exactly 100 ml of inoculums’ size was used for each Treatment the experimental setup were each filled with 11 liters of water in different water jars and were labelled A, B, C+D the control experiment was labelled H and it was contaminated with 32300 ppm of sterile bony light crude oil sample obtained from shell petroleum company heterotrophic bacteria, cynabacteria and aerobic heterotrophic + cynobacteria were inoculated in water samples A, B, C+D respectively. Control H was not inoculated with water sample, sample B was treated with 0.25 mg/ml of ciprofloxacin and NY statin sample A was treated CuSO4 and nystatin to inhibit the activities of cynobacteria and fungi in the experimental setup. Samples C+D were treated with 0.25 mg/m of nytatin only, whereas control setup which was labelled H was left utreated prior to the experimental setup. Containers for the treatments were thoroughly washed with detergents and rinsed repeatedly with distilled water.
Molecular analysis of bacterial and cyanobacteria isolates
The isolates were subjected to DNA extraction, polymerase chain reaction and gene sequencing as adopted by Ichor. Universal primers CYA 106F (CGC ACG GGT GAG TAA CGC GTG A and CYA 359F(GGG GAA TYT TCC GCA ATG GG) with a 40 nucleotide GC clamp (51 CGC CCG CCG CGC CCC GCG CCG GTC CCG CCG CCC CCG CCC G 31) on the 51 end forward primer and CYA 781R (equimolar mixture of CYA781Ra (GAC TAC TGG GGT ATC TAA TCC CAT T) and CYA 781Rb (GAC TAC AGG GGT ATC TAA TCC CTT T) reverse primers for amplification of a segment of cyanobacterial 16SrRNA gene (70) were synthesized. A semi nested PCR reaction was carried out with the first reaction using primers CYA 106F and CYA 781R and followed by a reaction with primers CYA 359F and CYA781R. The DNA of aerobic heterotrophic bacteria were extracted directly from water samples and filterates from sediment samples and polymerase chain reaction was performed using universal primers used for bacteria; Eub 27F (51-31.AGA GTT TGA TCC TGG CTC AG) forward primer and Eub 1492R (51-31.ACG GCT ACC TTG TTA CGA CTT) for reverse primers. Purification of the PCR sequence products was done using 2 M sodium acetate wash technique before sequencing. PCR was carried out in a 25 μl final volume of reaction mixture containing 100 ng of DNA 2.5 μl of 10x PCR buffer with 200 μg bovine serum albumin (nuclease free) and 0.2 u Taq DNA polymerase (Banngalore Genei, India) in a 1 cycler (BioRad, USA). The thermal cycling profile was thus; initial denaturation for 3 mins at 94°C followed by 35 amplification cycles each consisting of 1.5 min denaturation at 94°C, 1 min annealing at 59°C and a 2 min elongation at 72°C with a final 5 min elongation at 72°C [14]. Sequences generated were compared with known sequences in the Gen Bank using the basic local alignment search tool (BLAST) of the National Centre for Biotechnology Information (NCBI) and the species of aerobic heterotrophic bacteria and cyanobacteria were identified based on the percentage similarity with known sequences in the data base [14].
Results
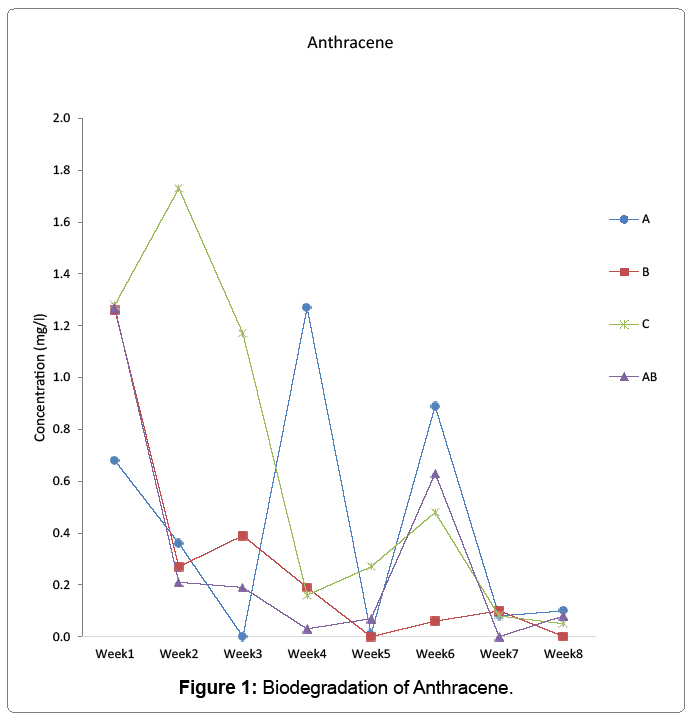
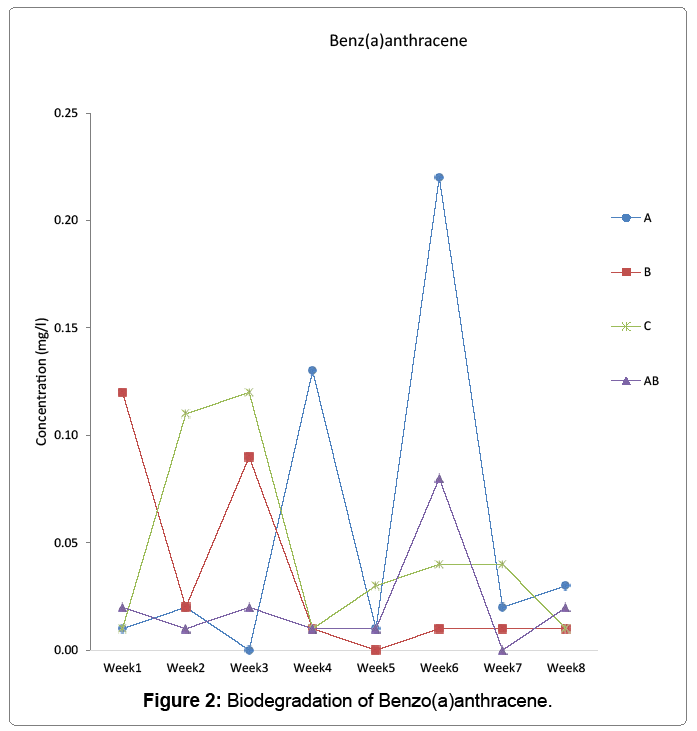
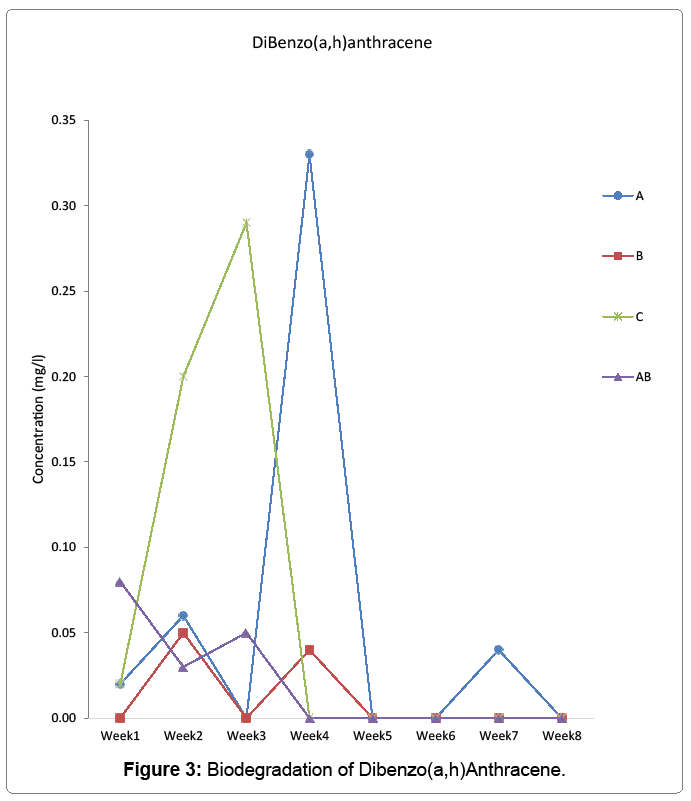
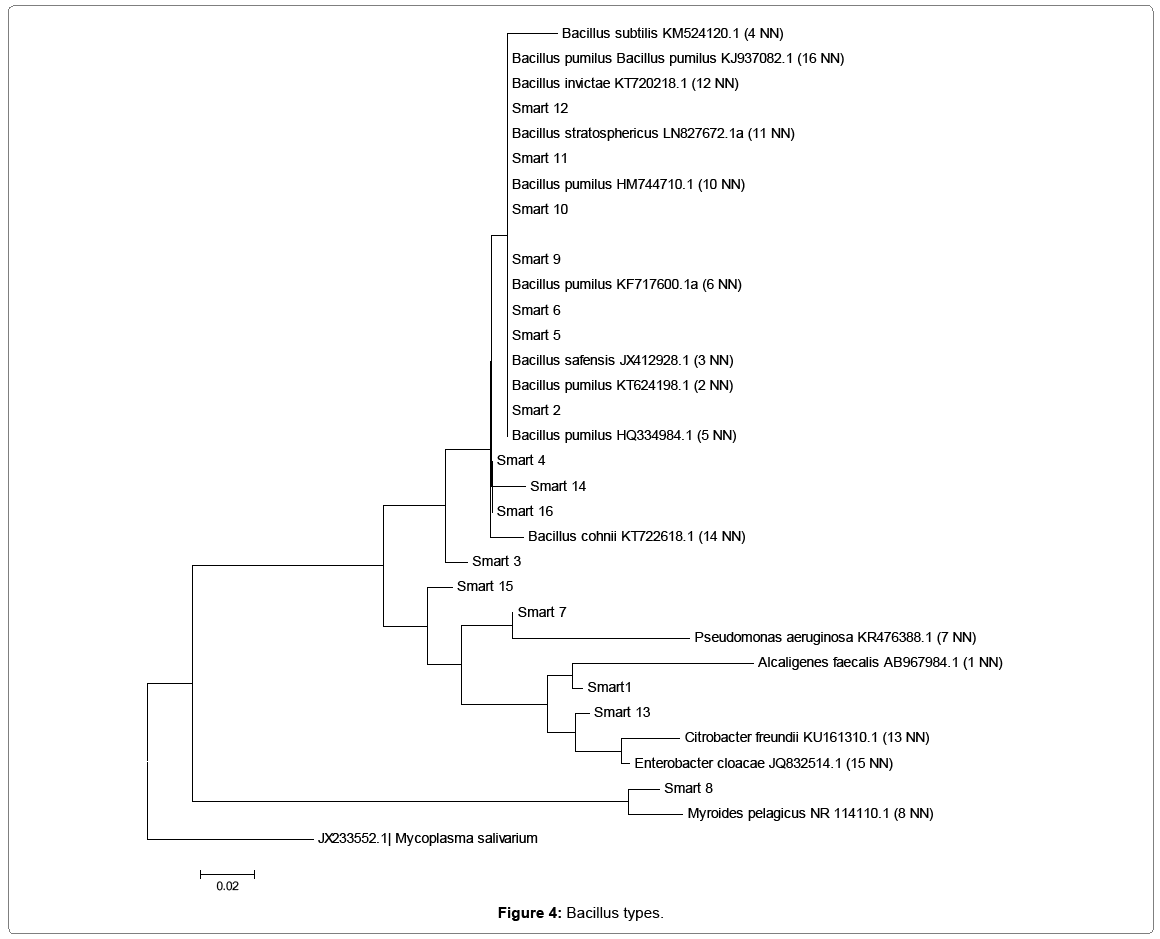
Biodegradation of anthracene, benzo(a)anthracene, and dibenzo(a,h) anthracene by aerobic heterotrophic bacteria, cyanobacteria and their consortium was undertaken and monitored using agilent 7890 model of GC-MS for 56 days. Anthracene reduced to 0.68 mg/l in week one and to 0 in week 3 for aerobic heterotrophic bacteria treatment. It resurfaced in week 4 and fluctuated throughout the period to 0.1 mg/l on the last day monitored. Cyanobacteria degraded anthracene from week 1 to 1.26 mg/l and further reduced to 0.27 in week 2 but rose to 0.39 in week 3 but reduced to 0 in week 5 and rose to 0.001 mg/l on the last day monitored. The consortium of aerobic heterotrophic bacteria and cyanobacteria reduced anthracene to 1.27 mg/l in week one and maintained the pattern of reduction to 0.07 mg/l in week 5. It increased further to 0.63 mg/l in week 6, reduced to 0 in week 7 and reappeared in week 8 to 0.08 mg/l. In the control, it was reduced to 1.28 mg/l in week1 but increased to 1.73 mg/l and reduced and fluctuated throughout the period to 0.05 mg/l on the last day it was monitored Figure 1. Benzo(a)anthracene was reduced by aerobic heterotrophic bacteria to 0.01 mg/l in week 1 and by week 3 reduced to 0. It increased to 0.13 mg/l in week 4 but reduced to 0.01 and increased to 0.22 mg/l by week 5 and 6 respectively, but further reduced to 0.02 mg/l on week 7 but increased to 0.03 mg/l on the last day monitored. Cyanobacteria reduced benzo(a)anthracene was reduced to 0.12 mg/l in week 1 and the degradation remained at 0.01 mg/l from week 4 up to the last day it was monitored. In the consortium, benzo(a)anthracene reduced to 0.02 mg/l in week 1 and fluctuated throughout the period to 0.02 mg/l on the last day monitored. The control however had 0.01 mg/l which increased to 0.12 on week 3 but fluctuated to 0.01 on the last day monitored Figure 2. Dibenzo(a,h)anthracene was degraded by aerobic heterotrophic bacteria to 0.02 mg/l in week 1 and fluctuated throughout the period to 0 on the last day monitored. The treatment option with cyanobacteria however, it observed that it had the value of 0 but reappeared as 0.05 mg/l in week 2, 0 in week 3 and 0.04 by week 4 which was degraded to 0 by week 5 and throughout to the last day monitored. The consortium of aerobic heterotrophic bacteria and cyanobacteria degraded it to 0.08 on week 1, by week 4 it reduced to 0 and maintained it up to the last day it was monitored. The control option had 0.02 mg/l in week 1 which increased through to 0.29 mg/l in week 3 but reduced and remained at 0 in week 4 through to the last day monitored Figure 3. The degradation of anthrancene, benzo(a) anthracene and dibenzo(a,h)anthracene did not vary significantly with time at p>0.05 in all the treatments monitored. Figure 4 shows the phylogenetic showing the relationship of the aerobic heterotrophic bacteria obtained from brackish waters of Bodo creek. The evolutionary history was inferred using the Neighbour-Joining method [15]. The optimal tree is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Jukes-Cantor method [16]. Evolutionary analyses were conducted in MEGA6 [17].
Discussion
The resident aerobic heterotrophic bacteria and cyanobacteria isolated from Bodo creek degraded anthracene, benz(a)anthracene and dibenz(a,h)anthracene. The capability of aerobic heterotrophic bacteria and cyanobacteria from Bodo creek to degrade poly aromatic hydrocarbons has been reported previously. Ichor, et al. [18-20] reported on biodegradation of phenanthrene, PAH’s, fluoranthene, benzo(b)fluoranthene and benzo(k)fluoranthene by aerobic heterotrophic bacteria and cyanobacteria resident in Bodo creek. The cyanobacteria isolates are as reported by Ichor and the consortium of aerobic heterotrophic bacteria and cyanobacteria evolutionary history inferred as contained in Ichor [19]. Earlier research findings implicated Mycobacterium sp strain PYR-1 in degradation of 92% anthracene [21- 23]. Alkaliphilic bacteria and Bacillus badius strain D1 Ahmed [24] was reported to have degraded anthracene variously. Mycobacterium sp strain S1 grew on commercial anthracene [25]. Species of the genera Spingobium, Rhodococcus, Pseudomonas, Mycobacterium and Norcadia reportedly degraded anthracene [26]. Previous results also indicated that Alcaligenes faecalis MVMB1 effectively degraded anthracene [27]. Most of the HMW PAH-degrading bacteria; Actinomycetes, a variety of non-actinomycete bacteria have also been reported to metabolize benz[a]anthracene, fluoranthene, chrysene and pyrene. Three Burkholderia cepacia strains isolated from soil were observed to have grow on pyrene at concentrations of up to 1,000 mg/ liter and also degraded fluoranthene and benz[a]anthracene as a sole source of carbon and energy [27]. Pseudomonas (Sphingomonas) paucimobilis, EPA 505 reportedly biotransformed benz(a)anthracene. Mueller et al. [28] reported mineralization benz[a]anthracene by bacteria as its sole source of carbon and energy and formed 14CO2. A soil bacteria, Alcaligenes denitrificans strain WW1 also degraded benz[a]anthracene. Mycobacterium strain RJGII-135 cometabolized benz[a]anthracene and formed three dihydrodiols [29]. S. paucimobilis was said to have mineralised dibenz(a,h)anthracene to 33.3, 12.5 and 7.8% 14CO2 after 16 hrs of incubation period [30]. Dibenz(a,h)and Bap were reportedly co- metabolised by B. cepacia strains and decreased at 41 and 52% in dibenz and Bap in 56 days. B. cepacia strain VUN 10003 degraded 1.65 mg of dibenz(a,h)anthracene per litres as a sole carbon oil energy source after 56 days [13]. The result of study which spanned for 56 days of monitoring degradation of anthracene, benzo(a)anthracene and dibenzo(a,h)anthracene in a moderate salt aquatic environment – Bodo creek revealed a fluctuation in quantity of PAH’s studied throughout the period monitored with significant reduction on the last day sampled in all the treatment without nutrient amendment [31-33]. This trend of degradation in natural brackish water was previously reported during degradation of Phenanthrene, poly aromatic hydrocarbons, fluoranthene, benzo(b)fluoranthene and benzo(k)fluoranthene [18-20].
Conclusion
Based on the results of the present study, crude oil contaminated bodo reek has interest microbial capacity for the biodegradation of PAH as an alternative to the physical and chemical methods. It indicates the aerobic heterotrophic bacteria and cyanobacteria singly and as a consortium effectively degraded anthracene, benzo(a) anthracene and dibenzo(a,h)anthracene without nutrient amendment and bio stimulation of the studied organisms implicated in the process. Strategies for enhancing the metabolic capacity of the microbes, bioavailability of polyaromatic hydrocarbons and understanding the metabolic pathway of the degradation are highly advocated.
References
- Vila J, López Z, Sabate J, Mingullón C, Solanas AM, et al. (2001) Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp strain AP1: actions of the isolate on two- and three-ring polycyclic aromatic hydrocarbons. Appl Environ Microbiol 67: 5497-5505.
- Sutherland JB (1992) Detoxification of polycyclic aromatic hydrocarbons by fungi. J Ind Microbiol 9: 53-62.
- Kanaly RA, Harayama S (2000) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. Journal of Bacteriology 182: 2059-2067.
- Iglesias GS, Manchado A, Rebolo R, Gonzalez JI, Garcia DA, et al. (2010) A search for interstellar anthracene toward the Perseus anomalous microwave emission region Monthly Notices of the Royal Astronomical Society 407: 2157-2165.
- Arulazhagan P, Vasudevan N, Yeom N (2010) Biodegradation of polycyclic aromatic hydrocarbon by a halo tolerant bacterial consortium isolated from marine environment. International Journal of Environmental sciences and Technology 7: 639-652.
- Mrozik A, Piotrowska SZ, Labuzek S (2003) Review: Bacterial Degradation of Polycyclic aromatic Hydrocarbons Polish. Journal of Environmental Studies 12: 15-25.
- Van HR Springael D, Slot P, Govers HAJ, Parsons JR (2003) Degradation of Anthracene by Mycobacterium sp Strain LB501T Proceeds via a Novel Pathway through o-Phthalic Acid. Applied and Environmental Microbiology 69: 186-190.
- Saito A, Iwabuchi T, Harayama S (2000) A novel phenanthrene dioxygenase from Nocardioides sp strain KP7: Expression in Escherichia coli. Journal of Bacteriology 182: 2134-2141.
- Kumar G, Singha R, Kumar R (2010) Plasmid Associated Anthracene Degradation by Pseudomonas sp Isolated from Filling Station Site. Journal of Nature and Science 8: 89-94.
- Matthew ONI, Amund DI (2000) Degradation of Anthracene by Bacteria Isolated from Oil Polluted Tropical Soils Z Naturforsch 55: 890-897.
- Jacques RJS, Santos EC, Bento FM, Peralba MC, Selbach RPA, et al. (2005) Anthracene biodegradation by Pseudomonas sp Isolated from a petrochemical sludge land farming site. International Journal of Bio deterioration & Biodegradation 56: 143-150.
- Oladimeji1 AT, Owabor C, Ngozi, Nwacha Richard (2012) Kinetics of degradation of anthracene by the activity of corynebacteria sp and pseudomonas putida in contaminated water. International Journal of chemical sciences and applications 3: 314-322.
- Juhasz AL, Britz ML, Stanley GA (1997) Degradation of fluoranthene pyrene benz(a)anthracene and dibenz(ah)anthracene by Burkholderia cepacia. J Appl Microbiol 83: 189-198.
- Ichor T, Okerentugba PO, Okpokwasili GC (2014) Biodegradation of total petroleum hydrocarbon by aerobic heterotrophic bacteria isolated from crude oil contaminated brackish waters of Bodo creek. Journal of Bioremediation and Biodegradation, Vol-5.
- Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees Molecular Biology and Evolution 4: 406-425.
- Jukes TH, Cantor CR (1969) Evolution of protein molecules In Munro HN editor Mammalian Protein Metabolism, pp. 21-132.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 60 Molecular Biology and Evolution30, pp. 2725-2729.
- Ichor T, Ebah EE, Azua ET (2016a) Aerobic Degradation of Fluoranthene Benzo(b)Fluoranthene and Benzo(k) Fluoranthene by Aerobic Heterotrophic Bacteria - Cyanobacteria Interaction in Brackish Water of Bodo Creek. Journal of Petroleum & Environmental Biotechnology, Vol-7.
- Ichor T, Gberikon GM, Dooshima N (2016b) Biodegradation of phenanthrene by a consortium of aerobic heterotrophic bacteria and cyanobacteria in petroleum hydrocarbon polluted brackish water of bodo creek. Microbiology Journal 6: 1-8.
- Ichor T, Gberikon GM, Terwase J (2016c) Time Dependent Influence of Aerobic Heterotrophic Bacteria - Cyanobacteria Interaction during Biodegradation of Polycyclic Aromatic Hydrocarbons. Journal of Petroleum and Environmental Biotechnology, Vol-7, p. 286.
- Moody JD (2001) Degradation of phenanthrene and anthracene by cell suspension of Mycobacterium sp strain PYR-1 Appl Environ Microbiol 67: 1476-1483.
- Leneva NA (2009) Phenanthrene and anthracene degradation by microorganisms of the genus Rhodococcus Prikl Biokhim Mikrobiol 45: 188-194.
- Kim E, Zylstra GJ, Freeman JP, Heinze TM, Deck J, et al. (1997) Evidence for the role of 2-hydroxychromene-2-carboxylateisomerase in the degradation of anthracene by Sphingomonas yanoikuyae B1 FEMS Microbiol Lett 153: 479-484.
- Ahmed T, Ahmed, Munif A, Othman, Vasudeo D, et al. (2012) Degradation of Anthracene by Alkaliphilic Bacteria Bacillus radius Environment and Pollution, VoL-1.
- Tongpim S, Pickard MA (1999) Cometabolic oxidation of phenanthrene to phenanthrene trans-9 10-dihydrodiol by Mycobacterium strain S1 growing on anthracene in the presence of phenanthrene. Can J Microbiol 45: 369-376.
- Takeuchi M, Hamana K, Hiraishi A (2001) Proposal of the genus Sphingomonas sensu stricto and three new genera Sphingobium Novosphingobium and Sphingopyxis on the basis of phylogenetic and chemotaxonomic analyses. Int J Syst Evol Microbiol 51: 1405-1417.
- Nadu T, Velan M (2011) Biodegradation of Anthracene: Influence Of selected physiochemical parameters and metabolism Green Technology and Environmental Conservation GTEC, p. 167-172.
- Mueller JG, Chapman PJ, Pritchard PH (1989) Action of a flueoranthene-utilizing bacterial community on plycyclic aromatic hydrocarbon components of creosote Applied and Environmental Microbiology 55: 3085-3090.
- Mahaffey WR, Gibson DT, Cerniglia CE (1988) Bacterial oxidation of chemical carcinogens: formation of polycyclic aromatic acids from benz[a]anthracene Appl Environ Microbiol 54: 2415-2423.
- Ye D, Siddiqi MA, Maccubbin AE, Kumar S, Sikka HC (1995) Degradation of polynuclear aromatic hydrocarbons by Sphingomonas paucimobilis. Environ Sci Technol 30: 136-142.
- Salleh AB, Ghazali MF, Zaliha NR, Basri M (2003) Bioremediation of petroleum hydrocarbon pollution. Indian Journal of Biotechnology 2: 411-425.
- Shingler V (2003) Integrated regulation in response to aromatic compounds: from signal sensing to attractive behaviour. Environ Microbiology 12: 1226-1241.
- Weissenfels WD, Beyer M, Klein J (1990) Degradation of phenanthrene fluorene and fluoranthene by pure bacterial cultures. Appl Microbiol Biotechnol 32: 479-484.
Relevant Topics
Recommended Journals
- Oil & Gas Research Journal
- Renewable Energy and Applications Journal
- Oceanography Journal
- Industrial Pollution Control Journal
- Coastal Zone Management Journal
- Climatology & Weather Forecasting Journal
- Geoinformatics & Geostatistics Journal
- Engineering and Technology Journal
- Petroleum & Environmental Biotechnology Journal
- Polymer Sciences Journal
Article Tools
Article Usage
- Total views: 4685
- [From(publication date):
March-2017 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 3469
- PDF downloads : 1216