Attrition of Iron-Induced Biochemical Injury in Mice Kidney by a Citrus Bioflavonoid, Hesperidin
Received: 02-May-2018 / Accepted Date: 23-Jul-2018 / Published Date: 31-Jul-2018 DOI: 10.4172/2168-9652.1000240
Keywords: Mice; kidney; Antioxidants; Glutathione; Catalase; lipid peroxidation; lactate dehydrogenase
Introduction
Iron is essential for the survival of all organisms. Iron is abundant in the biosphere and it is extremely important in carrying out various metabolic processes in all living organisms for their survival. Iron is primarily found in two forms: the ferrous (Fe+2) and the ferric (Fe+3) and this transition from one form to another puts it in a unique position of donating or accepting electrons depending on the situation in the cell. Iron plays a crucial role in a number of metabolic processes including nitrogen fixation, oxygen transport, electron transfer, and DNA synthesis [1]. “Free” iron generates hydroxyl radicals from superoxide and hydrogen peroxide via Fenton reaction inducing oxidative stress in the cells [2]. The hydroxyl free radicals produced due to participation of iron are highly reactive. They interact with lipids, proteins and DNA causing severe damage to these macromolecules and subsequently damage vital tissues and organs [3]. The cells have inherent capacity to protect itself from oxidative stress-induced by iron, where it is oxidized to Fe+3, which tightly combines with transferrin and is kept in a nontoxic redox-inert state [4]. Two-thirds of serum transferrin is present as apotransferrin and will quickly capture the free iron that may be released from the cell whenever it is required [5].
Iron is a vital micronutrient and it is indispensable part of several biological phenomena including mammalian cell growth, DNA and heme syntheses, cytochrome p450 enzyme activity, hypoxic response reactions, oxygen transport, and cell proliferation [6]. Iron serves as a prosthetic group in different enzymes eg. iron-sulphur and heme proteins of the respiratory chain, as well as ribonucleotide reductase. These enzymes are involved in the rate-limiting step in DNA synthesis [7,8]. Iron also acts as a common prosthetic group composed of protoporphyrin IX and a Fe2+ ion. Presence of iron is a double-edged sword, it is extremely indispensable to carry various important metabolic processes. Whereas the deficiency and overload of iron are the result of defective metabolism and are associated with several diseases [9]. Mismanagement of iron in the cells is the principal cause of underlying pathogenic events in oxidative stress that is harbinger of several diseases [10,11].
Iron pool in the body is tightly regulated due to existence of negative feedback mechanism [12]. However, the excess iron in the body is referred to as iron overload. The gene mutations are responsible for most of the iron overload in populations of northern Europe that leads in an amino acid substitution at position 282 of the HFE protein. The C282Y substitution is rare outside the white ethnicity although other mutations do exist [13]. Iron overload is rare in Asian populations and if it is, it may be due to abnormalities in non-HFE iron-related molecules [14]. Iron overload in the body is highly toxic as it may be associated with serious health conditions including aging, autoimmune nephrotic syndromes, diabetes, cataractogenesis, cardiovascular diseases, degenerative retinal damage, gastrointestinal tract disorders, heavy metal toxicity, Parkinson’s disease, ischemia reflow states, Alzheimer’s disease, bronchopulmonary dysphasia, kidney damage, macular degeneration, stroke and cancer [15- 24]. Therefore, it is imperative to reduce iron-induced oxidative stress by use of natural products. The dietary supplements may be of great value in arresting or treating iron-induced toxicity and subsequently the ironinduced disorders.
Plants synthesize a number of secondary metabolites, which are not essential for their survival but play other important roles during the life of plants. The citrus plants synthesize hesperidin as secondary metabolite, which is present in the fruit juices and rinds of these plants [25]. Hesperidin, a bioflavonoid protects plant against fungal and bacterial invasions [26]. The highest amount of hesperidin is found in sweet oranges (Citrus sinensis) and tangelos and orange juice with pulp has higher amount of hesperidin than the orange juice that is without pulp [27]. Usually one litre of orange juice contains 470-761 mg of hesperidin [28]. The ingestion of hesperidin leads to its conversion into the aglycone hesperitin in the colon by intestinal bacteria, which is degraded or absorbed [29]. Hesperidin is active against inflammation, oxidative stress, free radical generation and ulceration. It also blocks selected cytochrome p450 enzymes resulting in drug interactions [30,31]. Hesperidin increases capillary (blood vessels) permeability and is useful in absorption of vitamin C [32]. It has been indicted in hypertension in humans and it protects against haemorrhages, infections and heals ruptured capillaries and connective tissues [33]. The antiallergenic, analgesic, anti-cancerous, anti-inflammatory, antimicrobial anti-hypotensive and vasodilating activities of hesperidin have been reported in different study systems [34-38]. The daily intake of 500 mL orange juice consecutively for four weeks has been found to modulate the activation of 3422 genes in humans. However, only 1819 genes got activated when hesperidin was given in a similar fashion to the humans [39]. Hesperidin protected against oxidative stress, neurotoxicity, Herpes simplex virus infection, apoptosis, atherogenesis, inflammatory bowel disease, arthritis, platelet and erythrocyte aggregation and infection [40-50]. Hesperidin scavenged free various radicals in vitro and increased the wound healing of irradiated wounds. Oral administration of hesperidin was found to be non-toxic up to a dose of 2 g/kg body weight in mice [51,52]. Earlier sub chronic administration of 5% hesperidin for 13 weeks has been reported to be non-toxic in mice [53]. Kidney is an important organ of the body, which plays a major role in detoxification therefore, the present study was undertaken to obtain an insight into the effect of hesperidin on the iron-induced biochemical injury the mice kidney.
Materials and Methods
Chemicals
Ferric chloride, di-sodium hydrogen phosphate (Na2HPO4), hydrogen peroxide (H2O2), di-potassium hydrogen phosphate (K2HPO4), potassium di-hydrogen phosphate (KH2PO4), and carboxymethylcellulose (CMC) were procured from MERC, India Ltd., Mumbai, whereas hesperidin, 1-Chloro-2,4-Dinitrobenzene (CDNB), phenazine methosulphate, nitroblue-tetrazolium, sodium pyruvate, cumene hydroperoxide, thiobarbituric acid, 5-5’-dithiobis[2- nitrobenzoic acid, 5-thionitrobenzoic acid, mM tert-butylhydroperoxide and nicotinamide adenine dinucleotide (NADH) were supplied by the Sigma Aldrich Chemical Company, Kolkata, India
Animal care and handling
The animal care and handling were performed in accordance with the guidelines issued by the World Health Organization, Geneva, Switzerland and the INSA (Indian National Science Academy, New Delhi, India). Generally, six to eight weeks old inbred male and female Swiss albino mice (1:1) weighing 25 to 30 g were requisitioned from the colony maintained under the controlled conditions of temperature (23 ± 2°C), humidity (50 ± 5%) and 12 h of light and dark cycle, respectively. The animals were provided with standard food and water ad libitum. Five animals were housed in a polypropylene cage containing wood powder (procured locally) as bedding throughout the experiment. The Animal Ethics Committee of Mizoram University, Aizawl, India had approved the study.
Preparation of Drug and Mode of Administration
The required amount of hesperidin was dissolved in distilled water containing 1% CMC and the animals were administered with 250 mg/ kg body weight of hesperidin orally [52].
Experimental
The effect of hesperidin on iron-induced oxidative stress in the mouse kidney was studied by dividing the animals into the following groups:
Iron: The animals of this group were administered with 0, 5000, 10,000 and 20,000 ppm of ferric chloride in ordinary drinking water daily for 30 days consecutively.
Hesperidin+Iron: This group of animals was orally administered with 250 mg/body weight of hesperidin intraperitoneally for five days before administering the animals with 0, 5000, 10,000 and 20,000 ppm of ferric chloride in ordinary drinking water daily up to 30 days.
Thirty days after the administration of ferric chloride, the animals from both the groups were killed by cervical dislocation. The mice were dissected and perfused with ice cold saline transcardially. The kidney from both group of animals was removed blot dried. A total of 80 mice were used to complete this experiment.
Preparation of Homogenate
The kidney was weighed and 10 % homogenate was prepared in a phosphate buffer saline using an electrical homogenizer (REMI, Mumbai, India). Various biochemical parameters were estimated in the kidney homogenate after 30 days of iron treatment.
Total Proteins: The total proteins were estimated by the Lowry’s method with minor modification [54].
Glutathione: The glutathione (GSH) was assayed as described earlier with minor modifications [55]. In brief, the proteins were precipitated with 0.5 mL ice cold 10% 5-sulfosalicylic acid and the tubes were incubated on ice for 10 min, centrifuged (Sorvall Instruments RC5C, DuPont, Minnesota, USA) for 15 min at 15,000 rpm at 4°C. The protein free supernatant was collected and mixed immediately with 0.5 mL of NADPH (4 mg of reduced form was dissolved in 100 mL of 0.5% NaHCO3), 0.5 mL of glutathione reductase (6 units/mL in 0.1 M phosphate buffer, pH 7.0) and 1 mL of 0.6 M DTNB (prepared in 0.2 M phosphate buffer of pH 8). The formation of TNB was read against the blank in a UV-Visible double beam spectrophotometer (Shimadzu Corporation, Tokyo, Japan) at 412 nm. A sample without GSH was used as a blank. The GSH concentration has been expressed as μmol/mg protein. Standard curve was plotted using different concentrations of GSH.
Glutathione-S-Transferase: The glutathione-S-transferase (GST) activity was estimated as described by Habig and Pabst [56]. The tissue homogenate was mixed with 0.1 M potassium phosphate buffer, CDNB and 10 mM GSH, and kept in water bath for 10 min at 37°C. The absorbance was recorded at 1 min intervals against distilled water, which was used as a blank at 340 nm using a UV-VIS spectrophotometer. The GST activity has been expressed as nmol/mg of protein.
GST activity=Absorbance of sample–Absorbance of blank×1000/9.6×Vol of sample
Catalase: The catalytic reduction of hydrogen peroxide was used as a measure of catalase activity [57]. The hydrogen peroxide was mixed with tissue homogenate and incubated at 37°C. The hydrogen peroxide decomposition was monitored at 0.5 s, 10 s intervals up to 30 s and the absorbance was read against the phosphate buffer blank at 240 nm using a UV-VIS spectrophotometer. The average difference in absorbance in 30 s was calculated.
Superoxide Dismutase: The activity of superoxide dismutase (SOD) was determined as described earlier [58]. Briefly, the tissue homogenate was mixed with phenazine methosulphate, nitroblue tetrazolium and NADH. The whole mixture was incubated at 30˚C for 90 sec. The reaction was terminated by the addition of acetic acid and n-butanol. The blank was prepared in a similar fashion without the sample and the reaction was stopped by adding acetic acid and n-butanol. The sample absorbance was read against the blank at 560 nm in a UV-VIS spectrophotometer.
Lipid Peroxidation: The induction of lipid peroxidation (LOO) was measured as various thiobarbituric acid reactive substances (TBARS) including malondialdehyde, lipid hydroperoxides and aldehydes in the tissue homogenate [59]. The homogenate was heated with thiobarbituric acid (0.8%), sodium dodecyl sulphate (0.1%) and acetic acid (20%) in a boiling water bath for 30 min to precipitate the lipoproteins. The resultant mixture was cooled, extracted with n-butanol-pyridine, and the absorbance of the butanol layer was recorded at 532 nm using UVVisible double beam spectrophotometer. The resultant concentration of TBA reactive substances is expressed as nmol/mg protein obtained from a standard curve of tetraethoxypropane.
Statistical Analysis
The significance between the treatments was determined using the Student’s ‘t’ test and one-way ANOVA with Tukey’s post-hoc test. A p value of <0.05 was considered statistically significant. The Solo 4 statistical package (BMDP Statistical Software Inc, Los Angeles, CA, USA) was used for statistical analyses.
Results
The results of all biochemical analyses are represented as mean ± standard error of the mean in Tables 1-5 and Figures 1-5.
| FeCl3 (PPM) | Iron | Hesperidin + Iron |
|---|---|---|
| 0 | 12.10 ± 0.18 | 11.64 ± 0.22 |
| 5000 | 10.61 ± 0.10**2 | 12.02 ± 0.22d |
| 10000 | 9.42 ± 0.12** | 10.84 ± 0.16d |
| 20000 | 8.27 ± 0.08** | 10.60 ± 0.13d |
Level of significance= aP <0.05; bP < 0.01; cP<0.001; dP < 0.0001 When Iron group
compared with Hesperidin+Iron group;
*P<0.001; **P<0.0001, Iron group compared to control (0).
N=10
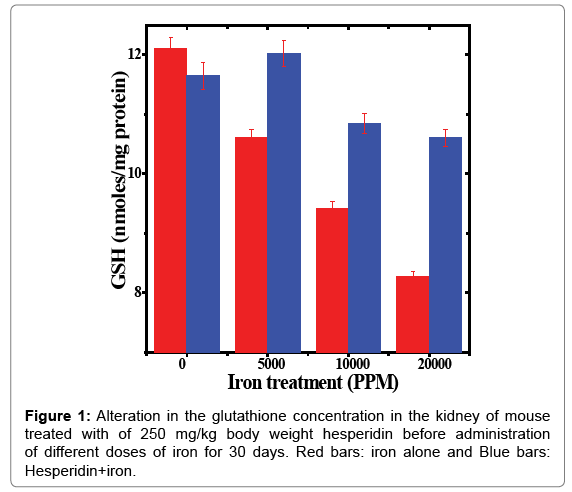
Table 1: Alteration in the glutathione concentration by 250 mg/kg body weight of hesperidin in the kidney of mouse treated with different doses of iron for 30 days.
| FeCl3 (PPM) | Iron | Hesperidin + Iron |
|---|---|---|
| 0 | 4.55 ± 0.13 | 5.37 ± 0.04d |
| 5000 | 2.14 ± 0.09** | 3.12 ± 0.16d |
| 10000 | 1.86 ± 0.14** | 2.31 ± 0.12a |
| 20000 | 1.25 ± 0.07** | 2.04 ± 0.09d |
Level of significance= aP <0.05; bP < 0.01; cP<0.001; dP < 0.0001 When Iron group
compared with Hesperidin+Iron group;
*P<0.001; **P<0.0001, Iron group compared to control (0).
N=10
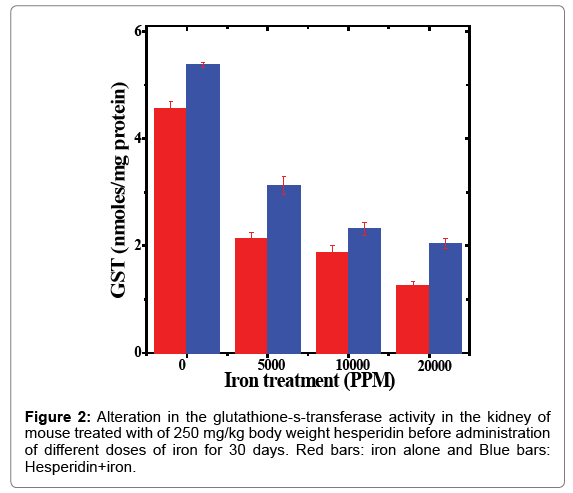
Table 2: Alteration in the glutathione-s-transferase activity by 250 mg/kg body weight of hesperidin in the kidney of mouse treated with different doses of iron for 30 days.
| FeCl3 (PPM) | Iron | Hesperidin + Iron |
|---|---|---|
| 0 | 86.09 ± 1.33 | 81.86 ± 1.96 |
| 5000 | 42.53 ± 3.65** | 73.96 ± 3.06c |
| 10000 | 30.32 ± 1.41** | 62.03 ± 0.94d |
| 20000 | 26.63 ± 1.21** | 53.54 ± 0.67d |
Level of significance= aP <0.05; bP < 0.01; cP<0.001; dP < 0.0001 When Iron group
compared with Hesperidin+Iron group;
*P<0.001; **P<0.0001, Iron group compared to control (0).
N=10
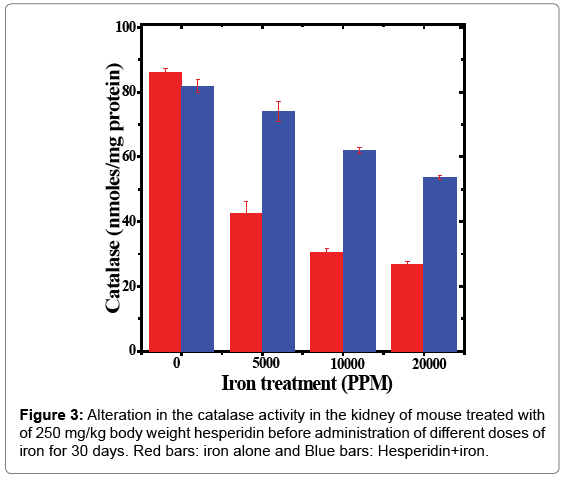
Table 3: Alteration in the catalase activity by 250 mg/kg body weight of hesperidin in the kidney of mouse treated with different doses of iron for 30 days.
| FeCl3 (PPM) | Iron | Hesperidin + Iron |
|---|---|---|
| 0 | 5.15 ± 0.23 | 4.93 ± 0.10 |
| 5000 | 1.98 ± 0.08** | 3.76 ± 0.08d |
| 10000 | 1.88 ± 0.02** | 3.20 ± 0.07 |
| 20000 | 1.51 ± 0.09** | 3.03 ± 0.12d |
Level of significance= aP <0.05; bP < 0.01; cP<0.001; dP < 0.0001 When Iron group
compared with Hesperidin+Iron group;
*P<0.001; **P<0.0001, Iron group compared to control (0).
N=10
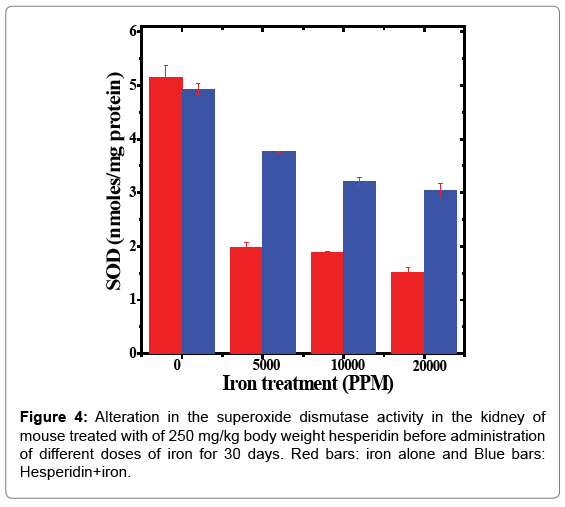
Table 4: Alteration in the superoxide dismutase activity by 250 mg/kg body weight of hesperidin in the kidney of mouse treated with different doses of iron for 30 days.
Glutathione (GSH)
The estimation of glutathione in kidney showed a spontaneous level of 12.10 ± 0.18 μmoles/mg protein in control animals and administration of hesperidin alone did not change GSH contents significantly when compared to non-drug treated control (Table 1). The chronic administration of different doses of ferric chloride for thirty days resulted in a dose dependent and significant decline in the GSH concentration when compared to untreated control (Table 1 and Figure 1). The administration of mice with hesperidin before iron overload significantly raised the glutathione contents (Table 1 and Figure 1).
Glutathione-S-transferase (GST)
The spontaneous glutathione-s-transferase activity in the kidney of mice was estimated to be 4.55 ± 0.13 nmol/mg protein and treatment of mice with hesperidin increased this activity significantly when compared to non-drug treated control (Table 2). The administration of mice with ferric chloride for 30 days alleviated the activity of the GST significantly (Table 2). This decline in the GST activity was dose dependent and a maximum decline of 3.6 folds was observed for 20000 ppm iron overload (Table 2 and Figure 2). Hesperidin treatment before iron overload elevated the GST activity significantly (p<0.05) and the maximum increase in the GST activity was recorded in the animals receiving 5000 ppm iron after hesperidin (p<0.0001) treatment (Figure 2). With increasing iron overload, the increase in GST activity by hesperidin was lesser (Table 2 and Figure 2).
Catalase
The spontaneous catalase activity in the mouse kidney has been found to be 86.09 ± 1.33 nmol/mg protein and administration of hesperidin alone did not alter this activity significantly when compared to non-drug treated control. The chronic administration of ferric chloride for 30 days reduced the catalase activity in a dose dependent manner (Figure 3). This decline was significantly higher than the noniron treated control. The maximum decline of 3.25 folds in catalase activity was observed for 20,000 ppm iron overload (Table 3 and Figure 3). Hesperidin treatment before iron overload elevated the catalase activity significantly (Table 3 and Figure 3). This rise in the catalase activity was approximately 2-fold for 10,000 and 20,000 ppm hesperidin and iron treated animals (Table 3).
Superoxide dismutase (SOD)
The spontaneous superoxide dismutase activity in the kidney of mouse was measured as 5.15 ± 0.23 nmol/mg protein and the administration of hesperidin alone did not alter this activity significantly when compared to non-drug treated control. The oral administration of different doses of ferric chloride for 30 days in drinking water reduced the SOD activity significantly (Table 4). The SOD activity depleted in iron dose dependent manner and a maximum decline was observed in the kidney of animals that received 20,000 ppm iron overload (Figure 4). The pattern of decline in the SOD activity in the hesperidin+iron group was similar to that of iron alone treatment (Figure 4). However, hesperidin treatment before iron overload significantly elevated the superoxide dismutase activity (Table 4).
Lipid peroxidation (LOO)
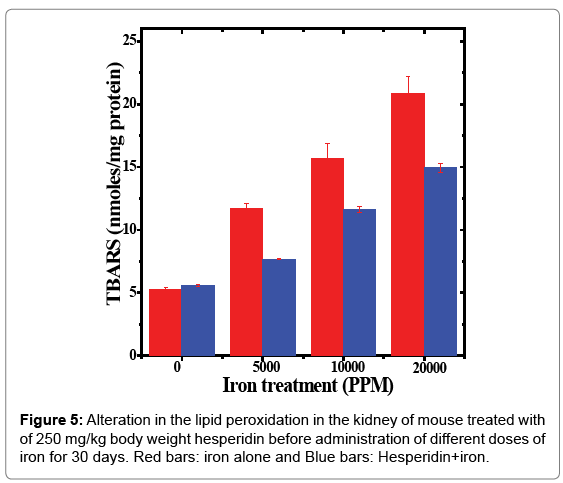
The spontaneous lipid peroxidation in the mice kidneys was estimated to be 5.24 ± 0.19 nmol/mg proteins (Table 5 and Figure 5). The oral feeding of different doses of ferric chloride for 30 days raised the lipid peroxidation in a dose dependent but significant manner when compared to untreated control (Table 5 and Figure 5). The iron overload increased the lipid peroxidation approximately by 2, 3 and 4 folds in the mice kidneys receiving 5000, 10,000 and 20,000 ppm of ferric chloride for 30 days (Table 5). The administration of hesperidin alone did not increase the level of lipid peroxidation which was almost similar to the spontaneous level (5.57 ± 0.07 nmoles/mg protein). Treatment of mice with hesperidin for five days before oral administration of different doses of ferric chloride in drinking water for 30 days significantly reduced lipid peroxidation when compared to iron loaded group (Table 5 and Figure 5). A maximum reduction of 1.5 folds was recorded for 5000 ppm iron overload, whereas it was 1.4 folds for 10,000 and 20,000 ppm iron overload, respectively (Table 5).
Discussion
Large amounts of ingested iron can cause excessive levels of iron in the blood [60]. High blood levels of free ferrous iron react with hydrogen peroxide to produce hydroxyl free radicals, which are highly reactive and can damage DNA, proteins, lipids, and other cellular components [61]. Thus, iron toxicity occurs when there is free iron in the cell, which generally occurs when iron levels exceed the capacity of transferrin to bind the iron [62]. Damage to the cells of the gastrointestinal tract can also prevent them from regulating iron absorption leading to further increases in blood iron levels [60]. Iron typically damages cells in the heart, liver and elsewhere, which can cause significant adverse effects including coma, metabolic acidosis, shock, liver failure, coagulopathy, adult respiratory distress syndrome, long-term organ damage, and even death [63]. The human body does not have any mechanism to remove excess iron, which is stored as a complex with ferritin protein and in certain conditions it is also stored as hemosiderin, a degradation product of ferritin [64,65]. This leads to iron-induced oxidative stress that subsequently damages several organs including kidneys, where it induces cancer and [64]. Iron is known to cause kidney injury in humans [60,66,67]. Therefore, present study was undertaken to evaluate the role of hesperidin, a citrus bioflavonoid in protecting the mice kidney against the iron-induced biochemical injury.
Glutathione or γ-glutamylcysteinylglycine (GSH) is a tripeptide, which controls thiol redox reactions and maturation of extramitochondrial iron sulphur clusters [68]. GSH exists in two forms the reduced and oxidized [68]. Glutathione insulates cytosolic function in iron metabolism by varying its concentration during redox stresses [69]. Glutathione participates in a number of redox reactions, which are vital to the cell. It plays a crucial role to neutralize the endogenous and exogenous toxic injuries in the cells [70]. Cells have inbuilt mechanisms to tightly regulate the concentration of iron. However, dysregulation of these mechanisms and iron overload stimulates production of free radicals leading to increased oxidative stress [71]. The endogenous glutathione may be active in neutralizing the free radicals produced by excess iron as a result there has been a dose dependent decline in the glutathione concentration in the mice kidney exposed to different doses of iron overload for 30 days. Iron treatment has been reported to reduce glutathione concentration in vitro and in vivo [72-74]. Pre-treatment of mice with hesperidin attenuated the iron induced decline in the GSH concentration in mice kidney. Similarly, quercetin has been reported to protect against the iron induced depletion in the glutathione in rat kidney [72]. Naringin has been found to alleviate the iron induced glutathione concentration in vitro [73,74].
Glutathione-s-transferases are a group of ubiquitous detoxifying enzymes, which are present in all aerobic eukaryotes, which protect cells from the toxic insults by deactivating hydrophobic cytotoxic, genotoxic compounds and reactive oxygen species [75]. The administration of mice with different doses of iron for one month in drinking water caused a dose dependent reduction in the glutathione-s-transferase activity in the kidney. A similar effect has been observed in the sera of Caucasian patients receiving iron therapy for the treatment of β-thalassemia, where GSTT1 and GSTM1 genes were down regulated [76]. This indicates that iron overload is detrimental to glutathione-stransferase activity due to its ability to induce oxidative stress in the cells. Hesperidin treatment for five days before iron overload increased the glutathione-s-transferase activity in mice kidney, when compared to iron treatment alone. The reports regarding the estimation of GST activity in hesperidin treated iron overloaded mouse kidney are unavailable. However, hesperidin has been reported to attenuate ironinduced decline in glutathione-s-transferase activity in rat liver earlier [77]
Catalase or oxidoreductase (EC. .1.11.1.6) is a tetrameric enzyme, found in all those organisms that utilize oxygen for respiration and energy production. Catalase detoxifies H2O2, a strong oxidizing agent, generated during respiration, which is the major cause of tissue pathogenesis. The catalase contains four porphyrin heme groups, which allow it to interacts with H2O2 and decomposes it into water and molecular oxygen [78,79]. The exposure of mice to different doses of iron for 30 days decreased the activity of catalase in the mouse kidney. Likewise, rats exposed to iron overload has shown a reduction in catalase activity in their kidneys and livers earlier [72,77]. The mice fed with iron overload diet for four months also showed a reduced catalase activity in the liver [80]. Similarly, iron overload has been reported to attenuate the catalase activity in vitro [73,74]. The hesperidin treatment arrested this decline in the catalase activity by elevating the activity of catalase in mice kidney significantly. Hesperidin treatment has been reported to elevated catalase activity in the iron overloaded liver earlier [77]. Quercetin has been reported to augment the activity of catalase in the kidney of iron overloaded rats [72]. A similar effect has been detected with naringin, a citrus bioflavonoid in HepG2 cells and isolated liver mitochondria in vitro [73,74].
Superoxide dismutases are ubiquitously present in all organisms that use oxygen for their energy requirement. SOD acts as a signaling molecule, toxic agent or a harmless species [81]. All organisms that employ oxygen for respiration generate superoxide radical (O2•-) during respiration. Although O2•- per se is not highly reactive, it becomes reactive when it interacts with transition metal complexes of iron, copper and manganese leading to increased oxidative stress [82,83]. The SOD dismutates O2- into less harmful product hydrogen peroxide and acts as a first line of defence against oxidative stress [82,83]. SOD also inactivates nitric oxide reducing oxidative stress. The iron overload in the mouse kidney has alleviated the SOD activity in a dose dependent manner in the present study. Iron overload has been reported to deplete the SOD activity in rat kidney [72]. Likewise, iron overload has been reported to reduce the SOD activity in rat and mice liver earlier [77,80]. Hesperidin treatment for five consecutive days before iron overload led to a rise in the SOD activity in mice kidney in the present study. In an earlier study hesperidin has been reported to elevate SOD activity in iron overloaded rat liver [77]. Quercetin has been found to increase the SOD activity in the rat kidney [72]. Similarly, naringin treatment has been reported to elevate the SOD activity in iron overloaded HepG2 cells and isolated mice liver mitochondria earlier [73,74].
The inactivation of hydroxyl and hydroperoxyl radicals results in the formation of lipid peroxidation. Hydroxyl radicals are formed due to the redox cycling of Fe2+ via Fenton reaction, where iron reacts with H2O2 to produce hydroxyl radical. The Haber Weiss reaction regenerates Fe2+ when O2•- reacts with Fe3+ [61,84]. The increased iron overload has been indicated in kidney cancer [64]. Therefore, it is pertinent to assay lipid peroxidation in the iron overloaded mice kidney. Iron overload has increased lipid peroxidation in the mouse kidney by 2 to 4 folds depending on the dose of iron. The rats receiving iron exhibited an increase in the lipid peroxidation in the rat kidney and liver [72,77,85]. Similarly, our earlier investigations have reported accelerated lipid peroxidation in vitro [73,74]. The lipid peroxidation showed an increase in mice liver fed iron diet for four months [80]. An increased lipid peroxidation was observed in the serum of iron overloaded patients [86]. The administration of hesperidin for five consecutive days retarded lipid peroxidation significantly in the kidney of iron overloaded mouse. A similar effect has been observed in iron overloaded rat liver treated with hesperidin earlier [77]. Quercetin has also been found to attenuate the iron-induced lipid peroxidation in the rat kidney in an earlier study [72]. Likewise, naringin has been reported to reduce lipid peroxidation in the HepG2 cells and isolated mice liver mitochondria in vitro [73,74].
The exact mechanism by which hesperidin reduced the iron induced oxidative stress is not clearly known. However, iron triggers the formation of free radicals via Fenton and Haber Weiss reactions during various metabolic activities [61,84] and presence of hesperidin before iron treatment might have blocked the generation of ironinduced free radicals resulting in the decline in iron-induced oxidative stress in the mice kidney. Our earlier study has shown that hesperidin inhibited the production of different free radicals in vitro [51]. The iron overload triggered the transcriptional activation of IKKβ resulting in the transcription of NF-κB, and TNF-α and also activation of COXII and prostaglandins that stimulate inflammation and are responsible for pathophysiology [87,88]. Hesperidin has been found to inhibit the stimulation of NF-κB and COX-II earlier [89,90], which may have led to the alleviation in the iron induced oxidant status in the kidney of hesperidin pre-treated group. The Nrf2 activation has been reported to inhibit iron accumulation in the mouse liver the site of iron accumulation and metabolism [91]. Hesperidin has been reported to stimulate Nrf2 in rat kidney, which may have reduced the accumulation of iron and reduced the iron-induced oxidative stress [92].
Conclusion
The present study demonstrates that hesperidin treatment reduced iron-induced biochemical injury in the mice kidney alleviating, lipid peroxidation and increasing the glutathione, glutathione-s-transferase, catalase and superoxide dismutase levels. This action of hesperidin may be mediated by free radical inhibition and suppression of NF-κB, COX-II, prostaglandins and TNF-α activation. The Nrf2 activation by hesperidin may have also contributed to reduced iron-induced oxidative stress in the mouse kidney. Our study indicates the utility of hesperidin to reduce iron-induced oxidative stress in clinical situation.
Acknowledgment
This work was carried out under grant no. F4-10/2010(BSR) UGC from the University Grants Commission, Government of India, New Delhi, India.
References
- Dlouhy AC, Outten CE (2013) The iron metallome in eukaryotic organisms. Met Ions Life Sci 12: 241-278.
- Kell DB (2009) Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics 2: 2.
- Galaris, D, Pantopoulos K (2008) Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci 45: 1-23.
- Bradley JM, Le Brun NE, Moore GR (2016) Commentary: Ferritins: furnishing proteins with iron. J Rare Dis Res Treat 1: 22-24
- Xu Z, Shi Z, Li Y (2014) The Crosstalk between micro RNA and iron homeostasis. Int J Genomic Med 1: 112.
- Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, et al.(2001) Ferritin and the response to oxidative stress. Biochem J 357: 241-247.
- Zhang C (2014) Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell 5: 750-760.
- Gozzelino R, Arosio P (2016) Iron homeostasis in health and disease. Int J Mol Sci 17: 130.
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, et al. (1999) Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson's disease. Neurotoxicology 20: 239-247.
- Yun S, Vincelette ND (2015) Update on iron metabolism and molecular perspective of common genetic and acquired disorder, hemochromatosis. Crit Rev Oncol/Hematol 95: 12-25.
- Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117: 285-297.
- Steiner M, Ocran K, Genschel J, Meier P, Gerl H, et al. (2002) A homozygous HFE gene splice site mutation (IVS5+ 1 G/A) in a hereditary hemochromatosis patient of Vietnamese origin. Gastroenterology 122: 789-795.
- Lok CY, Merryweather-Clarke AT, Viprakasit V, Chinthammitr Y, Srichairatanakool S, et al. (2009) Iron overload in the Asian community. Blood 114: 20-25.
- Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview, Methods Enzymol 186: 1-85.
- Swaminathan S, Fonseca VA, Alam MG, Shah SV (2007) The role of iron in diabetes and its complications. Diabet Care 30: 1926-1933.
- Chua AC, B Klopcic B, Lawrence IC, Olynyk JK,Trinder D(2010) Iron: an emerging factor in colorectal carcinogenesis. World J Gastroenterol 16: 663-672.
- Fleming RE, Ponka P (2012) Iron overload in human disease. New Eng J Med 366: 348-359.
- Martines Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13: 1045-1060.
- Gelfand BD, Wright CB, Kim Y, Yasuma T, Yasuma R, et al. (2015)Iron toxicity in the retina requires Alu RNA and the NLRP3 inflammasome. Cell Rep 11: 1686-1693.
- Tantiworawit A, Tapanya S, Phrommintikul A, Saekho S, Rattarittamrong E, et al. (2016) Prevalence and risk factors for cardiac iron overload and cardiovascular complications among patients with thalassemia in northern Thailand. Southeast Asian J Trop Med Pub Hlth 47: 1335-1342.
- Rank CU, Petersen J, Birgens HS, Nielsen OJ (2015) Hereditary hyperferritinemia-cataract syndrome. Eur Oncol Haematol 2: 147-149.
- Stugiewicz M, Tkaczyszyn M, Kasztura M, Banasiak W, Ponikowski P, et al. (2016)The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications. Eur J Heart Fail 18: 762-773.
- Neves J, Leitz D, Kraut S, Brandenberger C, Agrawal R, et al. (2017) Disruption of the hepcidin/ferroportin regulatory system causes pulmonary iron overload and restrictive lung disease. EBioMedicine 20: 230-239.
- Iglesias DJ, Cercós M, Colmenero-Flores JM, Naranjo MA, RÃos G, et al.(2007) Physiology of citrus fruiting. Braz J Plant Physiol 19: 333-362.
- Del Rio JA, Gomez P, Baidez AG, Arcas MC, Botia JM, et al. (2004) Changes in the levels of polymethoxyflavones and flavanones as part of the defense mechanism of Citrus sinensis (cv. Valencia Late) fruits against Phytophthora citrophthora. J Agr Food Chem 52: 1913-1917.
- Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, YANO M, et al. (2006) Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem 70: 178-192.
- D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, et al. (2007) Polyphenols, dietary sources and bioavailability. Ann Inst Super Sanita 43: 348-361.
- Jin MJ, Kim U, Kim IS, Kim Y, Kim DH, et al. (2010) Effects of gut microflora on pharmacokinetics of hesperidin: A study on non-antibiotic and pseudo-germ-free rats. J Toxicol Environ Health A 73: 1441-1450.
- Emim JA, Oliveira AB, Lapa AJ (1994) Pharmacological evaluation of the antiâ€inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J Pharm Pharmacol 46: 118-212.
- Crespo ME, Galvez J, Cruz T, Ocete MA, Zarzuelo A (1999) Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med 65: 651-653.
- Selsman GJ, Horoschak S (1950) The treatment of capillary fragility with a combination of hesperidin and vitamin C. Am J Diges Dis 17: 92-94.
- Ameer B, Weintraub RA, Johnson JV, Yost RA, Rouseff RL (1996) Flavanone absorption after naringin, hesperidin, and citrus administration. Clin Pharmacol Therapeut 60: 34-40.
- Miyagi Y, Om AS, Chee KM, Bennink MR (2000) Inhibition of azoxymethane-induced colon cancer by orange juice. Nutr Cancer 36: 224-229.
- Vabeiryureilai M, Lalrinzuali K, Jagetia GC (2015) Determination of anti-inflammatory and analgesic activities of a citrus bioflavanoid, hesperidin in mice. Immunochem Immunopathol 1: 107.
- Ahmadi A, Shadboorestan A, Nabavi SF, Setzer WN, Nabavi SM (2015) The role of hesperidin in cell signal transduction pathway for the prevention or treatment of cancer. Curr Med Chem 22: 3462-3471.
- DobiaÅ¡ L, Petrová M, Vojtko R, Kristová V (2016) Longâ€term treatment with hesperidin improves endotheliumâ€dependent vasodilation in femoral artery of spontaneously hypertensive rats: The involvement of NOâ€synthase and Kv channels. Phytother Res 30: 1665-1671.
- Iranshahi M, Rezaee R, Parhiz H, Roohbakhsh A, Soltani F (2015) Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci 137: 125-132.
- Milenkovic D, Deval C, Dubray C, Mazur A, Morand C (2011) Hesperidin displays relevant role in the nutrigenomic effect of orange juice on blood leukocytes in human volunteers: a randomized controlled cross-over study. PLoSOne 6: e26669.
- Zaragoza F, Fdez-Corbeira P, Iglesias I, Benedi J (1986) New natural inhibitors of platelet aggregation in vivo. Part I. Citroflavonoids and hesperidin. Ann Real Acad Farm 52: 497-504.
- Son HS, Kim HS, Ju JS (1991) Effects of rutin and hesperidin on total cholesterol concentration, transaminase and alkaline phosphatase activity in carbon tetrachloride treated rats. Hanguk Nonghwa Hakhoe Chi 34: 318-326.
- Galati EM, Monforte MT, Kirjavainen S, Forestieri AM, Trovato A, et al. (1994) Biological effects of hesperidin, a citrus flavonoid. (Note I): Antiinflammatory and analgesic activity. Farmaco 40: 709-712.
- Loguercio C, D'Argenio G, Delle Cave M, Cosenza V, Della Valle N, et al. (1996) Direct evidence of oxidative damage in acute and chronic phases of experimental colitis in rats. Dig Dis Sci. 41: 1204-1211.
- Tanaka T, Makita H, Kawabata K, Mori H (1997) Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 18: 957-965.
- Kawaguchi K, Mizuno T, Aida K, Uchino K (1997) Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas. Biosci Biotech Biochem 61: 102-104.
- Kawaguchi K, Kikuchi S, Takayanagi K, Yoshikawa T, Kumazawa Y (1999) Colony stimulating factor inducing activity of hesperidin. Planta Med 65: 365-366.
- Lee JH, Kim YS, Lee CK, Lee HK, Han SS (1999) Antiviral activity of some flavonoids on Herpes-simplex virus. Korean Pharmacog 30: 34-39.
- Cho J (2006) Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res 29: 699-706.
- Chen MC, Ye YI, Guang JI, Jian, Liu JW (2010) Hesperidin upregulates heme oxygenase-1 to attenuate hydrogen peroxide-induced cell damage in hepatic L02 cells. J Agric Food Chem.58: 3330-3335.
- Tamilselvam K, Braidy N, Manivasagam T, Essa MM, Prasad NR, et al. (2013) Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson’s disease.Oxidat Med cell Longev 102741.
- Jagetia GC, Rao KVNM (2017) Topical application of hesperidin, a citrus bioflavanone accelerates healing of full thickness dermal excision wounds in mice exposed to 6 Gy of whole body γ-Radiation. Clin Res Dermatol Open Access 4: 1-8.
- Jagetia GC, Rao KVNM (2018) Hesperidin, a Citrus bioflavonoid potentiates repair and regeneration of deep dermal excision wounds of mice whole body exposed to different doses of 60Co γ-radiation. Clin Dermatol J3:  000147.
- Kawabe M, Tamano S, Shibata MA, Hirose M, Fukushima S, et al. (1993) Subchronic toxicity study of methyl hesperidin in mice. Toxicol Lett 69: 37-44.
- Sandermann H, Stromiger J (1972) Purification and properties of C 55-isoprenoidalcohol phosphokinase from Staphylococcus aureus. J Biol Chem 247: 5123-5513.
- Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophy Acta 582: 67-78.
- Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation, J Biol Chem 249: 7130-7139.
- Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47: 469-474.
- Ohkawa ON, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 391-398.
- Fishbane S, Mathew A, Vaziri ND (2014) Iron toxicity: Relevance for dialysis patients. Nephrol Dial Transplant29: 255-259.
- Das TK, Wati MR, Fatima-Shad K (2015) Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch Neurosci 2: e20078.
- Roberts WL, Smith PT, Martin WJ, Rainey PM (1999) Performance characteristics of three serum iron and total iron-binding capacity methods in acute iron overdose. Am J Clin Pathol 112: 657-664.
- Weinberg ED (2009) Iron toxicity: New conditions continue to emerge. Oxid Med Cell Longev 2: 107-109.
- Nishida Y (2009) Structural Characteristic of iron (III) chelates to induce tissue damage and renal carcinoma; chemical origin of the iron toxicity. TCIMail 141: 1-5.
- Arosio P, Elia L, Poli (2017) Ferritin, cellular iron storage and regulation. IUBMB Life 69: 414-422.
- Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, et al. (2013) Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423-4434.
- Rostoker G, Vaziri ND, Fishbane S (2016) Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs 76: 741-757.
- Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids: 2012.
- Kumar C, Igbaria A, D'autreaux B, Planson AG, Junot C, et al. (2011) Glutathione revisited: A vital function in iron metabolism and ancillary role in thiolâ€redox control. EMBO J 30: 2044-2056.
- Aquilano K, Baldelli S, Ciriolo MR (2014) Glutathione: New roles in redox signaling for an old antioxidant. Front Pharmacol 5: 196.
- Lane DJ, Merlot AM, Huang MH, Bae DH, Jansson PJ, et al. (2015) Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim Biophys Acta (BBA)-Mol Cell 1853: 1130-1144.
- Singh D, Chander V, Chopra K (2005) Quercetin, a Bioflavonoid, Attenuates Ferric Nitrilotriacetateâ€Induced Oxidative Renal Injury in Rats. Drug Chem Toxicol 27: 145-156.
- Jagetia GC, Reddy TK, Venkatesha VA, Kedlaya R (2004) Influence of naringin on ferric iron induced oxidative damage in vitro. Clin Chim Acta 347: 189-197.
- Jagetia GC, Reddy TK (2011) Alleviation of iron induced oxidative stress by the grape fruit flavanone naringin in vitro. Chemico-Biol Interact 190: 121-128.
- Hayes JD, Flanagan JU, Jowsey IR (2005) “Glutathione transferases,†Annual Review of Pharmacol Toxicol 45: 51-88.
- Sclafani S, Calvaruso G, Agrigento V, Maggio A, Nigro VL, et al. (2013) Glutathione S transferase polymorphisms influence on iron overload in β-thalassemia patients. Thalassemia Rep 3: 6.
- Pari L, Karthikeyan A, Karthika P, Rathinam A (2015) Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol Rep 2: 46-55.
- Kirkman HN, Gaetani GF (1984) Catalase: A tetrameric enzyme with four tightly bound molecules of NADPH. Proc Nat Acad Sci 81: 4343-4347.
- Alfonso-Prieto M, Biarnés X, Vidossich P, Rovira C (2009) The molecular mechanism of the catalase reaction. J Am Chem Soc 131: 11751-11761.
- Liu D, He H, Yin D, Que A, Tang L, et al. (2013) Mechanism of chronic dietary overload induced liver damage in mice. Mol Med Rep 7: 1173-1179.
- Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, et al. (2014)Superoxide dismutases and superoxide reductases. Chem Rev 114: 3854-3918.
- Abreu IA, Cabelli DE (2010) Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim Biophys Acta (BBA)-Proteins and Proteomics 1804: 263-274.
- Azadmanesh J, Borgstahl GE (2018) A review of the catalytic mechanism of human manganese superoxide dismutase. Antioxidants 7: 25.
- Kehrer JP (2000) The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149: 43-50.
- Fischer JG, Glauert HP, Yin T, Sweeney-Reeves ML, Larmonier N, et al. (2002) Moderate iron overload enhances lipid peroxidation in livers of rats but does not affect NF-κB activation induced by the peroxisome proliferator, Wy-14,643. J Nutri 132: 2525-2531.
- De Souza GF, Ribeiro HL, De Sousa JC, Heredia FF, De Freitas RM, et al. (2015) HFE gene mutation and oxidative damage biomarkers in patients with myelodysplastic syndromes and its relation to transfusional iron overload: an observational cross-sectional study. BMJ open 5: e006048.
- She H, Xiong S, Lin M, Zandi E, Giulivi C, et al. (2002) Iron activates NF-κB in Kupffer cells. Am J Physiol Gastrointest Liver Physiol 283: G719-G726.
- Lousse JC, Defrère S, Ramos RG, Van Langendonckt A, Colette S, et al. (2009) Involvement of iron, nuclear factor-kappa B (NF-κB) and prostaglandins in the pathogenesis of peritoneal endometriosis-associated inflammation: A review. J Endometriosis 1: 19-29.
- Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S (2005) Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res 25: 3367-3374.
- Ghorbani A, Nazari M, Jeddi-Tehrani M, Zand H (2012) The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: involvement of PPARγ-dependent mechanism. Eur J Nutr  51: 39-46.
- Subramanian P, Anandan R, Jayapalan JJ, Hashim OH (2015) Hesperidin protects gentamicin-induced nephrotoxicity via Nrf2/HO-1 signaling and inhibits inflammation mediated by NF-κB in rats. J Funct Foods 13: 89-99.
- Okada K, Warabi E, Sugimoto H, Horie M, Tokushige K, et al. (2012) Inhibits hepatic iron accumulation and counteracts oxidative stress-induced liver injury in nutritional steatohepatitis. J Gastroenterol 47: 924-935.
Citation: Jagetia GC, Lalramthari (2018) Attrition of Iron-Induced Biochemical Injury in Mice Kidney by a Citrus Bioflavonoid, Hesperidin. Biochem Physiol 7: 240. DOI: 10.4172/2168-9652.1000240
Copyright: © 2018 Jagetia GC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5374
- [From(publication date): 0-2018 - Nov 30, 2025]
- Breakdown by view type
- HTML page views: 4418
- PDF downloads: 956