Biotransfromation of Dibenzothiophene by Resting Cells of a Newly Isolated Serratia marscens Sp. Strain Originated from Industrial Wastewater
Received: 23-Mar-2018 / Accepted Date: 30-Apr-2018 / Published Date: 02-May-2018 DOI: 10.4172/2155-6199.1000439
Abstract
Five isolates able to use dibenzothiophene (DBT) as a sole sulfur source with high rates were selected to investigate their potentialities as biocatalysts of biodesulfurization reactions. The desulfurizing activities of selected strains were investigated in growing and resting cell state. The biodegradation yields were considerably higher in resting cell reaction especially for two strains tentatively named S1 (98.8%) and S27 (97.5%). These results insinuated that biodegradation activity was mainly related to secondary metabolism on these strains. Their biotransformation potentialities were also evaluated under various conditions in order to evaluate their stability in both aqueous and organic media; and their sensitivity to the presence of squalene, used in this study as a representative of hydrocarbons in petroleum. The results showed that the 5 selected strains were still active in the presence of 95% of squalene but no transformation observed at 99% of squalene. The sulfur substrate selectivity was studied in presence of other organosulfur compounds such us dimethylsulfoxide DMSO and benzothiazole BTH. The presence of these substrates inhibited the DBT uptake by the bacteria and consequently decreased its degradation rate. Moreover, conventional analysis of 16S ribosomal DNA sequencing showed that the strain-with highest bioconversion rates-belonged to Serratia marcescens species. To far of our knowledge, Serratia sp. was rarely reported as DBT degrder strain. Thus, the rate and the extent of the biodesulfurization reaction, exhibited by the strain Serratia marcescens S27, suggested that it could be used in practical scale.
Keywords: Dibenzothiophene; Biodesulfurization; Organosulfur compounds; Xenobiotics; Biodegradation; Serratia sp
Introduction
Organic sulfur compounds including dibenzothiophene (DBT), benzothiophene (BT) and their alkylated derivatives in fossil fuels have been the major cause of worldwide environmental problems including air pollution and acid rain [1] and healthy problems such us carcinogenicity to humans [2,3]. In addition, with the increasing demands for energy and more stringent environmental policies, deep desulfurization of petroleum is becoming more and more required [4].
Biodegradation of organosulfur compounds has been previously studied using different strains likeCorynebacterium sp. [5,6], Paenibacillus sp. A11-2 [7], Pseudomonas sp. [8-10], Rhodococcus sp.[11], Mycobacterium sp. [12,13], Sphingomonas sp. [14], Bacillus subtilis WU-S2B [15], Gordonia sp. [16-18], and Brevibacterium sp. [ 19].
DBT has been used as a polyaromatic sulfur compound model for the isolation and characterization of bacteria capable of bioconversion of recalcitrant organosulfur compounds found in variety of fossil fuels [ 20,21]. The dibenzothiophene (DBT) shows high toxicity and mutagenicity which may affect human health and ecosystems in general [2]. A small number of genera such as Rhodococcus and Gordonia are known to remove sulfur from DBT via a sulfur-specific pathway [22,23]. Rhodococcus and Gordonia have the specificity to oxidize sulfur in DBT without cleaving the carbon skeleton into lowcarbon- number hydrocarbons. This property has a great advantage when applied to industrial processes.
DBT biodesulfurization pathway, so called 4S route, is based on multi-enzymatic system involving four consecutive reactions [24]. The reaction started by DBT oxidation using two monooxygenases: DBT monooxygenase (DszC) and DBT sulfone monooxygenase (DszA) and ended by the convertion to 2-hydroxybiphenyl as the end product using the desulfinase enzyme (DszB) [25].
Microbial desulfurization of DBT have an increasing interest as it is easy to maintain, does not require hydrogen gas, offers less environmental disturbance lower operating costs comparing to hydrodesulfurisation [20]. However, biodesulfurization yield could be limited due to the bacterial enzymatic activities and substrate transport rate across the cell membrane [26-28].
Considering the relevance of the overall impact of pollution generated by DBT, we report in this study the characterization of DBT bioconversion using five strains isolated from wastewater in minimum medium with DBT as the sole sulfur source. Examination of DBT consumption by selected strains was investigated under growing and resting cell state. DBT biodegradation in biphasic medium was also examined in order to simulate a real conditions and in presence of other organosulfur compounds to examine to broad specificity of the finally selected strain. Identification of stains was achieved using 16S rRNA identification. Detection of desulfurizing genes was also highlighted in order to characterize the metabolic pathway used by the strain with a high degradation potential.
Materials And Methods
Chemicals
Dibenzothiophene (DBT) (Wako Pure Chemicals Co., Japan), Benzothiazole (BTH, Wako Pure Chemicals Co., Japan) and Dimethylsulfoxide (DMSO; Wako Pure Chemicals Co., Japan) were of liquid chromatography grade and other chemicals were of analytical grade, commercially available. All other reagents were of the highest purity commercially available and were used without further purification. All media and solutions were prepared with deionized water.
Enrichment and isolation
Wastewater was obtained from different industrial facilities discharge (tannery, agro-food, plastic industry) in Tunisia. Several strains growing with DBT as a sole source of sulfur were isolated from sampled wastewater. Thus, 10 mL of wastewater were suspended in 100 mL of BHMS medium (composition: 1 g/L KH2PO4; 0.2 g/L K2HPO4; 0.2 g/L MgSO4H2O; 0.02 g/L CaCl2; 1 g/L NH4NO3; and 2 drops of FeCl3 60%), inoculated with 0.25 mM of DBT as a unique sulfur source and 5 g/L glucose as a carbon source. The resulted suspension was incubated on a rotary shaker (200 rpm) at 30°C until turbidity for 24 h. Then, the mixture was centrifuged at 14000 rpm for 3 min and the culture was then transferred into fresh medium which was inoculated with 5% v/v inocula followed by a sub-culturing with 2% v/v inocula five times [29]. Bacterial isolation was made by streaking a singlecolony onto the same medium containing 1.5% agar.
Culture conditions
The five selected strains cultivated in 50 mL of culture medium in 250 mL flasks were inoculated with 25 mg/L of DBT and incubated for 120 hours at 30°C under shaking conditions. During cultivation time, aliquots of the culture were sampled for measurements of DBT concentrations by HPLC and cell growth by turbimetric assay at an Optical Density of 660 nm (O.D.).
The culture medium was prepared as mentioned by Izumi et al. [30]. The standard minimal sulfate-free medium (SMM) contained: 5 g carbon source (glucose or glycerol), 0.5 g of KH2PO4, 4 mg of K2HPO4, 1 g of NH4Cl, 0.2 g MgCl2.6H2O, 0.02 g of CaCl2, 0.01 g of NaCl, 10 mL of metal solution in 1000 mL of deionized water (pH 7,7). The metal solution contained 0.5 g of FeCl2.4H2O, 0.5 g of ZnCl2, 0.5 g of MnCl2.4H2O, 0.1 g of Na2MoO4.2H2O, 0.05 g of CuCl2, 0.05 g of Na2WO4.2H2O and 120 mmol of HCl in 1000 ml of deionized water. The sole sulfur source in this experiment was DBT (25 mg.L-1 added in solution in ethyl alcohol).
DBT bioconversion by resting cells
Cells were cultivated in 50 mL of SMM in 250 mL flasks. They were harvested at the end of the growth phase by centrifugation (20000 g for 15 min), washed twice with sterilized deionized water and once with 0.1 M phosphate buffer (pH 7.0) and suspended in an appropriate volume of the phosphate buffer to adjust the cell concentration to an optical density at 660 nm of 10. The cell suspension was heated at 121°C for 5 min in case of heat inactivated cells to examine the possibility of substrates adsorption to the resting cells. The reaction was started by adding DBT used here as a substrate. DBT was added at a finale concentration of 1 g/L. The bioconversion experiments were carried out in 250 mL flasks at 30°C and 200 rpm. Samples were collected every 2 hours of intervals for assay of DBT by HPLC. Resting cells activity was determined by the rate of DBT consumption during 24 h of the experiment.
Bioconversion reactions in biphasic medium
The capability of selected strains to convert DBT in two-phase system was also investigated. Thus, we used a biphasic medium with squalene as a representative of hydrocarbons. The two-phase desulfurization systems contains resting cells suspended in 25 mL phosphate buffer (0.1 M; pH 7) inoculated with 3 mM DBT and the appropriate concentration of squalene: 0, 50, 90, 95, 99 vol.%; with respect to the total volume. The reaction was stopped by centrifugation and sampling of the organic phase. Substrate concentrations were determined in the organic phase by HPLC analysis. Residual DBT degradation activity was calculated as the percentage of the activity in presence of squalene relative to the activity measured in absence of squalene (100% activity=activity in aqueous phase).
Degradation of other organosulfur compounds
To investigate the range of organic sulfur compounds that can be assimilated by selected strains, two compounds were used: benzothiazole BTH and dimethylsulfoxide DMSO. The organic sulfur compounds; in addition to DBT were added as the sole sulfur source in growth experiments at a final concentration of 30 mg/L. The cultivation was extended for 7 days at 30°C. By the end of the experiment, HPLC analysis was performed using aqueous phase. Cell growth was estimated by measuring the optical density at 660 nm.
HPLC analysis
All compounds concentrations were measured by High Performance Liquid Chromatography (HPLC) (Type LC-10A, Shimadzu Co., Kyoto, Japan), equipped with a diode-array detector and an automatic injector.
To analyze by HPLC 1.5 mL of sample was centrifuged at 13000 rpm for 5 min, into Eppendhorf tubes (aqueous samples with acetonitrile, in ½ dilutions). When both phases were separated, the compounds in oil once dissolved was measured with a column for reverse-phase analysis (Type VP-ODS Shim-pack, 150 mm 4.6 mm, Shimadzu Co., Kyoto, Japan). In this case, isocratic elution was performed with a 55:45 (v/v) acetonitrile: water mobile phase at 1 mL min-1. DBT was detected at a wave length value of 280 nm.
16S rRNA identification
The Genomic DNA were prepared using ISOPLANT (Nippon-gene Co., Tokyo, Japan) and used for PCR. The 16S rRNA gene locus was amplified by PCR as described previously [31]. The blast program (http://ncbi.nlm.nih.gov/BLAST/NCBI, MD, USA) was used for the gene homology search with the standard program default. The phylogenetic tree based on the 16S rRNA gene sequence was constructed by the neighbor-joining method [32], with the Kimura two-parameter model as a distance corrector [33] after alignment of sequences with the CLUSTAL X multiple sequence alignment program [ 34].
Detection of dszA, dszB, and dszC genes based on PCR
isolated strain was screened by PCR for the presence of the desulfurizing genes; dszC, dszA and dszB genes. They are:
• dszA forward: 5’-TCGATCAGTTGTCAGGGG-3’, dszA reverse: 5’-GGATGGACCGACTGTTGAG-3’
• dszB forward: 5’-ATCGAACTCGACGTCCTCAG-3’, dszB reverse: 5’-GGAACATCGACACCAGGACT-3’
• dszC forward: 5’-CTGTTCGGATACCACCTCAC-3’, dszC reverse: 5’-ACGTTGTGGAAGTCCGTG-3’. All PCR amplifications were performed under conditions according to Duarte et al. [29] protocol.
Results
Isolation and selection of efficient strains for DBT biotransformation
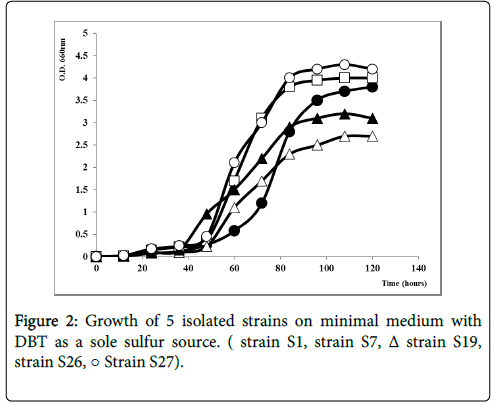
Five pure strains able to grow with DBT (Figure 1) as a sole sulfur source were isolated form industrial wastewaters facilities. Strains were subjected to the analysis of DBT consumption evaluated as DBT biodegradation. The bacterial growth was continued for 120 h, in the parallel way with the depletion of DBT. As shown in Figure 2, the strains tentatively named S1, S27 and S7 grew well on DBT as a sole sulfur source with a specific growth rate respectively equal to 0.055 h-1, 0.056 h-1 and 0.052 h-1. However, S19 and S26 had a slower growth under the same conditions with μmax of 0.036 h-1 and 0.049 h-1 respectively. The growth profile by S27 showed a Short lag phase of growth early in the first 10 hours, followed by the exponential growth phase from 30 hours. The maximum growth was observed after 80 hours of incubation followed by a small stationary phase maintained for 120 hours. In contrast, for the strain S26 and S1, there was a Slight inhibition in the period 0-8 hours of growth and the maximum growth rate was observed respectively at 96 h and 108 h. The selected strains belong probably to different species due to their different growth curve.
Table 1 showed the DBT degradation using bacterial growing cells supplemented with 0.25 mM DBT as the sole sulfur source and 5 g/L of glucose as carbon source. The degradation proceeded with the cell growth, and most of DBT was depleted at 120 h of reaction time. Under growing conditions the highest DBT bioconversion rate was observed for S27 and S1 which were respectively 93% and 90.7% within 120 hours of reaction time.
| Cell concentration | Residual DBT (mM) | Degradation (%) | |
|---|---|---|---|
| (OD 660) | |||
| S1 | 3.8 | 0.023 | 90.7 |
| S7 | 4 | 0.076 | 69.48 |
| S19 | 2.7 | 0.088 | 64.64 |
| S26 | 3.1 | 0.073 | 70.62 |
| S27 | 4.2 | 0.016 | 93.5 |
| no cells | -- | 0.25 | 0 |
Table 1: DBT degradation by growing cells of selected bacteria.
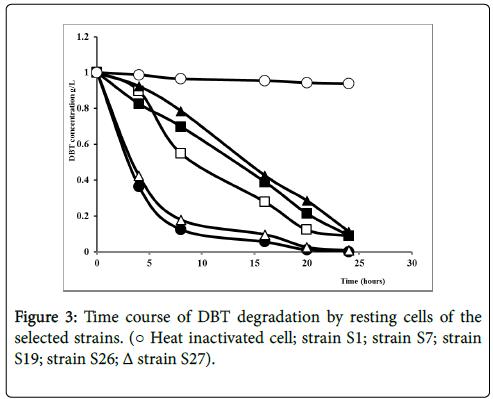
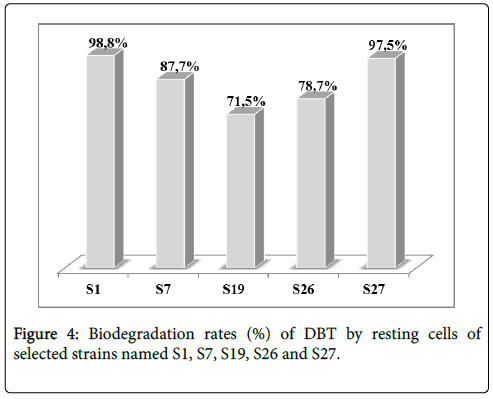
To better understand the biodegradation of dibenzothiophene using selected strains, the experiment was carried out with bacterial resting cells. Figure 3 showed the transformation of DBT by the resting cells of the 5 selected strains. Nearly, all the strains degrade the DBT after 25 hours of experiment time. Obviously, DBT conversion rate with resting cells was considerably higher for all strains comparing to the growing cell conditions in a shorter time. The resting cells of S1 and S27 displayed the highest degradation in a shorter time rate evaluated respectively as 98.8% and 97.5% as illustrated in Figure 4.
No transformation was observed with the heat inactivated cells, indicating that transformation was proceeded by a biochemical reaction; neither by absorption of substrate by the cells nor by spontaneous transformation.
DBT bioconversion in biphasic medium
The effect of the presence of an oil fraction is essential in this kind of processes as it can affect both the biocatalyst growth and the 4Spathway development. Mass transfer and toxic effects are expected [3]. Table 2 reported the desulfurization assay in aqueous and two-phase systems by resting cells of the 5 isolated strains. The effect of the presence of an organic medium on desulfurization activities of the strains was studied using squalene which is supposed to be a good model of diesel oil in terms of hydrophobicity. Various concentrations of squalene were used, ranging from 50% to 99% of the total reaction volume. Activities in squalene compared to activities in aqueous phase are shown in Table 2 with the consideration that: 100% activity=activity in aqueous phase.
| Squalene concentration (%) | Residual DBT-consuming activity (%) | ||||
|---|---|---|---|---|---|
| S1 | S7 | S19 | S26 | S27 | |
| 50 | 64 | 58 | 50 | 62 | 74 |
| 90 | 40 | 33 | 26 | 39 | 58 |
| 95 | 30 | 20 | 11 | 18 | 28 |
| 99 | 0 | 0 | 0 | 0 | 0 |
Table 2: Effect of squalene concentration on DBT-consuming activities in biphasic medium. Squalene concentration (vol.%) is calculated with respect to the total reaction volume. Residual activity is given as the percentage of the activity in presence of squalene relative to the activity measured in absence of squalene (100% activity=activity in aqueous phase).
According to the Table 2, DBT consuming activities of all strains decreased with the addition of squalene. As expected, no biotransformation activity was detected in micro-aqueous media with 99% squalene. However, all strains were still biologicaly active in 95% squalene with distinctive rates. DBT consumption yield was still high in the presence of 90% squalene for the strains S1 and S27; 40 and 58% respectively. However, the higher sensitivity of selected strains due to the presence of squalene was observed from 90% of squalene. The strain S27 was the strain displaying the highest activities activities in 95% squalene.
In fact, the effect of the presence of squalene on DBT-consuming activities depended upon strain [35]. DBT-degrading activity was less stable in the presence of the squalene. Nevertheless, the 5 selected strains were still active in the presence of 95% of squalene (more than 5% of the activity in aqueous medium). However, no activity was detected in microaqueous (99% squalene) media. Our results confirmed that an extended range of solvent can be used for a biodesulfurization process. Moreover, the sensitivity of the strains to the presence of solvent is variable. For instance, S1 was the strain displaying the highest DBT-degrading activity when the reaction medium contained 95% squalene whereas S27 was the best one in the presence of 50% solvent.
Characterization of S27 strain
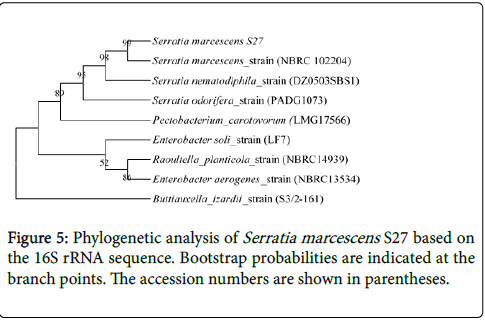
The isolate S27 was found to be aerobic, motile and Gram negative bacteria. 16S rRNA sequencing of isolate S27 showed a similarity of 99% with that of Serratia marcescens (Gene bank accession No. NBRC_102204) and hence categorized as Serratia marscens S27 strain (phylogenetic tree shown in Figure 5). The 16S rDNA sequence of isolate S27 has been submitted to the Gene bank (accession No. KY780304).
DBT desulfurization property of R. erythropolis IGTS8, first bacterium to be reported for possessing the ability of removing sulfur from DBT [22] has been shown to be due to the presence of the desulfurization genes i.e., dsz ABC operon [36]. Therefore, PCR was carried out for the molecular evidence for the presence of dsz genes in the isolate S27. Analysis of the sequences of the PCR products revealed 100% identity with the corresponding dsz genes (dsz A, dszB, dszC) from R. erythropolis IGTS8. This is in agreement with the conserved nature of the dsz genotype of desulfurizing bacteria. Genes involved in DBT metabolism have been found to be present in almost all of the DBT degrading bacteria and have been shown to have almost 70% of the sequence homology [1].
DBT bioconversion in the presence of various sulfur sources
Many types of organo-sulfur compounds apart from DBT such as BT and alkylated DBT’s derivatives have been shown to be present in diesel, crude oil and industrial wastewater [6]. To assess the broad specificity of the isolated stain; Serratia marscens S27 sp., the strain was subjected to a growth test in presence of DBT, Benzothiazole (BTH) as an aromatic organosulfur compound and Dimethylsulfoxide (DMSO) as an aliphatic organosulfur compound.
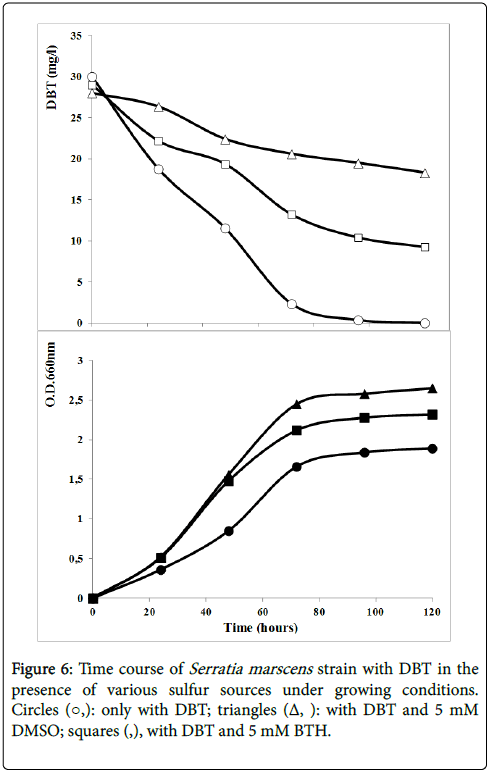
Figure 6 showed the DBT degradation by strain S27 in the presence of various sulfur sources under growing condition. DMSO is an aliphatic organosulfur compound. DMSO is more realistic sulfur source than DBT for the production of industrial amounts of biocatalyst because of its cost and availability. The strain grew considerably better with dimethyl sulfoxide and DBT than with DBT as the sole sulfur source. However, DBT consuming rate reached 33.9% in the presence of DMSO comparing to 98% with DBT only. The DBT degradation seemed to be inhibited by the presence of DMSO. Similar result was obtained in the presence of BTH. The bacterial growth was enhanced by the presence of the aromatic organosulfur compound BTH (Figure 6). However, DBT consumption was about 68% in the presence of BTH.
Discussion
Five strains named S1, S7, S19, S26 and S27; selected from the initial 35 isolates able to grow on DBT as the sole sulfur source were isolated from industrial wastewater samples. First steps of selection included high DBT biotransformation activity of growing and resting cells. The five selected strains had different DBT degradation rates. Further investigation of their properties confirmed that they had different behavior towards DBT. The desulfurizing activity of growing cells was considerably high for the two strains S1 and S27 (90.7 and 93.5% respectively). The DBT consuming rate was considerably relevant in resting cell state for all strains comparing to growing state. This behavior could be explained by the fact that degradation ability is probably due to a second metabolism on these strains. Knowing the suitable cell state for DBT biotransformation could have an impact for practical application.
Moreover, keeping high bioconversion activities in an organic medium is a prerequisite for an industrial biodesulfurization process. Thus, the effect of the presence of squalene was measured on the DBT consuming activity. The DBT degradation activity for all strains was reduced comparing to their activity in aqueous medium. Nevertheless, the five strains were still active in the presence of 95% squalene. This result is mainly due to the byproduct accumulation in the oil phase. However, almost no activity was detected in microaqueous (99% squalene) media for all strains; the organic phase prevents the substrate to reach the bacterial cell. In fact, bioconversion of DBT seems to be a too complex process involving many steps to be active in such a medium.
The strain S27 having the highest DBT bioconversion rate was identified as Serratia marcescens strain. The detection of the desulfurization genes i.e., dsz ABC operon lead us to conclude that 4S pathway is mainly involved in DBT degradation. Based on previous research, the 4S pathway is a specific pathway for biodesulfurization of DBT and its conversion into 2-Hydroxybiphenyl. In this case the carbon skeleton of DBT is released intact [2]. Furthermore, Bioconversion pathways of DBT by bacteria, has been widely studied, as: Arthrobacter [37], Brevibacterium [38], Mycobacterium and Rhodococcus rhodochrous IGTS8 [39]. These strains were only able to remove sulfur of DBT converting to compound hydroxybiphenyl 2(2- HBP) [40]. These microorganisms have the specificity to selectively remove organic sulfur without degrading the carbon atoms. To the best of our knowledge, very few reports have demonstrated the desulfurization potentials of genus Serratia sp. reported in this study.
Even if DBT is generally taken as the model compound of heterocyclic organosulfur OSC, other OSC represent a high proportion of these molecules, known to be recalcitrant to hydrodesulfurization. In this study two OSCs were taken into account: DMSO (aliphatic OSC) and BTH (aromatic OSC). The presence of BTH and DMSO has obviously enhanced the bacterial growth related to the abundance of sulfur element. The uptake of DMSO and BTH by the strain Serratia marscens S27 was considerably higher than DBT uptake. Consequently, DBT bioconversion considerably decreased. This suggested that the degradation activity of DBT on this strain seemed to be inhibited by these compounds and the strain predominantly utilized these compounds as sulfur source rather than DBT. This behavior could be a limitation when applied in practical scale containing a mixture of organosulfur compounds.
Conclusion
Five isolates able to desulfurize DBT in growing and resting state were isolated from industrial wastewater. The biotransformation rate was considerably higher in resting cell state than in growing cell state which meaned that DBT biodegradation rate belonged to the secondary metabolism on theses strains. The strain with high degradation rate was identified as Serratia marcescens strain. However, the bioconversion of DBT using this strain is highly influenced by the organosulfur compounds existant in the medium. Along with the fact that the strain expressed desulfurizing activity in a growing and resting cell state, as shown previously, this bacterium could contribute to the DBT containing wastewater treatment. Although it appears that the S. marcescens is capable of desulfurizing DBT, the bioconversion of this compound still required a lot of research to discover the real mechanism of the metabolic pathway of desulfurization of DBT.
References
- Monticello DJ (2000) Biodesulfurization and upgrading of petroleum distillates. Curr Opin Biotechnol 11: 540-546.
- Araújo HWC, Freitas Siva MC, Lins CIM, Nascimento AE, Silva CAA, et al. (2012) Oxidation of dibenzothiophene (DBT) by Serratia marcescens UCP 1549 formed biphenyl as final product. Biotechnology for biofuels 5: 33.
- Caro A, Boltes K, Leton P, Garcia-Calvo E (2008) Biodesulfurization of Dibenzothiophene by growing cells of Pseudomonas putida SCCT 5279 in biphasic medium. Chemosphere 73: 663-669.
- Bahuguna A, Lily MK, Munjal A, Singh R, Dangwal K (2011) Desulfurization of dibenzothiophene (DBT) by a novel strain Lysinibacillus sphaericus DMT-7 isolated from diesel contaminated soil. J Environ Sc 23: 975-982.
- Maghsoudi S, Kheirolomoom A, Vossoughi M (2000) Selective desulfurization of dibenzothiophene by newly isolated Corynebacterium sp. Strain P32C1. Biochem Engin J 5: 11-16.
- Omori T, Monna L, Saiki Y, Kodama T (1992) Desulfurization of dibenzothiophene by Corynebacterium sp. Strain SY1. App Environ Microbiol 58: 911-915.
- Konishi J, Ishii Y, Onaka T, Okumura K, Suzuki M (1997) Thermophilic carbon-sulfur bond targeted biodesulfurization. App Environ Microbiol 63: 3164-3169.
- El-Bassi L, Iwasaki H, Oku H, Shinzato N, Matsui T (2010) Biotransformation of benzothiazole derivatives by the Pseudomonas putida strain HKT554. Chemosphere 81: 109-113.
- Setti L, Lanzarini G, Pifferi PG (1997) Whole cell biocatalysis for an oil desulfurization process. Fuel Process Tech 52: 145-153.
- Luo MF, Xing JM, Gou ZX, Li S, Liu GL, et al. (2003) Effects of different sulfur sources on growth and desulfurization activity of Nocardia globerula R-9. J Chem Indus Engin 54: 1413-1417.
- Oshiro T, Izumi Y (1999) Microbial desulfurization of organic sulfur compounds in petroleum. Biosc Biotech Biochem 63:1-9.
- Kayser KJ, Cleveland L, Park H, Kwak J, Kolhatkar A, et al. (2002) Isolation and characterization of a moderate thermophile, Mycobacterium phlei GTIS10, capable of dibenzothiophene desulfurization. App Microb Biotech 59: 737-746.
- Li FL, Xu P, Ma CQ, Luo LL, Wang XS (2003) Deep desulfurization of hydrodesulfurization-treated diesel oil by a facultative thermophilic bacterium Mycobacterium sp. X7B. FEMS Microbiol Lett 223: 301-307.
- Lu J, Nakajima-Kambe T, Shigeno T, Ohbo A, Nomura N, et al. (1999) Biodegradation of dibenzothiophene and 4,6-dimethyl dibenzothiophene by Sphingomonas paucimobilis strain TZS-7. J Biosc Bioeng 88: 293-299.
- Kirimura K, Furuya T, Nishii Y, Ishii Y, Kino K, Usami S (2001) Biodesulfurization of dibenzothiophene and its derivatives through the selective cleavage of carbon-sulfur bonds by a moderately thermophilic bacterium Bacillus subtilis WUS2B. J Biosc Bioeng 91: 262-266.
- El-Bassi L, Shinzato N, Namihira T, Oku H, Matsui T (2009) Biodegradation of thiodiglycol, a hydrolyzate of the chemical weapon yperite, by benzothiophene-desulfurizing bacteria. Journal of Hazardous Materials 167: 124-127.
- Rhee SK, Chang JH, Chang YK, Chang HN (1998) Desulfurization of dibenzothiophene and diesel oil by a newly isolated Gordona strain, CYKS1. App Environ Microbiol 64: 2327-2331.
- Chang JH, Chang YK, Ryu HW, Chang HN (2000) Desulfurization of light gas oil in immobilized-cell systems of Gordonia sp. CYKS1 and Nocardia sp. CYKS2. FEMS Microbiol Lett 182: 309-312.
- Setti L, Farinelli P, Martino SD, Frassinetti S, Lanzarini G, et al. (1999) Developments in destructive and non-destructive pathways for selective desulfurization in oil-biorefining processes. App Microbiol Biotech 52: 111-117.
- Labana S, Pandey G, Jain RK (2005) Desulphurisation of dibenzothiophene and diesel oils by bacteria. Lett Appl Microbiol 40: 159-163.
- Li F, Zhang Z, Feng J, Cai X, Xu P (2007) Biodesulfurization of DBT in tetradecane and crude oil by a facultative thermophilic bacterium Mycobacterium goodie X7B. J Biotechnol 127: 222-228.
- Kayser KJ, Bielaga-Jones BA, Jackowski K, Odusan O, Kilbane JJ (1993) Utilization of organosulfur compounds by axenic and mixed cell cultures of Rhodococcus rhodochrous IGTS8. J Gen Microbiol 139: 3123-3129.
- Li W, Wang MD, Chen H, Chen JM, Shi Y (2006) Biodesulfurization of dibenzothiophene by growing cells of Gordonia sp. in batch cultures. Biotechnol Lett 28: 1175-1179.
- Caro A, Boltes K, Leton P, Garcia-Calvo E (2007) Dibenzothiophene biodesulfurization in resting cell conditions by aerobic bacteria. Biochem Eng J 35: 191-197.
- Davoodi-Dehaghani F, Vosoughi M, Ziaee AA (2010) Biodesulfurization of dibenzothiophene by a newly isolated Rhodococcus erythropolis strain. Bioresource technology 101: 1102-1105.
- Guobin S, Jianmin X, Chen G, Huizhou L, Jiayong C (2005) Biodesulfurization using Pseudomonas delafieldii in magnetic polyvinyl alcohol beads. Lett Appl Microbiol 40: 30-36.
- Mohebali G, Ball AS, Rasekh B, Kaytash A (2007) Biodesulfurization potential of a newly isolated bacterium, Gordonia alkanivorans RIPI90A. Enzyme Microbial Technol 40: 578-584.
- Rashtchi M, Mohebali GH, Akbarnejad MM, Towfighi J, Rasekh B, et al. (2006) Analysis of biodesulfurization of model oil system by the bacterium, strain RIPI-22. Biochem Eng J 29: 169-173.
- Duarte GF, Rosado AS, Seldin L, De Araujo W, Van Elsas JD (2001) Analysis of bacterial community structure in sulfurous-oil-containing soils and detection of species carrying dibenzothiophene desulfurization (dsz) genes. Appl Environ Microbiol 67: 1052-1062.
- Izumi Y, Oshiro T, Ogino H, Hine Y, Shimao M (1994) Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1. Appl Environ Microbiol 60: 223-226.
- Matsui T, Shinzato N, Tamaki H, Muramatsu M, Hanada S (2009) Shinella yambaruensis sp. nov., a 3-methyl-sulfolane assimilating bacterium isolated from soil. Int J Syst Evol Microbiol 59: 536-539.
- Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406-425.
- Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16: 111-120.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 25: 4876-4882.
- Abbad-Andaloussi S, Lagnel C, Warzywoda M, Monot F (2003) Multi-criteria comparison of resting cell activities of bacterial strains selected for biodesulfurization of petroleum compounds. Enz Microbiol Tech 32: 446-454.
- Denis-Larose C, Labbe D, Bergeron H, Jones AM, Greer CW, et al. (1997) Conservation of plasmid-encoded dibenzothiophene desulfurization genes in several rhodococci. Appl Environ Microbiol 63: 2915-2919.
- Jong-Su S, Young-Soo K, Qing XL (2006) Degradation of dibenzothiophene and carbazole by Arthrobacter sp P1-1. Inter biodetr biodegrad 58: 36-43.
- Van Afferden M, Schacht S, Klein JE, Truper HG (1990) Degradation of dibenzothiophene by Brevibacterium sp. Arch Microbiol 153: 324-328.
- Li F, Xu P, Ma C, Zheng Y, Qu Y (2003) Biodesulfurization of dibenzothiophene by a newly isolated bacterium Mycobacterium sp X7B. J Chem Enginee 36: 1174-1177.
- Wang P, Krawiec S (1994) Desulfurization of dibenzothiophene to 2-hydroxybiphenyl by some newly isolated bacterial strains. Arch Microbiol 161: 266-271.
Citation: El-Bassi L, Nefissi RO, Shinzato N, Ghrabi A (2018) Biotransfromation of Dibenzothiophene by Resting Cells of a Newly Isolated Serratia marscens Sp. Strain Originated from Industrial Wastewater. J Bioremediat Biodegrad 9: 439. DOI: 10.4172/2155-6199.1000439
Copyright: © 2018 El-Bassi L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5461
- [From(publication date): 0-2018 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 4461
- PDF downloads: 1000