Characterization of GA3 Insensitive Reduced Height Mutant of Emmer Wheat Var. NP200 (Triticum Dicoccum)
Received: 07-May-2014 / Accepted Date: 19-Jun-2014 / Published Date: 21-Jun-2014 DOI: 10.4172/2329-8863.1000132
Abstract
A γ-ray induced semi-dwarf mutant was obtained in tall emmer wheat variety NP200. The mutant was insensitive to externally applied gibberellin. An allele specific marker for dwarfing gene Rht-B1b was used to check the status of dwarfing gene in the mutant, semi dwarf and tall emmer and semi dwarf durum varieties. The primer showed amplification of Rht-B1b gene in semi dwarf durum and emmer varieties. The parent NP200 showed presence of wild type allele (Rht-B1a) with the primer pair BF-WR1. All semi dwarf emmer varieties showed a band of 237bp with primer pair BF-MR1showed presence of Rht-B1b. However, the mutant showed absence of amplification for both Rht-B1a and Rht-B1b alleles with respective primer pairs. The results indicated that the reduced height mutant carried a mutation different from the known allele Rht-B1b.
Keywords: Triticum dicoccum , GA3 insensitivity, Reduced height mutant, Rht genes, Yielding ability, Allele specific primer
404068Introduction
Emmer wheat (Triticum dicoccum Schubler) is cultivated in parts of peninsular India. Emmer wheats are reported to contain high amounts of protein and dietary fibre [1,2] and hence are being recommended for inclusion in diet [3]. T. dicoccum possesses better resistance to wheat rust and have more tolerance to high temperature than other species of wheat [4,5]. Traditional varieties of emmer are tall, susceptible to lodging and low yielding [6]. Tall Emmer variety NP200 is a selection from local wheat. Introduction of semi dwarf stature has resulted in improvement in harvest index and yield in bread wheat. Till date, 21 height reduction genes are known in wheat including two major genes Rht-B1b and Rht -D1b which are present in 90% of the semi-dwarf cultivars [7]. Search for alternative height reducing genes led to the discovery of two genes Rht-B1d and Rht 8 (located on 2DS) from varieties Saitama-27 and Akakomughi respectively. Introduction of dwarfing gene Rht-B1b has also shown improvement in emmer wheats [8]. It seems possible to generate variability for reduced height genes so that they can be used to increase option for the breeder to improve the emmer wheats [9]. A more comprehensive understanding of how the Rht-1 genes confer dwarfism will allow the development of novel alleles with improved specificity for agronomic traits [10,11]. Novel genetic variants of GA-insensitive Rht-1 genes in hexaploid Wheat characterized and their agronomic value have also been reported [12]. In this study, aγ-ray induced short statured mutant HW1095 was taken which is GA3 insensitive and was characterized using molecular markers.
Materials and Methods
An emmer wheat variety NP200 was used in this study. Seeds were subjected to 100, 200, 300 and 400 Gy ofγ-rays. The treated seeds were space planted in M1 generation. The M1 plants were selfed and harvested individually. In the M2 population of 200 Gy treatment, a reduced height mutant with vigorous growth and high tillering was observed. The mutant was carried forward as plant to row progeny. Subsequently, its progeny was designated as HW1095 [6]. The mutant was then tested in yield trials in five states representing peninsular and central parts of India.
GA3 insensitivity test
Twenty seeds were placed in blotting paper folds supported by stands. Seedlings were grown either in water (control) or 10-4 M GA3 (treatment). After 12 days, seedling height was recorded. Student’s t-test was performed to test if the differences in seedling height of GA3 treated and control were significant.
Rht genotyping: DNA was isolated from bulk of five plants according to Saghai-Maroof et al. [13]. The mutant along with parent (NP200), tall variety (NP201) and semi dwarf dicoccum varieties (DDK1009, DDK1025, HW5013, HW5301 and MACS2961) were subjected to Rht genotyping. The alleles Rht-B1b and Rht-B1a were amplified using allele specific primers using the primer combinations as follows: primer BF: (5’-GGTAGGGA GGCGAGAGGCGAG-3’) and MR1: (5’-CATCCCCATGGCCATCTCGAGCTA-3’) for Rht-B1b; primers BF and WR1 (5’-CATCCCCATGGCCATCTCGAGCTG-3’) for Rht-B1a [14]. DNA from the mutant along with tall and semi dwarf dicoccum varieties was also amplified with a different primer pair which differs from perfect pair at forward end (5’-TCTCCTCCCTCCCCACCCCAAC-3’). The reaction was performed in 25 µl containing 10 pmoles of each primer, 100 µM of dNTPs, and 2 mM of MgCl2, 1 unit of Taq DNA polymerase and 100 ng of template DNA. The PCR amplification was carried out in an Eppendorf Mastercycler according to [14]. The PCR products were separated on a 2% agarose gel prepared in 1X TBE buffer, visualized under UV light after Ethidium bromide staining and photographed [15].
Results and Discussion
The plant height of tall parent variety NP200 varied from 97.0 cm to 120 cm with a mean of 110 cm. The reduced height mutant showed mean height of 71.0 cm and showed 35.4 percent reduction in height over parent in M2 generation. The dwarf dicoccum mutant bred true to type in M3 generation with a plant height ranging from 63.0 to 79.0 cm with a mean of 70.7 cm. The mutant was also found to be lodging resistant (0-10%) as compared to parent which showed (0-90%) and the test varieties DDK1009, DDK1025 (T.diccocum), MACS2846 (T.durum) and MACS2496 (T.aestivum) with lodging resistance of 0-60%, 35-90%, 0-35% and 0%, respectively [16].
The dicoccum mutant along with tall parent NP200 and other emmer varieties were tested for their response to externally applied gibberellic acid. The reduced height mutant (HW1095) and dwarf emmer varieties showed no increase in height whereas tall varieties (NP200 and NP201) showed 29.2 and 20.4% increase in height in response to externally applied gibberellic acid (Table 1). This showed that mutant was insensitive to externally applied gibberellin. Reduction in plant height has been found to be effective in improving crop performance in higher input conditions in bread and macaroni wheats. T. dicoccum is gaining importance due to dietary requirement of low digestibility, low glycaemic index and therapeutic value for the management of diabetes [3] and therefore, yield improvement is needed. Transfer or induction of dwarfing genes can be useful in imparting lodging resistance and also improving efficiency of dry matter partitioning. Semi dwarf varieties of emmer wheat using existing Rht-B1b gene have been developed however, an alternative gene for height reduction through induced mutation is needed for increasing germplasm base.
| S.No. | Genotype | Control (cm) | Treated (cm) % increase | Significance |
|---|---|---|---|---|
| 1 | NP200 | 25.4 ±0.6 | 35.9±1.4 (29.2) | ** |
| 2 | HW1095 | 18.8±0.4 | 19.2±0.5 (01.8) | NS |
| 3 | NP201 | 24.2±0.3 | 30.4±0.6 (20.4) | ** |
| 4 | DDK1009 | 16.8±0.2 | 16.0±0.2 (-04.5) | NS |
| 5 | DDK1025 | 18.7±0.5 | 18.4±0.4 (-01.6) | NS |
| 6 | HW5013 | 17.1±0.3 | 16.1 ±0.4 (-05.8) | NS |
| 7 | HW5301 | 16.6±0.3 | 14.5±0.3 (-14.9) | NS |
| 8 | MACS2961 | 16.3±0.4 | 15.2±0.5 (-06.9) | NS |
Table 1: Response of genotypes to externally applied GA3.*Values in parenthesis indicate % increase in seedling height over corresponding control
A reduced height phenotype can result from introgression of Rht-B1b throughout-crossing or due to induced mutation. To check whether height reduction in the putative mutant was due to mutation or introgression of semi dwarfing gene Rht-B1b, the allele specific markers for major dwarfing genes Rht-B1b developed by Ellis et al. were used [14].
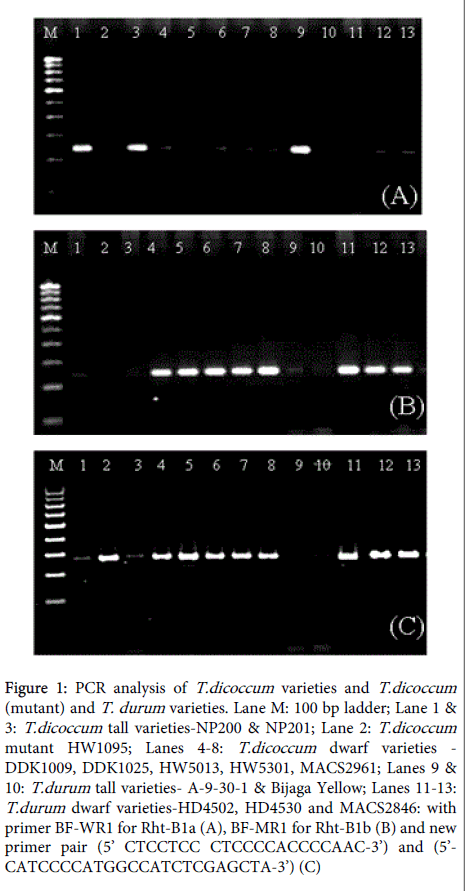
The specificity of these markers has been validated on bread and durum wheat varieties (Figure 1B). Use of the allele specific markers has not been reported on emmer wheats.
In this study, the allele specific markers were used to check the status of Rht genes in tall emmer varieties (NP200 and NP201), five semi dwarf emmer varieties (DDK1009, DDK1025, HW5013, HW5301 and MACS2961) and the newly developed dwarf mutant (HW1095) (Figure 1A and 1B). The validity of primers in tall emmer varieties for Rht-B1a and semi dwarf emmer varieties for Rht-B1b was confirmed. All semi dwarf emmer varieties showed a band of 237bp with primer pair BF-MR1 for presence of Rht-B1b. The parent variety NP200 showed amplification of wild type allele (Rht-B1a), however, the mutant (HW1095) showed absence of amplification for both Rht-B1a and Rht-B1b alleles with respective primer pairs. The PCR amplification was repeated on DNA extracted from next generation seedlings and results were found to be repeatable. This indicated that the mutant carried a different mutation than the existing allele (Rht-B1b). This was further confirmed by amplifying with another primer pair which covers a longer stretch of DNA segment (Figure 1C). In the mutant, this primer amplified a product of about 400bp as compared to 237bp with perfect primer pair (BF-MR1). Since the primer amplified the mutant allele, it is possible that mutation does not involve a large deletion. The amplification will enable further characterization of induced mutation. A variation was also observed in case of tall durum cultivar Bijaga Yellow where a band of 237 bp was expected with the primer pair BF-WR1, however, no amplification was observed with either primer pairs (Figure 1A and 1B).
Figure 1: PCR analysis of T.dicoccum varieties and T.dicoccum (mutant) and T. durum varieties. Lane M: 100 bp ladder; Lane 1 & 3: T.dicoccum tall varieties-NP200 & NP201; Lane 2: T.dicoccum mutant HW1095; Lanes 4-8: T.dicoccum dwarf varieties - DDK1009, DDK1025, HW5013, HW5301, MACS2961; Lanes 9 & 10: T.durum tall varieties- A-9-30-1 & Bijaga Yellow; Lanes 11-13: T.durum dwarf varieties-HD4502, HD4530 and MACS2846: with primer BF-WR1 for Rht-B1a (A), BF-MR1 for Rht-B1b (B) and new primer pair (5’ CTCCTCC CTCCCCACCCCAAC-3’) and (5’- CATCCCCATGGCCATCTCGAGCTA-3’) (C)
This indicated that the mutant carried a different mutation than from the existing major gene mutations (Rht-B1b). The GA3 sensitivity test showed that the two tall emmer varieties responded to externally applied gibberellin resulting in significant increase in seedling height. The semi dwarf emmer varieties and the new mutant showed no significant difference in mean seedling height for the water grown control and the GA3 treatment.
A comparative yield trial, which included eight released varieties of emmer wheat, one bread, wheat and one macaroni wheat variety as control, NP200 parent and the mutant HW1095, was conducted in five diverse locations. The results showed that the mutant gave highest yield in three of the five locations among all the varieties. The mutant gave higher yield than the parent and other emmer wheat varieties at all the locations (Table 2). For mean of all the locations the mutant was at second place in ranking at 40.0 qt/ha compared to the bread wheat variety MACS 2496 which gave highest average yield of 40.4 qt/ha. These results showed that the mutant has high yielding potential in the tested locations.
| Genotype | Gujarat State (qt/ha) |
Karnataka (qt/ha) |
Maharashtra (qt/ha) | Tamil Nadu (qt/ha) |
Peninsular Zone (qt/ha) | Mean of All Zones (qt/ha) |
|---|---|---|---|---|---|---|
| DDK1028 | 22.7 | 37.8 | 37.9 | 22.1 | 37.6 | 33.2 |
| DDK1030 | 23.5 | 39.7 | 41.2 | 7. 2 | 40.3 | 33.6 |
| DDK1009 | 23.5 | 38.5 | 40.2 | 29.4 | 39.2 | 35.1 |
| MACS-2947 | 26.8 | 39.5 | 40.9 | 27.2 | 40.0 | 36.2 |
| DDK1025 | 21.7 | 42.5 | 39.4 | 31.8 | 44.1 | 36.3 |
| MACS-2956 | 25.4 | 40.0 | 39.6 | 35.9 | 39.7 | 36.6 |
| DDK1029 | 31.5 | 41.4 | 46.5 | 10.9 | 43.5 | 37.9 |
| MACS-2961 | 26.8 | 42.7 | 46.0 | 23.4 | 44.1 | 38.6 |
| NP200 (parent) | 22.6 | 34.4 | 42.7 | 20.6 | 37.9 | 33.2 |
| HW1095 (mutant) | 29.0 | 43.3 | 49.1 | 21.6 | 45.8 | 40.0 |
| MACS-2846 (T.durum) | 34.7 | 38.6 | 46.4 | 12.4 | 41.8 | 37.5 |
| MACS-2496 (T.aestivum) | 45.3 | 40.0 | 47.0 | 12.5 | 43.2 | 40.4 |
| S.E. mean | 0.64 | 0.66 | 1.08 | 0.44 | 0.44 | |
| CD | 1.8 | 1.8 | 3.0 | 1.3 | 1.2 |
Table 2: Grain yield of reduced height mutant (HW1095), parent and released varieties across different emmer growing regions of India
Conclusion
The study reported here showed induction of a mutation resulting in about 30% reduction in height. The mutant was insensitive to externally applied gibberellin. The absence of amplification with primers specific to wild type allele or the known allele causing height reduction and insensitivity to externally applied gibberellin indicated that there was a different mutation at the Rht-B1 locus.
References
- Galletti GC, Bocchni P, D’ Autunono LF (1996) Fiber composition of a neglected wheat species (Triticum dicoccum Schubler) as determined by pyrolysis /gas cromatography / mass spectroscopy. J Agric Food Chem 44: 3133-3135.
- D’ Autuono LF, Galletti GC, Bocchini P (1998) Fiber quality of emmer (Triticum dicoccum Schubler) and einkorn wheat (T. monococcum L.) landraces determined by analytical pyrolysis. J Sci Food Agric 88: 213-219.
- Yenagi NB, Hanchinal RR, Patil CS, Koppikar V (2001) Glycemic and lipidemic responses to dicoccum wheat (Triticum dicoccum) in the diet of diabetic patients. International Journal of diabetes in developing countries. 21:153-155.
- Spielmeyer W, Sharp PJ, Lagudah, ES (2003) Identification and validation of markers linked to broad-spectrum resistance gene Sr2 in wheat (Triticum aestivum L.). Crop Science 43: 333-336.
- Hussein S, Spies JJ, Pretorius ZA, Labuschagne MT (2005) Chromosome locations of leaf rust resistance genes in selected tetraploid wheats through substitution lines. Euphytica 141: 209-216.
- Nayeem KA, Sivasamy M (2006) HW1095 Pusa dwarf dicoccum has high yield performance across the Indian zones. Annual Wheat Newsletter 52: 66-67.
- Worland AJ, Korzun V, Roder MS, Ganal MW, Law CN (1998) Genetic analysis of dwarfing gene Rht8 in wheat. Part II. The distribution and adaptive significance of allelic variants at the Rht8 locus of wheat as revealed by microsatellite screening. Theoretical and Applied Genetics 96: 1110-1120.
- Hanchinal RR, Lohithaswa HC, Desai SA, Math KK, Patil BN, et al. (2004) Current status of emmer wheat (Triticum dicoccum) (Schrank.) (Schulb.) cultivation in India. : In Nayeem KA, Sivasamy M, Nagarajan S (eds), Wheat for Tropical Areas, 117-125. Laserpark printers, Coimbatore, India.
- Jin X J, Sun DF, Li HY, Yang Y, Sun GL (2013) Characterization and molecular mapping of a dwarf mutant in wheat. Genetics and Molecular Research 12: 3555-3565.
- Li Y, Xiao J, Wu J, Duan J, Liu Y, et al. (2012) A tandem segmental duplication (TSD) in green revolution gene Rht-D1b region underlies plant height variation. New Phytologist 196:282-291.
- Pearce S, Saville R, Vaughan SP, Chandler PM, Wilhelm EP, et al.(2011) Molecular characterization of Rht-1 dwarfing Genes in hexaploid wheat. Plant Physiology 157: 1820–1831.
- Tan M-K, Koval J, Ghalayini A (2013) Novel Genetic Variants of GA-Insensitive Rht-1 Genes in Hexaploid Wheat and Their Potential Agronomic Value. PLoS ONE 8:e99375.
- Saghai-Maroof MA, Soliman K, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosome location and population dynamics. Proc Natl Acad Sci USA 81: 8014-8018.
- Ellis MH, Spielmeyer W, Gale KR, Rebetzke, GJ, Richards RA (2002) Perfect markers for Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor Appl Genet 105: 1038-1042.
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- Nayeem KA, Sivasamy M (2004) Pusa dwarfing genes in T. turgidum var. dicoccum through nuclear technique and their inheritance. : In Nayeem KA, Sivasamy M, Nagarajan S (eds), Wheat for Tropical Areas, 117-125. Laserpark printers, Coimbatore, India.
Citation: Bakshi S, Nayeem KA, Bhagwat SG, Shitre A and Das BK (2014) Characterization of GA3 Insensitive Reduced Height Mutant of Emmer Wheat Var. NP200 (Triticum Dicoccum). Adv Crop Sci Tech 2:132. DOI: 10.4172/2329-8863.1000132
Copyright: © 2014 Suman Bakshi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16154
- [From(publication date): 8-2014 - Jul 09, 2025]
- Breakdown by view type
- HTML page views: 11386
- PDF downloads: 4768