Characteristics of HIV/TB Co-Infected Patients with Data of T-Spot TB Testing: Review of Practice Pattern in an HIV Outpatient Clinic in New Orleans, Louisiana
Received: 12-Oct-2017 / Accepted Date: 20-Nov-2017 / Published Date: 27-Nov-2017
Abstract
Objectives: We studied the characteristics of a cohort of HIV/ TB Infection co-infected patients in a HIV Outpatient Clinic in New Orleans to identify the methodology of screening, diagnosis and treatment of TB Infection (TBI).
Methods: A three-year retrospective review of 1,937 HIV-infected subjects co-infected with Mycobacterium tuberculosis (MTB) in which a T-SPOT®.TB test was done, and a borderline or positive T-SPOT®.TB result was found, were selected. HIV status, adherence to HIV antiretroviral therapy (ART), and clinic follow-up information were abstracted from provider notes. When repeat T-SPOT®.TB tests were performed, the results were abstracted to determine concordance and rates of conversion and reversion.
Results: Out of 1,937 HIV-infected subjects reviewed from 1/2012 to 12/2014, 68 (3.5%) showed a positive or borderline T-SPOT®.TB test. The cumulative prevalence of positive T-SPOT®.TB tests was 2.4%. In 19 subjects who were initially T-SPOT®.TB-test positive and had serial testing done, 11 tests (58%) reverted to negative. In 15 subjects with borderline test results the first time, tests in 10 subjects (67%) changed to negative; 2 changed to positive, and these subjects then progressed to active TB during the following 14 months. Eighty-seven percent of patients in this clinic were treated for TBI.
Conclusion: Other than HIV viral load and CD4-cell count, maintenance of normal weight and adherence to medical care may have also played a part in preventing the progression of TBI to active TB in this cohort. The propensity for change in the T-SPOT®.TB from borderline to negative and the rate of reversion from positive to negative confirms the importance of the test’s borderline category. The reasons for these variations warrant further understanding and review. Although the variations observed in some test results may impede screening for TBI with its limitations notwithstanding, targeted screening, especially in this cohort, remains the backbone of TB control.
Keywords: Tuberculosis; MTB; HIV; IGRA; TB infection
Introduction
Human immunodeficiency virus (HIV) infection is an important risk factor for the progression or reactivation of latent tuberculosis infection (TBI) [1]. In the US, the incidence of tuberculosis (TB) disease among HIV-infected subjects is more than double the rate of non-HIV infected patients (6.8 versus 3.0 per 100,000 population) and is a prevalent cause of death [2,3].
Screening for and treating TBI play an important role in helping to control TB [4]. The progression of TBI to active TB disease is more common among the elderly and in immune-compromised patients whose TBI is undiagnosed and who are therefore not receiving preventive therapy [4-8]. Among HIV patients receiving treatment for TBI, the expected incidence of TB disease is 5.2/100 p-y (95% CI: 3.4- 7.8) [9]; however, without treatment the progression of LTB to disease ranges from 7% to 20% [10-12]. The progression to active disease is also higher among those patients with poor adherence to medical care, erratic compliance to antiretroviral therapy (ART) for HIV, and/ or intermittent adherence to TBI prophylaxis [13]. The rate of noncompletion of isoniazid preventive therapy (IPT) among patients with HIV was reported being as high as 41% [14]. This non-adherence to therapy is associated with double the rate of TBI’s progression to active TB disease [9].
The T-helper cell count (CD4) is considered a marker of clinical evolution for the progression of TBI to active disease, and the development of active TB is a criterion for the definition of AIDS [15]. However, there is evidence that although CD4 T-cells play a significant role in the immune defense against Mycobacterium tuberculosis (MTB), TBI may progress to primary/lymph node mediastinal TB in patients with a CD4 T-cell count higher than 200 cells/mL [6,16] and to parenchymal pulmonary TB among HIV patients with a CD4 T-cell count between 171 and 962 cells/mL [6,17,18]. Furthermore, a higher CD4 T-cell count was found to be associated with previous tuberculosis infection and a lower CD4 T-cell count associated with the recurrence of TB in high-prevalent settings [13]. These findings suggest that there may be concomitant factors other than cell-mediated immunity and CD4 T-cell count involved in the progression or non-progression of TBI to active TB in HIV populations.
Materials and Methods
We studied a co-infected HIV-TBI cohort of patients from 2012 to 2015 to determine the incidence and progression of TBI to active TB disease among HIV-infected patients and assess other concomitant factors that may play a part in the progression or lack of progression of TBI to active TB.
A retrospective cohort study was implemented to collect clinical data from HIV-infected subjects with positive or borderline interferongamma release assay (IGRA) tests (T-SPOT®.TB test, Oxford Immunotec, Ltd.) performed between January 2012 and December 2014. The T-Spot TB test is an enzyme-linked immunospot assay on peripheral blood mononuclear cells using MTB Complex specific antigen peptides. The result is reported as spot forming cells representing number of interferon-gamma producing T cells. A positive result is when the spot count in TB antigen wells exceeds the specific threshold compared to control.
Clinical and laboratory information was abstracted from the first documented positive or borderline IGRA test and continued to be abstracted until September 2015. Data on demographics, including age, race, sex and country of origin, as well as history of BCG vaccination, history of treatment for TBI and/or active TB. Clinical status was assessed by abstracting health parameters at the time of the first TB screening showing either a positive or borderline T-SPOT®.TB result. These parameters included CD4 count, viral load (VL), height and weight on the first and last visits, and the highest and lowest values of VL and CD4 T-cells between the date of the first positive or borderline IGRA result and the last visit before September 2015. Concurrent medical conditions that may have affected the progression of TBI to active TB were also identified.
Adherence to medical care was defined as the patient’s attending the HIV outpatient clinic. Compliance with medication was assessed as positive if it was documented in the physicians’ assessment notes [19-21].
Serial T-SPOT®.TB test results in the observation period were abstracted along with the corresponding dates. Conversion was defined as a negative result with a subsequent positive result on serial repeat testing, and reversion was defined as a positive result with a subsequent negative result. The cutoff point for a borderline T-SPOT®.TB test result was between 5 and 7 spots, and a positive result was equal to or higher than 8 spots as per FDA-approved T-SPOT®.TB test characterization.
Follow-up times and subjects lost to follow-up were assessed by collecting dates of the first and last visit attended. Minimal time of follow-up after a TBI diagnosis was set at 9 months to include patients in the analysis of data. The time between the date of the HIV diagnosis and the first T-SPOT®.TB test date was defined as length of time being HIV infected.
Statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc. Cary, NC). Continuous variables were summarized by median, mean, and range; proportions were compared using chi-square or Fisher’s exact tests, and t-statistic was used to test for group mean differences. Multivariable linear regression was used to test for the association of continuous outcomes with predictor variables; p values were two tailed, and statistical significance was set at 0.05. The study was approved by the University Medical Center and the Louisiana State University Health Sciences Center IRB.
Results
A total of 1,937 HIV-infected patients was analyzed to select those with a positive or borderline T-SPOT®.TB test during the study period (n=68) (3.5%). The other 1,869 (96.5%) patients remained with a negative T-SPOT®.TB test or were not tested with the T-SPOT®.TB test throughout the observation period.
The average time of patient follow-up was 2.4 years and varied between 9 months and 3 years. Most subjects were males (72%) of African-American (AA the) race (75%) who were US born (97%) and had a median age of 51 years and an average of 12 years since being diagnosed with HIV. Adherence to HIV medication was 67%, based on provider documentation. Adherence to HIV care, as determined by HIV medical visits, was 90%. The median CD4 cell count at the time of first TB test was 482 cells/mL, and the median VL was 198 copies/ mL; the median of highest VL test during the observed period was 424 copies/mL, and the median of lowest CD4 T-cell values was 379 (Tables 1 and 2).
| Characteristic | Patient n | % | LTB test (+) (n=42) | (Brdl) (n=23) | TBdx (n=3) |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 49 | 72 | 74% | 70% | 67% |
| Female | 19 | 28 | 26% | 30% | 33% |
| Race | |||||
| AA | 52 | 75 | 79% | 70% | 67% |
| W | 12 | 19 | 10% | 22% | 0 |
| Other | 4 | 6 | 2% | 8% | 33% |
| Age (Years) | |||||
| (median, range) | 51 | 28-72 | 51 | 53 | 42 |
| Country of Birth | |||||
| US | 66 | 97 | 100% | 96% | 67% |
| Not US | 2 | 3 | 0% | 4% | 33% |
*brdl = borderline result TBdx = Active TB Diagnosis
Table 1: Demographic characteristics (n=68).
| Characteristic | Measure | Range, (%) | LTB test (+) (n=42) | (Brdl) (n=23) | TBdx (n=3) |
|---|---|---|---|---|---|
| BMI (W/H2) | |||||
| At LTBI diagnosis (md) | 27 | 16-43 | 26 | 27 | 27 |
| At end of study (md) | 26 | 15-41 | 26 | 27 | 24 |
| HIV diagnosis (Y) | 12 | 0.5 – 27 | 13 | 11 | 11 |
| Study FU period (mo) (md) | 28 | 3 – 46 | 28 | 35 | 23 |
| <9 months | 6 | 10 (%) | 4 | 2 | 0 |
| 9+ months | 62 | 90 (%) | 38 | 21 | 3 |
| CD4 count (md, range) | |||||
| At first LTBI test | 482 | 95 – 1297 | 550 | 468 | 235 |
| Lowest count in period | 379 | 14 – 1278 | 434 | 374 | 235 |
| Highest count in period | 583 | 95 – 1394 | 640 | 552 | 563 |
| VL copies (md, range) | |||||
| At first LTBI test | 198 | nd* – 694100 | 196 | 204 | 11330 |
| Lowest value in period | nd | nd – 1093 | nd* | 33 | nd* |
| Highest value in period | 424 | nd – 694100 | 362 | 497 | 117538 |
| Adherence to HIV treatment (prvdr’s assessm notes) | |||||
| Yes | 47 | 69 (%) | 67% | 74% | 67% |
| No | 21 | 31 (%) | 33% | 26% | 33% |
| Adherence to HIV care (visits every 6 months) | |||||
| Yes | 61 | 90 (%) | |||
| No | 7 | 10 (%) | 7% | 17% | 0% |
*Non-detectable **md=median; brdl=borderline
Table 2: Clinical characteristics (n=68).
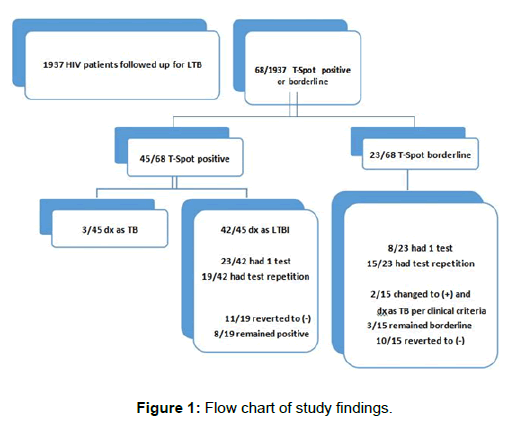
Sixty-eight of the 1,937 HIV-infected patients assessed (3.5%) showed an initial T-SPOT®.TB test with positive or borderline results at some point in the period of observation. Forty-five were positive (45/68 or 66%), and 23 were borderline (23/68 or 34%). Out of 45 subjects with a positive, 42 were classified as TBI and 3 were diagnosed as active TB (Figure 1).
Thirty-four (50%) of the 68 patients analyzed with either a positive or borderline initial test had a T-SPOT®.TB test repeated during the observed period. The median time that elapsed between tests varied between 299 and 360 days; mean intervals between tests were shorter when the results were borderline than when they were initially positive (312 vs. 490 days, p=0.06).
A sub-analysis of the serial testing results showed that out of 42 patients with an initial positive test, 19 had repeat testing. The reasons for the repeat testing were not clear. Eleven of those 19 (58%) reverted to a negative result, and 8 remained positive. Out of the 23 patients with an initial borderline test, 15 had repeat testing. Of these 15 cases, 10 changed to negative (67%), 2 converted from borderline to positive and then progressed to active TB, and 3 remained as borderline (Figure 1 and Table 3).
| Test Series Result | n | % |
|---|---|---|
| A: Initially Positive (n=19) | ||
| Reversion (positive to negative) | 11 | 58% |
| Concordant positive | 8 | 42% |
| B: Initially Borderline (n=15) | ||
| Change of result (borderline to negative) | 10 | 67% |
| Concordant borderline | 3 | 20% |
| Change of result (borderline to positive) | 2 | 13% |
| Total # of Tests Repeated | 34 | |
Table 3: Results with serial repeat T. spot TB test during study period (n=34).
The 68 patients with either a positive or borderline T-SPOT®.TB test analyzed at the outset were further classified in 5 clinical groups, as follows: Group A: patients with active TB at the beginning of the study (n=3), Group B: patients with a history of prior TB disease (n=3), Group C: patients with a history of prior TBI (n=23), Group D: patients diagnosed with TBI for the first time (n=21), and Group E : patients with borderline T-SPOT®.TB results on initial evaluation (n=18) (Table 4).
| **TB stage | n | % | Repeat testing | Change to (-) (%) | Change pattern | |
|---|---|---|---|---|---|---|
| A: Active TB | 3 | 4 | - | - | - | |
| B: Prior TB disease | 3 | 4 | 2 | 2/2 | (100%) | + to |
| C: Prior H of LTBI | 23 | 34 | 12 | 5/12 | (42%) | + to |
| 1/12 | (8%) | b to | ||||
| D: New LTBI Dx | 21 | 31 | 8 | 4/8 | (50%) | + to |
| E: Non- TB with borderline result | 18 | 27 | 12 | 9/12 | (75%) | b to - |
| Total | 68 | 100 | 34 | 21/34 | (62%) | |
** By Medical History; b= borderline
Table 4: Initial characteristics of study population related to stage/diagnosis of TB and test change rate from initial positive and borderline on repeat testing.
At the first T-SPOT®.TB test, laboratory readings were higher among active TB patients (median: 48 spots), followed by those with prior TBI disease (median 24 spots). Those subjects diagnosed as having current TBI showed the least number (median 12 spots). On serial testing, the percentage of T-SPOT®.TB test result changes was highest among patients with prior TB disease and treatment (100%) (Table 4, Group B) and was 50% and 42% among subjects with current or prior diagnoses of TBI, respectively. In total, 21/34 (62%) showed change in readings on serial testing irrespective of clinical classification (Tables 3 and 4).
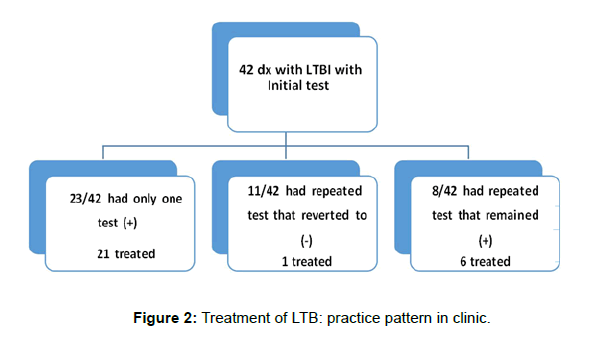
The clinic-practice pattern of TBI treatment in this cohort is shown in Figure 2. Of the 23 patients diagnosed as TBI based on an initial and the only positive T-SPOT®.TB test, 21 were treated; 11 had repeat testing and reverted to negative, and of these one was treated. Eight remained positive on repeat testing, and 6 of those 8 were treated for TBI. Thus 27/31 patients with defined TBI were treated.
Clinical differences between patients with TBI (defined as those with one positive T-SPOT®.TB test or consistent positive tests) who did not progress to active TB and those TBI patients who did progress to active TB were identified. These differences showed that the group that did not progress to active TB maintained similar weight over time (BMI change: -0.04 vs. -3.3, p=0.1) and better adhered to HIV medications and follow-up. Multivariate analyses of data revealed CD4 cell count did not correlate either with the progression of TBI to active TB or with the T-SPOT®.TB test result (Table 5).
| Clinical Factor | SPOT®.TB test positive | |||||
|---|---|---|---|---|---|---|
| Progression to TB (n=2) | No progression to TB (n=42) | p | ||||
| CD4 count | 296 | 527 | 0.1 | |||
| HIV-Viral Load | 235460 (Md:11330)† | 498 (Md: 190) | 0.4 | |||
| BMI_start (W/H2) | 27.3 | 26.1 | 0.6 | |||
| BMI_end (W/H2) | 24 | 26 | 0.6 | |||
| BMI change (W/H2) | -3.3 | -0.36 | 0.1 | |||
| T-Spot result (spot count) | 36 | 21 | 0.1 | |||
| Compliance with ARV (Y/N) | 66% | 68% | 0.9 | |||
| Association of T-Spot.TB test result and CD4 count | ||||||
| Correlation* | p | Regression-coeff‡ | p | R2 (p) | ||
| CD4 count | -0.12 | 0.4 | -0.002 | 0.8 | 0.19 (-0.03) | |
| Compliance with HIV-ARV | -0.2 | 0.1 | -4.43 | 0.3 | ||
| Time since HIV diagnosis (Y) | 0.02 | 0.9 | 0.40 | 0.1 | ||
| Age (Y) | -0.35 | 0.00 | -0.55 | 0.00 | ||
| Gender (M/F) | 0.14 | 0.3 | 2.84 | 0.44 | ||
†Md: median; *Pearson correlation coefficient; BMI: body mass index; ARV: anti-retroviral medication; ‡ linear regression model: SPOT®.TB test result = CD4 count + compliance with HIV-ARV + Time elapsed since HIV diagnosis + Age + Gender
Table 5: Clinical characteristics of patients with LTBI diagnosis by TB status and association between SPOT®.TB test and CD4 T-cell count.
Discussion
These findings align with previously published reports [19], which found a 10% rate of progression of TBI to active TB among HIV patients over an observation period of 12 months. In our study, the follow-up time may not have been long enough for TBI to progress to active TB. In those cases which progressed to TB after TBI diagnosis, the time elapsed between the diagnosis of TBI and the appearance of TB signs or symptoms varied between 4 and 14 months (n=2).
T-SPOT®.TB test repetitions serve as a surrogate for the confirmation of infection with Mycobacterium TB, with increased test sensitivity to 85.2% [18]. The rate of T-SPOT®.TB test reversion from positive to negative was noted to be 58%. Eighty percent of the borderline tests resulted in either a positive or a negative result upon repeat testing, with a change to negative in 67% of cases and to positive in 13% of subjects. Similar findings were noted in previously reported publications [18,20-22].
We also observed that the higher T-SPOT®.TB test readings (spot numbers) correlated more strongly with patients’ history of TB disease than to their current TBI status. This observation needs to be validated further.
The pre-analytical process and analytical phases of the T-SPOT®.TB test used in this study were constant. During the observation period, the phlebotomist team was the same, blood-sample handling and routine for the preparation of blood tubes was standardized, and blood samples arrived at the main laboratory in Memphis, TN (Oxford Diagnostic Laboratory) within 36 hours. However, reproducibility of the test could be influenced by lot-to-lot variability, intrinsic immunomodulation due to patient comorbidities, variable release of interferon-gamma, microbial contamination of blood samples, or blood-storagetemperature variation and other factors. [23-26]. The reasons for the variability in results thus remain to be yet fully understood.
As observed by others, the analysis of the effect of CD4 T-cell counts on the quantification of the T-SPOT®. A TB result was not statistically significant [19,22,27]. It is important to note that this cohort of HIVinfected patients was not in an advanced stage of their HIV/AIDS disease and had medium to elevated levels of CD4 cell counts (90% >200 cells/mL).
The results of our study showed that the T-SPOT®.TB results were independent of the level of immunosuppression status in HIVpositive patients. This was different from previous observations where that led to the conclusion that TB screening test performance is only meaningful in HIV patients with a cell count >300 [28-30]. Thus, there may be other factors that affect the test performance.
Conclusion
T-SPOT®.TB test results are independent of the immunosuppression status of patients with HIV. Prognostic biomarkers other than HIV viral load and CD4 cell count may explain the progression or lack thereof of TBI to TB disease in this endemic setting. Clinicians specializing in HIV/AIDS and TB may repeat the T-SPOT®.TB test as means of determining true sensitivity in selected cases and better identifying patients with unequivocal TBI. The propensity for change in the T-SPOT®.TB from borderline to negative and the rate of reversion from positive to negative validates the importance of the test’s borderline category. The variations observed in some test results may impede screening for TBI at a clinical level. The reasons for these variations in interferon-gamma release assays warrant further understanding and review. Nevertheless, its limitations notwithstanding, targeted screening with IGRA, especially in this cohort, remains the backbone of TB control.
Acknowledgment
The authors wish to acknowledge Dr. Lynn Besch, LSUHSC Section of Infectious Diseases, for her review and suggestions related to the preparation of this manuscript.
References
- Markowitz N, Hansen NI, Hopewell PC, Glassroth J, Kvale PA, et al. (1997) Incidence of tuberculosis in the United States among HIV-infected persons. Ann Intern Med 126: 123-132.
- Alami NN, Yuen M, Miramontes R,Pratt R, Price SF, et al. (2014) Trends in tuberculosis - United States 2013. MMWR 63: 229-233.
- Gupta RK, Lucas SB, Fielding KL, Lawn SD (2015) Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: A systematic review and meta-analysis. AIDS 29: 1987-2002.
- Lawn SD, Bekker LG, Wood R (2005) How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS 19: 1113-1124.
- Palmisano L, Vella S (2011) A brief history of antiretroviral therapy of HIV infection: Success and challenges. Ann 1st Super Sanita 47: 44-48.
- Jones BE, Young SMM, Antoniski D, Davidson PT, Kramer F, et al. (1993) Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Resp Dis 148: 1292-1297.
- Karo B, Hass W, Kollan C, Bartmeyer BG, Hamouda O, et al. (2014) Tuberculosis among people living with HIV/AIDS in the German ClinSurv Cohort: Long-term incidence and risk factors. BMC Infect Dis 14: 148.
- Assebe LF, Reda HL, Wubeneh AD, Lerebo WT, Lambert SM (2015) The effect of isoniazid preventive therapy on incidence of tuberculosis among HIV-infected clients under pre-ART care in Jimma, Ethiopia: A retrospective cohort study. BMC Public Health 15: 346.
- Pulido F, Peña JM, Rubio R, Moreno S, González J, et al. (1997) Relapse of tuberculosis after treatment in human immunodeficiency virus-infected patients. Arch Intern Med 157: 227-232.
- Sester M, Van Leth F, Bruchfeld J, Bumbacea D, Cirillo D, et al. (2014) Risk assessment of tuberculosis in immunocompromised patients: A TABNET study. Am J Resp Crit Care Med 190: 1168-1176.
- Aichelburg MC, Rieger A, Breitenecker F, Pfistershammer K, Tittes J, et al. (2009) Detection and prediction of active tuberculosis disease by a whole-blood interferon-gamma release assay in HIV-1-infected individuals. Clin Infect Dis 48: 954-962.
- Yirdaw KD, Jerene D, Gashu Z, Edginton ME, Edginton ME, et al. (2014) Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS One 9: e104557.
- Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, et al. (2007) The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 21: 1441-1448.
- Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, et al. (2009) Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: A prospective cohort. AIDS 23: 631–636.
- Lado FL, Barrio GE, Carballo AE, Barron ACOD (1999) Clinical presentation of tuberculosis and the degree of immunodeficiency in patients with HIV infection. Scand J Infect Dis 31: 387-391.
- Keiper MD, Beumont M, Elshami A, Langlotz CP, Miller Jr WT, et al. (1995) CD4 T lymphocyte and the radiographic presentation of pulmonary tuberculosis: A study of the relationship between these factors in patients with human immunodeficiency virus infection. Chest 107: 74-80.
- Luey C, Milne D, Briggs S, Thomas M, Handy R, et al. (2015) HIV-associated tuberculosis in Auckland. NZMJ 128: 36-43.
- Dorman SE, Belknap R, Graviss EA, Reves R, Schluger N, et al. (2014) Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Res Crit Care Med 189: 77-87.
- No Authors Listed (2014) Recommendations for HIV prevention with Adults and Adolescents with HIV in the United States. MMWR 63: 1180-1180.
- US Department of Health and Human Services (2015) HIV/AIDS bureau performance measures.
- Clark SA, Martin SL, Pozniak A, Steel A, Ward B, et al. (2007) Tuberculosis antigen-specific immune responses can be detected using enzyme-linked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease. Clin Exp Immunol 150: 238-244.
- Elzi L, Steffen I, Furrer H, Fehr J, Cavassini M, et al. (2011) Improved sensitivity of an interferon-gamma release assay (T-SPOT.TB®) in combination with tuberculin skin test for the diagnosis of latent tuberculosis in the presence of HIV co-Infection. BMC Infect Dis 11: 319.
- Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, et al. (2006) Latent tuberculosis in HIV positive diagnosed by the M. tuberculosis specific interferon gamma test. Respir Red 7: 56-63.
- Luetkemeyer AF, Charlebois ED, Flores LL, Bangsberg DR, Deeks SG, et al. (2007) Comparison of an interferon-γ release assay to tuberculin skin testing in HIV-infected individuals. Am J Resp Crit Care Med 175: 737-742.
- Tagmouti S, Slater M, Benedetti A, Kik SV, Banaei N, et al. (2014) Reproducibility of interferon gamma (IFN-γ) release assays: A systematic review. Ann Am Thorac Soc 11: 1267-1276.
- King TC, Upfal M, Gottlieb A, Adamo P, Bernacki E, et al. (2015) T-SPOT.TB interferon-γ release assay performance in healthcare worker screening at nineteen U.S. hospitals. Am J Respir Crit Care Med 192: 367-373.
- Grey J, Reves R, Johnson S, Belknap R (2012) Identification of false-positive Quantiferon-TB Gold In-Tube assays by repeat testing in HIV-infected patients at low risk for tuberculosis. Clinic Infect Dis 54: 20-23.
- Pullar ND, Steinum, Bruun JN, Dyrhol-Riise AM (2014) HIV patients with latent tuberculosis living in a low-endemic country do not develop active disease during a 2-year follow-up: A Norwegian prospective multicenter study. BMC Infect Dis 14: 667.
- Leidl L, Mayanja-Kizza H, Sotgiu G, Baseke J, Ernst M, et al. (2010) Relationship of immunodiagnostic assays for tuberculosis and numbers of circulating CD4+ T-cells in HIV infection. Eur Respir J 35: 619-626.
Citation: Frontini M, Ige M, Ali J (2017) Characteristics of HIV/TB Co-Infected Patients with Data of T-Spot TB Testing: Review of Practice Pattern in an HIV Outpatient Clinic in New Orleans, Louisiana. J Tuberc Ther 2: 108.
Copyright: © 2017 Frontini M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5130
- [From(publication date): 0-2017 - Dec 18, 2025]
- Breakdown by view type
- HTML page views: 4106
- PDF downloads: 1024