Bioremediation of Tannery Effluents for Sustainable Production of Leather in Uganda: Literature Review
Received: 23-May-2018 / Accepted Date: 18-Jun-2018 / Published Date: 20-Jun-2018 DOI: 10.4172/2155-6199.1000448
Keywords: Tannery industry; Bioremediation technology; Human health and safety; Hides and skins
Introduction
Bioremediation process
Bioremediation process has been applied worldwide using active microbes to manage terrestrial and aquatic ecosystems said by Ang et al., Dua et al. and Gavrilescu [1-3]. It was also asserted that, bioremediation uses biological process to overcome environmental problems like treating waste or pollutants by the use of microorganisms (as bacteria) that can break down the undesirable substances [4]. Alexander [5] further said that bioremediation relies largely on the enzymatic activities of living organisms, usually microbes, to catalyze the destruction of pollutants and transformation of pollutants to less harmful forms. Then Das and Chandran [6] stressed that it is a complex process depending on many factors including ambient environmental conditions (such as pH, Temperature, nutrients and molecular oxygen), Composition of the microbial community and the nature and amount of pollution present. McCullough et al. [7] said among the microbial communities (organisms) that have been tested and proved as successful bioremidiators include algae, protozoa, bacteria, archaea and fungi. However, bacteria has been found to be the most successful bioremideator due to their mode of reproduction and nutrition [8]. This build the foundation of the work undertaken in this research because it is intended to use bacteria as the active microorganisms for bioremediation of tannery waste. The advantage of using bioremidiators to restore the environment is that the ecosystem is left with metabolites that are environmentally friendly [9]. Most of the metabolites left are usually biodegradable [10]. Microorganisms are so important in this process because they have extraordinary metabolic diversity [11]. Bioremediation has proved a better option in cleanups. For instance, natural attenuation that involves dispersion, biodegradation, irreversible sorption, and/or radioactive decay of contaminants in soils and ground waters has been made possible. Studies have showed that it causes a net reduction of contaminant toxicity, human and ecological risk. Despite the above advantages, environmental remediation has rarely been taken advantage of especially in tropics [12]. Furthermore, bioventing process has shown in a recent study by Hoeppel et al. [13], that it has a potential of stimulating the natural in situ biodegradation of contaminants in soil by providing air or oxygen to existing soil microorganisms. The study further added that it has been found out to be using low air flow rates to provide only enough oxygen to sustain microbial activity in the vadose zone. Another study by Benner et al. [14] showed that air sparging also known as in situ air stripping and in situ volatilization is an in situ remediation technique. It is used for the treatment of saturated soils and groundwater contaminated by volatile organic compounds (VOCs) like petroleum hydrocarbons which is a widespread problem for the ground water and soil health. Das and Chandran [6], showed that biostimulation that is to say, the addition of nutrients rich in nitrogen and phosphorus to a contaminated environment can stimulate microorganisms capable of biodegradation of the pollutants such as oil. Several developed countries have been noticeable to be using bioremediation for treatment of waste water. For example North America by Corseuil and Alvarez [15], Pakistan by Malik et al. [16] and China by Zhou et al. [17] have demonstrated bioremediation potential to clean up water containing heavy metals and organic pollutants. In North America, bioremediation has been used to clean up Canada’s DEW line sties in the arctic and has been used to clean up after oil spills off of Alaska [18].
Measuring extent of bioremediation
In several studies for example Tang et al. [19], McCullough et al. [7] and Kasiri et al. [20] that have been done on bioremediation, physiochemical parameters have been measured using spectrophotometry or chromatographic techniques with Mwinyihija et al. [21] emphasizing the use of biosensors. Bio-sensor is a sensor that integrates a biological element with a physiochemical transducer to produce an electronic signal proportional to a single analyte which is then conveyed to a detector [22]. Typical Sensing Techniques for Biosensors include Fluorescence, DNA Microarray, SPR Surface Plasmon resonance, Impedance spectroscopy’ SPM (Scanning probe microscopy, AFM, STM), QCM (Quartz crystal microbalance), SERS (Surface Enhanced Raman Spectroscopy) and Electrochemical techniques [22,23]. In the studies [22,24] biosensors were used and found to be successful at quantifying the physio-chemical parameters. Besides, biosensors are found to be portable, easy to manage with less training, faster at reading results and less costly. In addition, biosensors use portable batteries instead of power lines. These features make them suitable in the tropical setting where people are not very trained to operate sophisticated equipment. Also, the tropical setting is characterized with high load shading (power fluctuations). In fact, most of the bioremediation is done in rural settings with no power lines to support the spectrophotometry and chromatographic methods which are commonly used in the analysis. Biosensors can be fitted into the system as chips to give online processing readings as water flows in the treatment system enabling immediate action [25]. With this feature, it can be incorporated into the automated self-check system for easy effluent management from water treatment plants [26]. This would improve on the healthy sea food production if the effluent to lakes and rivers is made suitable to enter the aquatic environment. Although, biosensors are ought to have all the above advantages, no comparison has been made on the efficiency of the three methods in Uganda. This will be the focus of the current study.
Effluents from tanneries
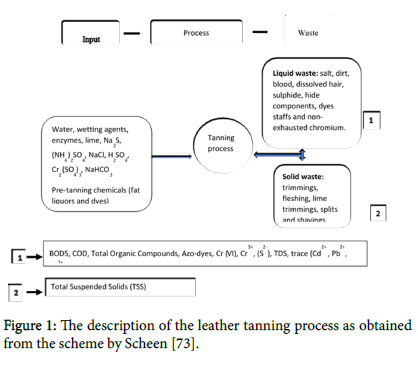
Water, wetting agents, enzymes, lime, sulphide, Na2S, ammonium sulphate, (NH4)2SO4, sodium chloride, NaCl, sulphuric acid, H2SO4, chromium sulphate Cr2(SO4)3 and sodium bicarbonate, NaHCO3 as well as pre-tanning chemicals like fat liquors and dyes are the most common inputs of tanneries as in the study by Amarnath and Krishnamoorthy [27] and summarized in the Figure 1 below.
Figure 1: The description of the leather tanning process as obtained from the scheme by Scheen [73].
The flow chart in Figure 1 shows the inputs of a leather tanning process and the associated wastes that can be either liquid waste or solid wastes. Liquid waste affects the following water parameters: BOD5, COD, Total organic compounds, Azo-dyes, Chromium (III), Chromium (VI), sulphide (S2-), Cadmium, Lead, Mercury and TDS while the solid waste does affect basically suspended solids accumulation.
It has been asserted by Sreeram and Ramasami [28] that the consumption of chromium during tanning process under optimal conditions of tanning is of the order of 40–70% of chromium that was added. This would mean 30–60% of chromium can be lost as waste to the surroundings after any tanning operation as described by Kolomazník et al. [29]. If the effluents are released to the environment with incomplete treatment, the amount of trivalent chromium may increase enormously within a year. This can be oxidized to hexavalent chromium by oxidizing agents to cause the contamination of soils and water, hence posing risk to human health and safety. Wastes are usually discharged into the environment with incomplete treatment. Thus, polluting aquatic and terrestrial ecosystems plus the atmosphere, making leather production unsustainable [30]. To solve this, an environmentally friendly method is needed i.e., bioremediation.
Characteristics of effluents
Nacheva et al. [31] asserted that all the stages of the 10 steps of the tanning process as in Table 1 are capable of producing enormous effluents that affect a vast number of environmental parameters. These parameters have an effect on the quality of water for drinking, soil for agriculture and air for breathing.
| Parameter | Process effluents | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| soak | liming | de-liming | pickling | Cr tanning | Tannins tanning | Re-tanning | Fat liquoring | Dyeing | |
| pH | 7.5 | 12.9 | 8.2 | 2.4 | 4.3 | 4.9 | 4.68 | 4.9 | 4.28 |
| TSS, mg/l | 6955 | 25310 | 1613 | 1315 | 1200 | 9350 | 960 | 675 | 521 |
| COD, mg/l | 9266 | 29600 | 8800 | 7620 | 11400 | 38570 | 5151 | 7587 | 8033 |
| BOD5, mg/l | 7920 | 10780 | 4200 | 3880 | 5700 | 12970 | 2230 | 2517 | 3607 |
| O &G, mg/l | 243 | 828 | 199 | 148 | 412 | 480 | 0 | 114 | 200 |
| S2-, mg/l | 5 | 3660* | 430 | 2 | 30 | 0 | 171 | 149 | 141 |
| k, mS/cm | 64.3 | 32.9 | 27.2 | 79.4 | 77.1 | 76.7 | 11.0 | 8.7 | 9.0 |
| TKN, mg/l | 380 | 2730 | 2205 | 1108 | 820 | 1266 | 510 | 275 | 220 |
| NH4-N, mg/l | 174 | 742 | 1210 | 947 | 719 | 940 | 255 | 140 | 189 |
| Cr3+, mg/l | 0 | 0 | 0 | 0 | 4500 | 0 | 50 | 1 | 1 |
Table 1: Characteristics of effluents in bovine tanneries with the complete manufacturing process as reported by Nacheva et al. [31]. *When hairburn method is used. The average obtained for hair-save spent liquors was 1860 mg/l.
Rationale
Leather tanning steps
Leather tanning process involves about ten steps as identified from Tünay et al. [32], on the study of the use and minimization of water in leather tanning processes and Thanikaivelan et al. [33] on the progress and recent trends in biotechnological methods for leather processing, this can be seen in Table 1 above. Each step uses a number of chemicals which are also released in effluents as depicted by Porter and Van der Linde [34] on their study on the trend towards a new conception of the environment-competitiveness relationship. The effluents can be in liquid and solid state in any tannery operation as it was said by Hu et al. [35] and Fathima et al. [36].
Emphasis of other researchers
In Uganda researchers have been putting much emphasis on numbers of tannery industries and not paying much attention to the quality of their effluents as said by Temsch and Marchich [37] in the UNIDO report on the strengthen the leather sector in Uganda project. For instance, it was reported that our Uganda has seven large tanneries and two small ones with installed capacity to process close to 10,000 hides and 47,000 skins per day as was reported in the newspaper entitled the daily monitor- Uganda on Dec 7, 2016. On the other hand, there isn’t any research focusing on the treatment of tannery effluents from these factories and tracing their danger to communities. On interviewing district residents, it was found out that some large-scale tanneries have a well-constructed effluent treatment plant but on the other hand others just release the effluents to the nearby swamp as reported by local residents. This gives a view that could be the source a serious stench in the villages along the wetland next to the tanneries.
Affected areas and their level of awareness
Locals in the areas affected include Ngendo-Masaka and those along the swamp and on a sad note some interviewed locals appeared not to know the cause of stench in the area and the operators of the tannery appear not to be concerned with the effects of the chemicals disposed to the swamp on the residents. On a surprise note, no researcher has picked courage to find out how much of the pollutants in the effluents are present in this wet land which is a water source of the locals in the area. On the other hand, Jinja has five large scale tanneries of which those near jinja municipality are also producing stench as reported by the residents interviewed from there and similarly no research is being conducted to follow the source and the effect of the stench. These concerns from local residents suggest a possibility of some of the tanneries to be operating without properly constructed effluent treatment plants.
Risk of tannery effluents
The fear is that people will continue to be exposed to the tannery effluents which posed a risk to human health and safety [38] that may lead to various infections in the country as stated by Wabinga et al. [39] and Wabinga et al. [40]. For this reason, the focus is to assess the suitability of bioremediation as a potential sustainable treatment technology for tannery effluents [41] and provide a solution to the affected tanneries in order to save the environment and residents at large. The point of contention is to curry out the baseline study on tannery effluents treatment status in Uganda in the same way as describe in Ali et al. [42] more still to characterize and determine physical chemical composition of tannery effluents as depicted in the study by Cooman et al. [43], further to devise culturing bacteria for bioremediation of the selected tannery pollutants following Tripathi et al. [44] and also identification of selectable marker for bacteria capable of bioremediation of heavy metals following Pieper and Reineke [45] method at the tannery sites. Conditions at the local site may not favour bioremediation active bacterial, therefore carrying out bioremediation at varying selected physical-chemical conditions as described by Noorjahan [46] and optimizing the synergic effect of the combined cultured bioremediation active bacteria to treat the selected pollutants in the tannery effluent in accordance with Verma and Singh [47].
Noticeable bioremediation studies
Researchers in several developed countries have been noticeable on bioremediation studies for treatment of tannery effluents. For example North America [15], Pakistan [16] and China [17] have carried out intensive research on bioremediation potential to clean up heavy metals and organic pollutants from tanneries before reaching the environment. Since the studies overseas have shown that it works, it can work also in Uganda.
To save Ugandans we need to research on both the numbers of tanneries producing leather and also on their ability to treat their effluents in order to keep the environment clean from tannery pollutants. The current work is addressing the treatment of tannery effluents option to make leather production in Uganda sustainable and marketable both nationally and internationally.
Methods
Literature search strategy
Relevant literature was selected from PubMed and google scholar because of their multidisciplinary nature of being used by researchers in the world while EndNote was selected to arrange the citations, references and bibliography.
Science Citation Index was also used, the database allows a researcher to identify which later articles have cited any particular earlier article, or have cited the articles of any particular author, or have been cited most frequently.
Endnote x7 was also adapted to fit citations into our text in this work. EndNote is a commercial reference management software package, used to manage bibliographies and references when writing essays and articles.
Selection of articles for review
Relevant studies were searched from PubMed and we were able to identify 135 articles all reflecting tannery effluent treatments methods. Unfortunately, none on PubMed reflected bioremediation of tannery effluent treatment technology method. On the other hand, google scholar reflected 5,100 articles with bioremediation technology as a possible method of treating tannery effluents. Neither PubMed nor google scholar reflected bioremediation technology as a method to treat tannery effluents in Uganda.
Results
Our literature review from PubMed and google scholar articles identified several provisions and mechanisms on which bioremediation of tannery effluents treatment is based. These mechanisms included Carrying out a baseline study on tannery effluents treatment status in Uganda; characterizing and determining physical chemical composition of tannery effluents; culturing bacteria for bioremediation of the selected tannery pollutants and Identification of selectable marker for bacteria capable of bioremediation of heavy metals at the study sites; carrying out bioremediation at varying selected physical-chemical conditions and optimizing the synergic effect of the combined cultured bioremediation active bacteria to treat the selected pollutants in the tannery effluent.
Selected literature also reflected that, Uganda has 7 large scale tanneries [48] which if not managed properly could pose a risk to Lake Victoria and hence on River Nile. The tanneries are indicated in the Table 2 below.
| Name | Products | Location of industry |
|---|---|---|
| Uganda Leather Industries Ltd, Jinja | Wet blue and finished leather | Jinja district |
| Sky Fat Tannery Co. Ltd (SFT) | Wet Blue Hides and Wet Blue Skins | Jinja district |
| Novelty Investment Tannery Ltd | Wet blue and Hides/skins | Masaka district |
| Uganda Fish Leather Tannery Ltd, Jinja |
Crust and finished skin from Nile Perch | Jinja district |
| Balawi Hides and Skins | Wet blue and Hides/skins | Busia District |
| SWT Tanners Ltd. | Wet blue and Hides/skins | Jinja District |
| Elgon Leather U Ltd | Wet blue and Hides/skins | Masaka district |
Table 2: Tanneries in Uganda. Source: National Association of Professional Environmentalists (NAPE)-Uganda.
Carrying out a baseline study on tannery effluents treatment status in Uganda
Locals of the affected areas in jinja and Masaka revealed that community knowledge about the tannery effluents in districts is very low but perception of tannery benefits is at large. In addition, resident’s knowledge about monitoring and specifically, community-based monitoring is very low as well as knowledge about implementers and donors, including who they perceive to be responsible for monitoring projects. We propose to carry out a baseline study on tannery effluents treatment status in the two most affected districts in the country, that is, Masaka and Jinja.
Characterizing and determining physical chemical composition of tannery effluents
It was revealed in the study by Gandini [49] that leather processing uses renewable resources for this reason it should provide a sustainable yield (the rate of harvest should not exceed the rate of regeneration). It was further stressed by Patel et al. [50] that there should be equivalent development of renewable substitutes for non-renewable resources to counteract environmental stress. The study by Islam et al. [51] suggested that the waste generation should not exceed the assimilative capacity of the environment. Kneese et al. [52] on the study of tannery effluents management stated that sustainable development primarily calls for renewable raw materials (controlled levels of chemicals), reduction and recycling of waste. It was reported by Kalyanaraman et al. [53] on tannery treatment study that controlling the limits of BOD5, COD, Total organic compounds, Azo-dyes, suspended solids, chromium(III), Cr3+, chromium(VI), Cr6+, sulphide, S2-, Cadmium, Lead, Mercury and total dissolved solids, TDS to recommended levels makes the tanning process sustainable. It was also stressed by Batzias et al. [54] on the study of tannery treatment methods that, BOD5, COD, Suspended Solids, Cr3+, S2-, TDS are the most important parameters to control when disposing the effluents to the environment. It was reported by Leta et al. [55] that BOD5 and COD levels in tannery effluents can be controlled with bioremediation techniques using microorganisms without affecting the environment. A study on the control of organic substances in tannery effluents revealed that Total organic compounds, can also be best controlled by the bioremediation techniques while preserving the environment [56].
Culturing bacteria for bioremediation of the selected tannery pollutants and identification of selectable marker for bacteria capable of bioremediation of heavy metals at the study sites
Bacteria of the type Pseudomonas aeruginosa were found to have activity with hydrocarbons in the study by Kiraye et al. [57] and also can use carbon from other organic compounds as their energy source [58]. Other species of Pseudomonas family like Pseudomonas fluorescens, Pseudomonas ambigua, Enterobacter cloacae and Bacillus subtilis were reported to have the ability to covert chromium (VI) to chromium (II) [59]. It was reported that Cadmium can be used by bacteria thereby reducing it in the environment [60] the case in place being Rhodobacter sphaeroides . It was further found out by Abbas et al. [61] on the isolation, Identification, and Characterization of Cadmium Resistant Pseudomonas sp. study that, Pseudomonas sp. can remove almost 70% of cadmium from samples in their log-phase and this is affected by temperature and pH of the surroundings. Lead [62] asserted that Lead (Pb) is among the most largely existing heavy and toxic metals in the environment. It was also pointed out by further explained in his study that, utilization of lead resistant bacteria to remove this toxic metal could be exploited in the lead bioremediation method. Arsenic from the effluents can be effected by processes that favour simultaneous removal of iron [63] and on comparing what Cavalca et al. [64] did on the study of arsenic remediation, it was revealed that Rhizobacterial community can do the work. It was further reported in the study of mercury bioremediation that, Pseudomonas putida has the ability to reduce divalent mercury sulphides to Mercury metal, in this way mercury ions as well as sulphides can be bioremediated using these bacteria species [65]. Gremion et al. [66] on his analytical study on soil bacterial communities using 16S rRNA clone libraries revealed that actinobacteria might be a dominant part of the metabolically active bacteria in heavy metal remediation. Furthermore, Nogales et al. [67] with a study on Combined use of 16S ribosomal DNA and 16S rRNA to study the bacterial community of polychlorinated biphenyl-polluted soil also pointed out that Actinobacteria might be a dominant part of the metabolically active bacteria in dealing with heavy metal remediation.
Carrying out bioremediation at varying selected physicalchemical conditions
A study on in-situ bioremediation of organic pollutants asserted that treating waste or pollutants by the use of microorganisms that can break down the undesirable substances need specific physical-chemical conditions [68]. According to Oller et al. [69], the specific physical chemical conditions of the tannery effluents at the point of disposal would be the most ideal as the microbial load for the study is used to the local conditions of the area. Furthermore, Saxena and Bharagava [70] on the study of similar context showed that using the microorganisms of the area achieves better results if they happen to be bioengineered for bioremediation of the tannery effluents. These studies are in line with the overall focus of this study.
Optimizing the synergic effect of the combined cultured bioremediation active bacteria to treat the selected pollutants in the tannery effluent
The author was reported by Popp et al. [71] on the study entitled Bacterial diversity in the active stage of a bioremediation system that, combined effect of cultured bioremediation active bacteria show a good degradation power of effluent pollutants. Shah [72] on optimization of parameters in a tannery effluent treatment plants also asserted that, it is laborious to optimize new strains of bacteria to perform bioremediation of these effluents than to use dominant micro organised that have already colonized the area. This is in agreement with the current study, because the focus is to isolate the dominant bacteria which will later be transformed by plasmid insertion carrying bioremediation trait and reintroducing them back to the site to do the job.
Comments
The status of the country in as far as tannery effluents is concerned needs to be deeply explained in order to maintain the environmental cleanliness. The levels of contamination of Masaka wetland and lake Victorian needs to be studied as these are sources of food and water for the residents. Failure to have information on how much pollution tanneries have contributed to these resources might continue to increase the unexplained occurrences of unclear illnesses to people hence creating sick communities that may fail to be productive to the country. In addition, the areas affected in central Uganda serve as food baskets of the region. Polluting soils and water of this area will have a pronounced effect on the agricultural and horticultural activities hence jeopardizing food security of the region as it is noticed in some parts of these villages.
References
- Ang EL, Zhao H, Obbard JP (2005) Recent advances in the bioremediation of persistent organic pollutants via biomolecular engineering. Enzyme Microb Technol 37: 487-496.
- Dua M, Singh A, Sethunathan N, Johri A (2002) Biotechnology and bioremediation: successes and limitations. Appl Microbiol Biotechnol 59: 143-152.
- Gavrilescu M (2010) Environmental biotechnology: achievements, opportunities and challenges. Dynamic Biochemistry, Process Biotechnology and Molecular Biology 4: 1-36.
- Gavrilescu M, Chisti Y (2005) Biotechnology—a sustainable alternative for chemical industry. Biotechnology Advances 23: 471-499.
- Alexander M (1999) Biodegradation and Bioremediation. Gulf Professional Publishing, Houston, Texas, USA.
- Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int 2011: 1-13.
- McCullough J, Hazen T, Benson S (1999) Bioremediation of metals and radionuclides: what it is and how it works. Lawrence Berkeley National Laboratory.
- Malik A (2004) Metal bioremediation through growing cells. Environ Int 30: 261-278.
- Mitsch WJ, Jørgensen SE (2004) Ecological engineering and ecosystem restoration. John Wiley & Sons, Hoboken, New Jersey, USA.
- Bouwer EJ, Zehnder AJ (1993) Bioremediation of organic compounds—putting microbial metabolism to work. Trends Biotechno 11: 360-367.
- Jain RK, Kapur M, Labana S, Lal B, Sarma PM, et al. (2005) Microbial diversity: application of microorganisms for the biodegradation of xenobiotics. Curr Sci 89: 101-112.
- Boopathy R (2000) Factors limiting bioremediation technologies. Bioresour Technol 74: 63-67.
- Hoeppel RE, Hinchee RE, Arthur MF (1991) Bioventing soils contaminated with petroleum hydrocarbons. JÂ Ind Microbiol 8: 141-146.
- Benner ML, Stanford SM, Lee LS, Mohtar RH (2000) Field and numerical analysis of in-situ air sparging: a case study. J Hazard Mater 72: 217-236.
- Corseuil HX, Alvarez PJ (1996) Natural bioremediation perspective for BTX-contaminated groundwater in Brazil: effect of ethanol. Water Sci Technol 34: 311-318.
- Malik AH, Khan ZM, Mahmood Q, Nasreen S, Bhatti ZA (2009) Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J Hazard Mater 168: 1-12.
- Zhou Y, Yang H, Hu H, Liu Y, Mao Y, et al. (2006) Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of north China. Aquaculture 252: 264-276.
- Poland JS, Mitchell S, Rutter A (2001) Remediation of former military bases in the Canadian Arctic. Cold Reg Sci Technol 32: 93-105.
- Tang L, Zeng G, Liu J, Xu X, Zhang Y, et al. (2008) Catechol determination in compost bioremediation using a laccase sensor and artificial neural networks. Anal Bioanal Chem 391: 679-685.
- Kasiri S, Doudi M, Tabatabaee M (2016) Bioremediation of Wastewater Contaminated by Crude Oil by Bacillus Isolated from Esfahan Oil Refinery.
- Mwinyihija M, Strachan NJC, Meharg AA, Killham KS (2005) Biosensor based toxicity dissection of tannery and associated environmental samples. Journal of the American Leather Chemists Association 100: 380-395.
- Bosch ME, Sánchez AJR, Rojas FS, Ojeda CB (2007) Recent development in optical fiber biosensors. Sensors 7: 797-859.
- Nobile J, Roth T, Rearick T, Schultz J, Rothberg J, et al. (2014) Apparatus and methods for performing electrochemical reactions. Patent and Trademark Office, USA.
- Ravindra NM, Prodan C, Fnu S, Padronl I, Sikha SK (2007) Advances in the manufacturing, types, and applications of biosensors. Jom 59: 37-43.
- Diamond D, Coyle S, Scarmagnani S, Hayes J (2008) Wireless sensor networks and chemo-/biosensing. Chemical Reviews 108: 652-679.
- Rodriguez-Mozaz S, de Alda MJL, Barceló D (2007) Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography–mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J Chromatogr A 1152: 97-115.
- Amarnath JS, Krishnamoorthy S (2001) Study on relationship between productivity, inputs and environmental quality in tannery effluent affected farms of Tamil Nadu. Water Resources Management 15: 1-15.
- Sreeram KJ, Ramasami T (2003) Sustaining tanning process through conservation, recovery and better utilization of chromium. Resources, Conservation and Recycling 38: 185-212.
- Kolomaznik K, Adámek M, Andel I, Uhlirova M (2008) Leather waste—potential threat to human health, and a new technology of its treatment. J Hazard Mater 160: 514-520.
- Harrison RM (1982) Pollution: causes, effects and control. Royal Society of Chemistry, UK.
- Nacheva PM, Chávez GM, Herrera MJ (2004) Alternative treatment strategy for tannery water reuse and material recovery. Water Sci Technol 50: 121-130.
- Tünay O, Kabdaşlı I, Orhon D, Cansever G (1999) Use and minimization of water in leather tanning processes. Water Sci Technol 40: 237-244.
- Thanikaivelan P, Rao JR, Nair BU, Ramasami T (2004) Progress and recent trends in biotechnological methods for leather processing. TRENDS Biotechnol 22: 181-188.
- Porter ME, Van der Linde C (1995) Toward a new conception of the environment-competitiveness relationship. J Econ Perspect 9: 97-118.
- Hu J, Xiao Z, Zhou R, Deng W, Wang M, et al. (2011) Ecological utilization of leather tannery waste with circular economy model. J Clean Prod 19: 221-228.
- Fathima N, Rao R, Nair BU (2012) Tannery solid waste to treat toxic liquid wastes: a new holistic paradigm. Environ Eng Sci 29: 363-372.
- Temsch R, Marchich M (2002) Unido programs funded by austria to strengthen the leather sector in Uganda.
- Harrison RM (2001) Pollution: causes, effects and control. Royal Society of Chemistry, UK.
- Wabinga H, Parkin D, Wabwire-Mangen F, Nambooze S (2000) Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer 82: 1585-1592.
- Wabinga HR, Mugerwa JW, Parkin DM, Wabwireâ€Mangen F (1993) Cancer in Kampala, Uganda, in 1989–91: changes in incidence in the era of AIDS. Int J Cancer 54: 26-36.
- Mwinyihija M (2007) Assessment of anaerobic lagoons efficacy in reducing toxicity levels of tannery effluent in Kenya. Res J Environ Toxicol 1: 167-175.
- Ali Z, Malik RN, Qadir A (2013) Heavy metals distribution and risk assessment in soils affected by tannery effluents. Chem Ecol 29: 676-692.
- Cooman K, Gajardo M, Nieto J, Bornhardt C, Vidal G (2003) Tannery wastewater characterization and toxicity effects on Daphnia spp. Environmental Toxicology: An International Journal 18: 45-51.
- Tripathi M, Vikram S, Jain RK, Garg SK (2011) Isolation and growth characteristics of chromium (VI) and pentachlorophenol tolerant bacterial isolate from treated tannery effluent for its possible use in simultaneous bioremediation. Indian J Microbiol 51: 61-69.
- Pieper DH, Reineke W (2000) Engineering bacteria for bioremediation. Curr Opin Biotechnol 11: 262-270.
- Noorjahan C (2014) Physicochemical characteristics, identification of fungi and biodegradation of industrial effluent. J Environ Earth Sci 4: 32-39.
- Verma T, Singh N (2013) Isolation and process parameter optimization of Brevibacterium casei for simultaneous bioremediation of hexavalent chromium and pentachlorophenol. J Basic Microbiol 53: 277-290.
- Muramuzi F (2009) The leather tannery industry in Uganda: risks to the environment and to human health.
- Gandini A (2008) Polymers from renewable resources: a challenge for the future of macromolecular materials. Macromolecules 41: 9491-9504.
- Patel M, Crank M, Dornburg V, Hermann B, Roes A, et al. (2006) Medium and long-term opportunities and risks of the biotechnological production of bulk chemicals from renewable resources.
- Islam MS, Khan S, Tanaka M (2004) Waste loading in shrimp and fish processing effluents: potential source of hazards to the coastal and nearshore environments. Mar Pollut Bull 49: 103-110.
- Kneese AV, Ayres RU, d'Arge RC (2015) Economics and the environment: a materials balance approach. Routledge, Abingdon, UK.
- Kalyanaraman C, Kanchinadham SBK, Vidya Devi L, Porselvam S, Rao JR (2012) Combined advanced oxidation processes and aerobic biological treatment for synthetic fatliquor used in tanneries. Ind Eng Chem Res 51: 16171-16181.
- Batzias FA, Sidiras DK, Politi DV (2012) Contribution to Tannery Waste Water Treatment for Chromium Removal/Recycle by Means of Cation Exchange Resins. Chem Eng Trans 29: 1297-1302.
- Leta S, Gumaelius L, Assefa F, Dalhammar G (2004) Identification of efficient denitrifying bacteria from tannery wastewaters in Ethiopia and a study of the effects of chromium III and sulphide on their denitrification rate. World J Microbiol Biotechnol 20: 405-411.
- Murugananthan M, Raju GB, Prabhakar S (2004) Separation of pollutants from tannery effluents by electro flotation. Sep Purif Technol 40: 69-75.
- Kiraye M, John W, Gabriel K (2016) Bioremediation Rate of Total Petroleum Hydrocarbons from Contaminated Water by Pseudomonas aeruginosa Case Study: Lake Albert, Uganda. J Bioremed Biodeg 7: 335.
- Samanta SK, Singh OV, Jain RK (2002) Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol 20: 243-248.
- Chandra R, Bharagava RN, Kapley A, Purohit HJ (2011) Bacterial diversity, organic pollutants and their metabolites in two aeration lagoons of common effluent treatment plant (CETP) during the degradation and detoxification of tannery wastewater. Bioresour Technol 102: 2333-2341.
- Bai HJ, Zhang ZM, Yang GE, Li BZ (2008) Bioremediation of cadmium by growing Rhodobacter sphaeroides: kinetic characteristic and mechanism studies. Bioresour Technol 99: 7716-7722.
- Abbas SZ, Rafatullah M, Ismail N, Lalung J (2014) Isolation, identification, and characterization of cadmium resistant Pseudomonas sp. M3 from industrial wastewater. J Waste Manage 2014: 1-6.
- Kang CH, Oh SJ, Shin Y, Han SH, Nam IH, et al. (2015) Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol Eng 74: 402-407.
- Zouboulis AI, Katsoyiannis IA (2005) Recent advances in the bioremediation of arsenic-contaminated groundwaters. Environ Int 31: 213-219.
- Cavalca L, Zanchi R, Corsini A, Colombo M, Romagnoli C, et al. (2010) Arsenic-resistant bacteria associated with roots of the wild Cirsium arvense (L.) plant from an arsenic polluted soil, and screening of potential plant growth-promoting characteristics. Syst Appl Microbiol 33: 154-164.
- Essa A, Macaskie L, Brown N (2002) Mechanisms of mercury bioremediation. Portland Press Limited, UK.
- Gremion F, Chatzinotas A, Harms H (2003) Comparative 16S rDNA and 16S rRNA sequence analysis indicates that Actinobacteria might be a dominant part of the metabolically active bacteria in heavy metalâ€contaminated bulk and rhizosphere soil. Environ Microbiol 5: 896-907.
- Nogales B, Moore ER, Llobet-Brossa E, Rossello-Mora R, Amann R, et al. (2001) Combined use of 16S ribosomal DNA and 16S rRNA to study the bacterial community of polychlorinated biphenyl-polluted soil. Appl Environ Microbiol 67: 1874-1884.
- Perelo LW (2010) In situ and bioremediation of organic pollutants in aquatic sediments. J Hazard Mater 177: 81-89.
- Oller I, Malato S, Sánchez-Pérez J (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409: 4141-4166.
- Saxena G, Bharagava RN (2015) Persistent organic pollutants and bacterial communities present during the treatment of tannery wastewater. CRC Press, Boca Raton, USA.
- Popp N, Schlömann M, Mau M (2006) Bacterial diversity in the active stage of a bioremediation system for mineral oil hydrocarbon-contaminated soils. Microbiology 152: 3291-3304.
- Shah S (2002) Structural And Functional Characterization Of Pentachlorophenol-Degrading Bacterial Consortium For Treatment Of Tannery Effluent. Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India.Â
Citation: Kiraye M, Wanasolo W, Nalwanga R, Mwinyihija M (2018) Bioremediation of Tannery Effluents for Sustainable Production of Leather in Uganda: Literature Review. J Bioremediat Biodegrad 9: 448. DOI: 10.4172/2155-6199.1000448
Copyright: © 2018 Kiraye M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7055
- [From(publication date): 0-2018 - Jul 04, 2025]
- Breakdown by view type
- HTML page views: 5860
- PDF downloads: 1195