Bioelectricity Generation and Dye Decolorization by Aspergillus niger and Trichoderma harzianum
Received: 01-Jun-2018 / Accepted Date: 13-Jun-2018 / Published Date: 15-Jun-2018 DOI: 10.4172/2155-6199.1000446
Keywords: Decolorization; Bioelectricity; Acid black 172
Introduction
Dyes are widely used in the textile industry and 10-15% of these dyes are lost in effluent during the dyeing process [1]. Azo dyes are a group of poorly biodegradable synthetic colorants that are often found in the textile wastewater [2]. These compounds are highly resistant to aerobic biodegradation by microorganisms but can be easily reduced by anaerobic microorganisms to form aromatic amines, which are a group of carcinogens that are stable under anaerobic conditions and must be returned to aerobic conditions before they can be further degraded and mineralized [3]. A range of physicochemical methods exists to remove color from dye containing effluents [4]. The most extensively used are coagulation and flocculation processes. They require significant quantities of chemicals and produce notable amounts of sludge, requiring further handling and disposal. Enzymatic decolorization is now widely used for the decolorization of dye wastewater. However, this method is also facing several problems such as cost of enzymes, enzymes stability and product inhibition [5]. On the other hand, biological processes provide a low-cost and efficient alternative for simultaneous color removal. In recent years, microbial fuel cell (MFC) technology has explored extensively for their innovative features and environmental benefits [6]. This work presents an efficient and cost effective approach to establish an MFC-assisted electrochemical oxidation process for the decolorization of azo dye.
Methodology
Dyes
Five model textile dyes (obtained from Moket Mac Company at 10th of Ramadan City, Egypt) were used for testing decolorization capacity of Aspergillus niger and Trichoderma harzianum including: Acid red 399, Acid yellow 235, Acid yellow 218, Acid blue 296, and Acid black 172.
Sample collection and cultivation conditions
A fungal species was collected from wastewater of an Egyptian Company for artificial Carpet at 10th of Ramadan City, in sterile clean glass bottles then, stored at 4°C, cultivated in petri-dishes containing Dox᾽s agar medium at 25°C and stocked in slant tubes containing Czapek᾽s yeast agar solid medium under refrigeration at 4°C. Initial selection was based on maximum voltage production and dye decolorization. For submerged cultures, a pre-inoculum of young mycelium disk of 0.5 cm of diameter was obtained from 2 days-old colonies for Aspergillus niger , while in case of Trichoderma harzianum two young mycelium disks of 0.5 cm of diameter were obtained from 4 days-old colonies in solid medium. Disks were inoculated into flasks with 50 ml of fresh medium containing 15 g/L glucose, 2 g/L NaNO3, 1 g/L KH2PO4, 0.5 g/L KCL and 5 g/L yeast extract in 50 mM phosphate buffer (pH 7) and incubated at 25°C for 96 h for Trichoderma harzianum , while Aspergillus niger was incubated at 35°C for 48 h. After this period of time, flasks were used for inoculation of 250 ml flasks (bottles) containing 50 ml Acid black 172 dye (which has been prepared using 10 mg/L) of anodic compartment of fungal microbial fuel cells. Each culture was incubated under maximum incubation temperature.
Construction of fungal microbial fuel cell
The dual chambered MFC was constructed using two air tight laboratory glass bottles (anode and cathode) of 250 ml capacity connected via a glass tube (Length=11 cm, width=6.6 and diameter=0.5 cm) that is heated and bent into a u-shaped, filled with 2% agar gel in saturated KCL solution and inserted through the lid of each bottle. The anodic and cathodic electrodes were graphite rods (surface area=5.5 cm). Copper wires were soldered to the electrodes by using a conductive epoxy. A multimeter with a data logger system (MT-1820 ProsʹKit, Taiwan) was used as the automated data acquisition system.
Operation of a fungal microbial fuel cell
The fuel cell compartments were cleaned and the electrodes were autoclaved for 20 min. The anode solution (50 mL) contained the following components (per liter) 15 g C6H12O6, 2 g NaNO3, 1 g KH2PO4, 0.5 g KCL, 5 g yeast extract in 50 Mm phosphate buffer (pH 7) and fungal biomasses. The compartment was inoculated with 50 ml acid black 194 which prepared as mentioned previously, finally anode volume (100 ml).
The cathode solution on the other hand, consisted of 100 ml of Potassium Ferricyanide (K3(Fe(CN)6) solution (10 mmol/L) prepared in distilled water. The initial pH of Potassium Ferricyanide solution (pH 2) at cathode was adjusted using 0.5 M HCL which acted as the electron acceptor.
The MFCs with inoculation were first incubated with electrolytes described above for 4 days, then evaluated the decolorization rate of Acid black 172 and electricity generation. The external resistance was fixed at 1000 Ω and in some case the MFCs were operated under different external resistances to obtain the polarization curves. The temperature of MFCs was kept at 35°C for Aspergillus niger and at 25°C for Trichoderma harzianum using the laboratory incubators both the two inoculated MFCs and the two control sets were operated under identical conditions respectively, and average results were reported.
Electrochemical analyses
Cell voltages were recorded every 20 minutes after a stable open circuit potential was achieved using a data logger system. Current (I) was calculated at a resistance(R) from the voltage (V) by I=V/R, and current density (I/A2), where A is the projected anode surface area.
Acid black 172 decolorization
The absorption of A. black 172 in the visible light range peaks at a wavelength of 572 nm was measured using a T60 UV-VIS spectrophotometer and used to calculate A. black 172 from Eq. (1) where A0 and A are the initial and final absorptions, respectively.
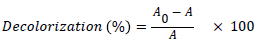
Results And Discussion
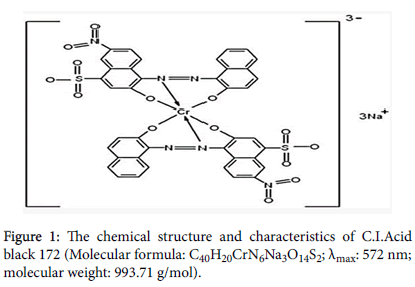
Figure 1 shows the chemical structure and characteristics of C.I.Acid black 172:
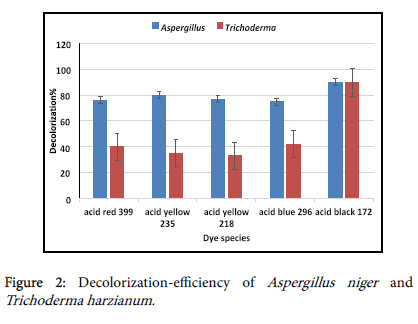
Decolorization-efficiency of Aspergillus niger and Trichoderma harzianum
Aspergillus niger and Trichoderma harzianum were selected to investigate their effect on decolorization rate of the following dyes: (1) Acid red 399, Acid yellow 235, Acid yellow 218, Acid blue 296, and Acid black 172. The initial concentration was (10 mg/L) amended in flasks containing 100 ml of Dox's broth medium. The flasks were inoculated as previously mentioned. The results in Figure 2 show variation in decolorization abilities. Aspergillus niger was able to remove more than 50% of these acid dyes. On other hand, Trichoderma harzianum was able to remove less than 50% of acid red 399, acid yellow 235, acid yellow 218 and acid blue 296 dyes. The results showed that, Aspergillus niger and Trichoderma harzianum were effectively able to remove more than 85% of acid black 172. Accordingly, it is selected for further investigations. These results may be due to chemical structures of dyes significantly affecting the performance of dye biodecolorization. For similar structures of azo dyes, monoazo dyes are easily biodecolrized than polyazo dyes [7]. Also, our results agree with Bor et al. [8] who state that color removal percentages of Reactive Blue 160 for Proteus hauseri still achieved above 90%.
Effect of acid black 172 concentration on dye decolorization and voltage output (OCV)
Comparison on performance of voltage output in a fungal microbial fuel cell at various concentrations of acid black 172 (i.e., 5, 10, 30 and 50 mg/L; Table 1) showed that the decolorization percent and voltage output (OCV) for Aspergillus niger and Trichoderma harzianum reached its maximum value (95%) and (890 mV) respectively at dye concentration 10 mg/L. Also showed that increasing in the dye concentration resulted in a decrease in the decolorization percent and voltage output (OCV). The results are in agreement with the findings of Bor et al. [8] that observed the decreased peak output voltage at high loading of Reactive Blue 160 might explain that decolorization and bioelectricity generation of Proteus hauseri are competitive to each other as both reactions all utilized electrons released from bacterial oxidation of organic matter. In addition, possibly due to accumulation of the decolorized intermediates of Reactive Blue 160 as mediators, the steadystate (SS) cell-voltages were stabilized at slightly higher levels than the SS-voltage in Reactive blue 160 free cases. In general, the performance of an MFC depends on the concentration and the type of dye used; Mu et al. [9] showed that during closed-circuit operation, decolorization efficiency decreased from 78.7% to 35% with an increase in influent dye concentration. Sun et al. [10] reported that the percent decolorization decreased with increase in Active Brilliant Red X-3B concentration.
| Dye concentration (mg/L) |
Decolorization (%) | Voltage(OCV) | ||
|---|---|---|---|---|
| Aspergillus | Trichoderma | Aspergillus | Trichoderma | |
| 5 | 93 | 94 | 850 mV | 870 mV |
| 10 | 95 | 95 | 890 mV | 890 mV |
| 30 | 80 | 75 | 810 mV | 800 mV |
| 50 | 65 | 61 | 750 mV | 720 mV |
Table 1: Effect of acid black 172 concentration on dye decolorization and voltage output (OCV)
Effect of co-substrate on decolorization of acid black 172 and voltage output (OCV)
Different co-substrates such as glucose, and sucrose were individually added as equimolecular to 30 g sucrose per liter were tested to determine the optimum co-substrate which supported the maximum decolorization and voltage output of the tested fungi. The results in Table 2 showed that, the decolorization percent for Aspergillus niger and Trichoderma harzianum reached its maximum value (95% and 98%) respectively using glucose as co-substrate, also showed voltage output value reached its maximum value (899 mV and 895 mV) for Aspergillus niger and Trichoderma harzianum respectively. The results similar to those obtained by Sun et al. [10] who stated that, the maximum decolorization rate of ABRX3 observed with glucose, also in agreement with our results Cao et al. [11] found that, the power density was highest for glucose during decolorization of Congo red.
| Co-substrate | Decolorization (%D) | Voltage (OCV) | ||
|---|---|---|---|---|
| Aspergillus | Trichoderma | Aspergillus | Trichoderma | |
| Glucose | 95 | 98 | 899 mV | 895 mV |
| Sucrose | 90 | 92 | 820 mV | 800 mV |
| Control | 80 | 75 | 700 mV | 620 mV |
Table 2: Effect of co-substrate on decolorization of Acid black 172 and voltage output (OCV).
Simultaneous dye decolorization and current generation
Dye decolorization rate and current generation were measured after operation for three days. The results in Table 3 showed that decolorization rate and current density were decreased after sixty hours of operation. These results in contrast with Chen and Chen et al. [12,13] who mentioned that impulse additions of C.I. reactive red 141 significantly enhanced increasing the rate of oxidative phosphorylation of P. hauseri to accelerate electron transport in the respiratory chain of immobilized cells on the anodic biofilm for bioelectricity production in MFC.
| Time (h) | Decolorization (%) | Current density (mA/m2) | ||
|---|---|---|---|---|
| Aspergillus | Trichoderma | Aspergillus | Trichoderma | |
| 0 | 95 | 94 | 163 | 160 |
| 12 | 93 | 94 | 149 | 154 |
| 24 | 92 | 93 | 147 | 154 |
| 36 | 92 | 92 | 145 | 145 |
| 48 | 90 | 90 | 145 | 136 |
| 60 | 90 | 90 | 136 | 131 |
| 72 | 88 | 85 | 133 | 129 |
Table 3: Simultaneous dye decolorization and current generation.
Conclusion
The microbial fuel cell (MFC) with a fungal anode achieved more than 84% decolorization of azo dye acid black 172 in three days of continuous operation with current density more than 128 MA/m2. Also a maximum open circuit voltage(OCV) was obtained with 10 g/L of dye concentration was 890 mV and maximum open circuit voltage(OCV) was obtained with Co-substrate with glucose was more than 894 mV for the two fungal species.
Acknowledgments
This research work was supported by Helwan university of Egypt as academic study. The authors also appreciate technical supports by scientific laboratory of Helwan university. Significant comments from the anonymous reviewers are also very much appreciated.
References
- Rajaguru P, Kalaiselvi K, Palanivel M, Subburam V (2000) Biodegradation of azo dyes in a sequential anaerobic-aerobic system. Appl Microbiol Biotechnol 54: 268-273.
- Cai MQ, Wei XQ, Du CH, Ma XM, Jin MC (2014) Novel amphiphilic polymeric ionic liquid-solid phase micro-extraction membrane for the preconcentration of aniline as degradation product of azo dye Orange G under sonication by liquid chromatography- tandem mass spectrometry. J Chromatograghy A 1349: 24-29.
- Garcia MY, Bengoa C, Stuber F, Fortuny A, Font J, et al. (2015) Biodegradation of acid orange 7 in an anaerobic-aerobic sequential treatment system. Chem Eng Process 94: 99-104.
- Mu Y, Yu HQ, Zheng JC, Zhang SJ (2004) TiO2-mediated photocatalytic degradation of orange II with the presence of Mn2+ in solution. J Phtochem Photobiol 163: 311-316.
- Husain Q (2010) Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: a review. Rev Environ Sci Biotechnol 9: 117-140.
- Rozendal RA, Leone E, Keller J, Rabaey K (2009) Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem Commun 11: 1752-1755.
- Hsueh CC, Chen BY, Yen CY (2009) Understanding effects of chemical structure on azo dye decolorization characteristics by Aeromonas hydrophila. J Hazard Mater 167: 995-1001.
- Bor YC, Meng Z, Chang TC, Yongtao D, Kae LL, et al. (2010) Assessment upon azo dye decolorization and bioelectricity generation by Proteus hauseri. Bioresource Technology 101: 4737-4741.
- Mu Y, Rabaey K, Rozendal R, Yuan Z, Keller J (2009) Decolorization of azo dyes in bioelectrochemical systems. Environ Sci Technol 34: 5137-5143.
- Sun J, Hu Y, Bi Z, Cao Y (2009) Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber microbial fuel cell. Bioresour Technol 100: 3185-3192.
- Cao Y, Hu Y, Sun J, Hou B (2010) Explore various co-substrates for simultaneous electricity generation and Congo red degradation in air- cathode single-chamber microbial fuel cell. Bioelectrochemistry 79: 71-76.
- Chen BY, Yen CY, Chen WM, Chang CT, Wang CT, et al. (2009) Exploring threshold operation criteria of biostimulation for azo dye decolorization using immobilized cell systems. Bioresour Technol 100: 5763-5770.
- Chen BY (2006) Toxicity assessment of aromatic amines to Pseudomonas luteola: chemostat pluse technique and dose-response analysis. Process Biochem 41: 1529-1538.
Citation: Osman ME, Khattab OH, Elnasr AA, Basset AS (2018) Bioelectricity Generation and Dye Decolorization by Aspergillus niger and Trichoderma harzianum. J Bioremediat Biodegrad 9: 446. DOI: 10.4172/2155-6199.1000446
Copyright: © 2018 Osman ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5479
- [From(publication date): 0-2018 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 4491
- PDF downloads: 988