Characterization of Slow Rusting Resistance Against Stem Rust (Puccinia graminis f. sp. tritici) in Selected Bread Wheat Cultivars of Ethiopia
Received: 30-Jul-2018 / Accepted Date: 28-Aug-2018 / Published Date: 05-Sep-2018 DOI: 10.4172/2329-8863.1000389
Keywords: AUDPC; Infection rate; Cultivars; Parameters
Introduction
Wheat (Triticum aestivum) is one of the major food crops in the world. It is produced across a wide range of agro-ecological and crop management regime. Ethiopia is the largest wheat producer in sub- Saharan Africa in 2016 [1,2]. About 5 million Ethiopian farmers produce 4.3 million tons of wheat across 1.7 million hectares of land under rain-fed conditions [3]. Its popularity comes from the versatility of its use in the production of a wide range of food products, such as injera, breads, cakes, pastas, etc. Wheat ranks third in area coverage and total production after teff and maize. Although the productivity of wheat has increased in the last few years in Ethiopia; it is still very low as compared to other wheat producing countries. The national average productivity is estimated to be 2.54 t ha-1 [3]; which is by far below experimental yields of over 5 tons ha-1 [4-6].
The low productivity is attributed to a number of factors including biotic (diseases, insects, weeds and etc.) and abiotic (low and high rainfall, temperature, low adoption of new agricultural technologies and etc.). Among the biotic factors, wheat stem rust, also known as black rust, caused by the fungus Puccinia graminis f. sp. tritici (Pgt) has been the most devastating disease in Ethiopia causing upto 100% yield losses on susceptible cultivars during epidemic years [7,8]. This biotroph reduces the total photosynthetic area, utilizes plants assimilates and interrupts the normal growth of the host leading to reduction of yield. According to Singh et al. [9] Ethiopia is considered as a hot spot for the development of stem rust races diversity. Studies carried out in Ethiopia showed that most previously identified races such as TTKSK, TKTTF, TTTTF, TRTTF, RRTTF and others were virulent on most varieties grown in the country.
Breeding of wheat cultivars with durable resistance to rust diseases is the best control strategy [10-12]. Generally, two types of resistance have been described in wheat. The first type of resistance is race specific, which is controlled by genes that act in a gene-for-gene manner with the rust fungus. These genes generally confer high level of resistance to specific rust biotypes, both at the seedling and adult plant stages. The second type is race non-specific which is controlled by minor genes and is usually most discernable in adult plants as partial and slow rusting resistance [11,13,14]. Race non-specific resistance is controlled by multiple genes [15] and remains effective against all races of the pathogen. This type of resistance confers durable resistance [12]. So far, few race non-specific genes have been characterized and catalogued in wheat [16-19].
Resistance breeding for wheat rusts in Ethiopia has been based on major genes for a long time. However, durable resistance to stem rust has been re-emphasized with the occurrence and spread of new races of Pgt. Hence, this study was designed to assess the levels of slow rusting resistance in some bread wheat cultivars to stem rust under green-house and field conditions and the information provided here will be important for developing potentially durable combinations of stem rust resistance genes in cultivars.
Materials And Methods
Green-house evaluation of bread wheat cultivars for their seedling resistance to stem rust
Green-house evaluations were done at Ambo Plant Protection Research Center (APPRC), Ethiopia by using 24 bread wheat varieties and one susceptible check (McNair). Seven seeds of each wheat variety and a susceptible check were planted in 3 cm diameter plastic pots separately in three replications. Seven-days-old seedlings (the first leaves were fully expanded, and the second leaves were just emerged to grow) were inoculated with spores of Virulent race, TTKSK (Ug99). Inoculated plants were then moistened with fine droplets of distilled water by using atomizer and placed in dew chamber for 18 hours in a dark at 18-22°C. Upon removal from chamber, seedlings were exposed to 3 hours of fluorescent light to dry dew on the leaves. Following this, the seedlings were transferred to the green-house where conditions were regulated at 12 hours photoperiod, at temperature range of 18-25°C and RH of 60-70%. Data on infection types (IT) were recorded 14 days after inoculation using 0-4 scale [20].
The IT readings of 3 (medium-size uredia with/without chlorosis) and 4 (large uredia without chlorosis or necrosis) were regarded as compatible reactions. Other readings, that are 0 (immune or fleck), 1 (small uredia with necrosis) and 2 (small to medium uredia with chlorosis or necrosis) were considered as incompatible. The infection types were defined by modifying characters as follows: (-) uredinia somewhat smaller than normal; (+) uredinia somewhat larger than normal for the infection type.
Field evaluation of bread wheat cultivars for their slow rusting resistance to stem rust at adult plant growth stage
The field experiment was conducted at Adet Agricultural Research Center. The center is located 11°17' N latitude and 37°43' E longitude at an altitude of 2240 m.a.s.l. The center receives moderate and pleasant climate with temperature ranging from 8-25°C and annual rainfall of 1270.5 mm.
Experimental materials
The twenty-four bread wheat varieties tested in the greenhouse were also tested against stem rust disease under field condition (Table 1). In the field the susceptible variety Morocco was used as a comparative control. All varieties except Morocco were under production. Morocco was planted perpendicular to the experimental blocks one week before the experimental plots to serve as spreader row. The spreader rows were then inoculated artificially with TTKSK race when most plants were at the stem elongation.
| S No | Cultivar | Year of Release | Source Center |
|---|---|---|---|
| 1 | Pavon-76 | 1982 | KARC/EIAR |
| 2 | Kakaba | 2010 | KARC/EIAR |
| 3 | Danda’a | 2010 | KARC/EIAR |
| 4 | Shorima | 2011 | KARC/EIAR |
| 5 | Huluka | 2012 | KARC/EIAR |
| 6 | Hoggana | 2011 | KARC/EIAR |
| 7 | Bonny | 1995 | KARC/EIAR |
| 8 | Africa Mayo | 2005 | KARC/EIAR |
| 9 | Medawalabu | 1999 | SARC/OARI |
| 10 | Galil | 2010 | Hazera Genetics Ltd |
| 11 | Tuse | 1997 | KARC/EIAR |
| 12 | Senkegna | 2005 | ADARC/ARARI |
| 13 | Kingbird | 2015 | EIAR |
| 14 | Hidase | 2012 | KARC/EIAR |
| 15 | Tay | 2005 | ADARC/ARARI |
| 16 | Dinkinesh | 2007 | SRARC/ARARI |
| 17 | Gassay | 2007 | ADARC/EIAR |
| 18 | Abolla | 1997 | KARC/EIAR |
| 19 | Millenium | 2007 | KARC/EIAR |
| 20 | Alidoro | 2007 | HARC/EIAR |
| 21 | Ogolcho | 2012 | KARC/EIAR |
| 22 | Densa | 2010 | ADARC/ARARI |
| 23 | Guna | 2001 | ADARC/ARARI |
| 24 | Shina | 1999 | ADARC/ARARI |
| 25 | Morocco (Sucpt.ck) |
Table 1: List of wheat cultivar used for evaluation of slow rusting resistance to stem rust under field and greenhouse conditions. Source: Crop variety register. Issue No. 16, 2013.
Experimental design and field plots
The experiment was laid out using randomized complete block design (RCBD) with three replications. The test materials were sown during the main cropping season of 2016 under rain fed conditions. There were a total of 75 plots comprising 24 bread wheat varieties and one susceptible check (Morocco). Each plot consisted of four rows with a size of 0.8 m × 1.5 m and with a spacing of 1.5 m between blocks and 0.4 m between plots. The inter row spacing was 0.2 m. The recommended fertilizer rate (92/46 N/P2O5 ha-1) and seed rate of 100 kg ha-1 were used. Urea was applied in split. Weeds were controlled three times by hand weeding.
Disease assessment
Disease severity: Stem rust severity, estimated as a proportion of the stem of the plant affected by the disease, were recorded using the modified Cobb’s scale where 0%=immune and 100%=completely susceptible [21]. Disease severity was assessed three times at 20 days interval from 10 randomly pre-tagged plants in the central two rows of each plot. It was recorded from the time of disease appearance until the crop attains its physiological maturity and the mean of the ten plants were calculated. Host plant response to infection was scored using the description of Roelfs et al. [22], where, immune=0.0, R=0.2, MR=0.4, MR-MS=0.6, MS=0.8, MS-S=0.9 and S=1.0.
Average coefficient of infection (ACI)
Average coefficient of infection was calculated by multiplying the percentage severity by a constant for host response [23]. The ACI for each variety was computed from three severity observations and ACI was used for calculating AUDPC for each variety.
Area under disease progress curve (AUDPC)
The data collected were entered in excel worksheets and AUDPC values were generated using the formula below [24].
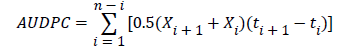
Where, Xi is the cumulative disease severity expressed as a proportion at the ith observation; ti is the time in days after appearance of the disease and n is total number of observations.
Disease progress rate (Inf-rate)
The three disease severity observations records at 20 days interval were regressed over time and the apparent infection rates as the coefficient of the regression line, ln [X/(100-X)], where X is average coefficient infection plotted against time in days [25] were calculated for each variety.
Grain yield and thousand kernel weight
Thousand kernel weights and grain yield were recorded from the middle two rows of each experimental unit as follows;
• Grain yield (GY): The central two rows of each entry were harvested, and their grains weighted for conversion to tones per hectare.
• Thousand kernel weight (TKW): One thousand grains select at random were weighted in grams.
Data analysis
Relative forms of the epidemiological parameters were generated by comparing the respective values of each entry with the susceptible variety Morocco. Coefficient of correlation was done using SPSS software version 15 [26] to determine the relationship between disease parameters and the relationship between disease and yield parameters. Seedling evaluation results were analyzed by using the descriptive statistics. Analysis of variance (ANOVA) was used for yield parameters of the field experiment as randomized block design (RCBD) for one factor for yield parameters, following the procedure described by Gomez et al. [27] using SAS computer software. When the variable was significant, mean separations were conducted based on LSD at 5% probability level.
Results And Discussion
Seedling reaction test
Results from the green-house test showed that the bread wheat cultivars differed in their reaction to the TTKSK stem rust race. Out of 25 wheat cultivars tested in a greenhouse 11 (Shorima, Hoggana, Galil, Hidase, Tay, Gassay, Abolla, Millenium, Ogolcho, Densa and Guna) showed resistance reactions (1+ to 2+), 7 cultivars (Kakaba, Danda’a, Hulluka, Bonny, Africa Mayo, Alidoro and Shina) had susceptible reactions (3-) and 6 varieties (Pavon-76, Madawalabu, Tuse, Senkegna, Kingbird and Dinkinesh) had mixed reactions (2+ and 3-) at the seedling stage. The susceptible check, Morocco was susceptible to the race, displaying infection type 3+ at the seedling stage (Table 2). The wheat cultivars which showed resistance reaction at seedling stage implied the presence of major gene resistance towards the race.
| No | Cultivar | TTKSK | TRS† | Infection Response | CI |
|---|---|---|---|---|---|
| 1 | Pavon-76 | 2+3- | 50.0 | MS | 40.0 |
| 2 | Kakaba | 3- | 45.0 | MS | 36.0 |
| 3 | Danda’a | 3- | 45.0 | MS | 36.0 |
| 4 | Shorima | 1+ | 40.0 | MR | 16.0 |
| 5 | Hulluka | 3- | 45.0 | MR | 18.0 |
| 6 | Hoggana | 1+ | 35.0 | MR | 14.0 |
| 7 | Madawalabu | 2+3- | 45.0 | MR-MS | 27.0 |
| 8 | Galil | 1+ | 30.0 | MR | 12.0 |
| 9 | Tuse | 2+3- | 55.0 | MR-MS | 33.0 |
| 10 | Senkegna | 2+3- | 40.0 | MR | 16.0 |
| 11 | Kingbird | 2+3- | 50.0 | MR-MS | 30.0 |
| 12 | Hidase | 1+ | 35.0 | MR | 14.0 |
| 13 | Tay | 2 | 35.0 | MR | 14.0 |
| 14 | Bonny | 3- | 50.0 | MS-S | 45.0 |
| 15 | Africa Mayo | 3- | 50.0 | MS-S | 45.0 |
| 16 | Dinkinesh | 2+3- | 30.0 | MR | 12.0 |
| 17 | Gassay | 2 | 40.0 | MR | 16.0 |
| 18 | Abolla | 22+ | 65.0 | MR-MS | 39.0 |
| 19 | Millenium | 1+ | 30.0 | MR | 12.0 |
| 20 | Alidoro | 3- | 45.0 | MS | 36.0 |
| 21 | Ogolcho | 2- | 45.0 | MR-MS | 27.0 |
| 22 | Densa | 1+ | 35.0 | MR | 14.0 |
| 23 | Guna | 1+ | 35.0 | MR | 14.0 |
| 24 | Shina | 3- | 30.0 | MR | 12.0 |
| 25 | Morocco | 3+ | 85.0 | S | 85.0 |
Table 2: Summary of wheat seedling reaction tests to stem rust under greenhouse and different disease parameters under field conditions on 25 tested wheat cultivars. †TRS=Terminal rust severity; MR=moderately resistant; MR-MS=moderately resistant to moderately susceptible; MS=moderately susceptible; MS-S=moderately susceptible to susceptible; S=Susceptible, CI=Coefficient of infection.
Field experiment
Slow rusting cultivars were identified on the bases of their terminal rust severity (TRS), coefficient of infection (CI), area under disease progress curve (AUDPC) and rate of rust progress (Inf-rate value).
Terminal severity of stem rust
There was wide variation in the stem rust severities ranging from 30% to 85% during the 2016 main cropping season at the Adet Research Center. Different field reactions ranging from moderately resistance (MR) to susceptible (S) responses were also observed at the trial. The observed stem rust severities of the cultivars and their infection types are presented in Table 2. Terminal rust severity represents the cumulative result of all resistance factors during the progress of epidemics [28]. Based on terminal rust severity, the tested wheat cultivars were grouped into three groups of slow rusting resistance, i.e., high, moderate and low levels of partial resistance having 1-30%, 31-50% and >50% TRS, respectively.
During the 2016 cropping season four wheat cultivars (Galil, Dinkinesh, Millenium and Shina) displayed disease severities of 30% with moderately resistant (MR) field responses. Of these, Dinkinesh and Shina had susceptible infection types at seedling stage. Similar trends were previously observed with Ethiopian wheat lines showing susceptible infection types at the seedling stage and maintaining low severity to stem rust in the field [29], confirming that these cultivars have race non-specific resistance to the disease. On the other hand, twenty cultivars were included in the second group with final rust severities ranging from 35-50% and were regarded as possessing moderate levels of slow rusting resistance. Of these, eight cultivars (Pavon-76, Kakaba, Danda’a, Madawalabu, Kingbird, Bonny, Africa Mayo and Alidoro) had compatible field (MR-S to MS-S) and seedling (2+3- to 3-) reactions and are of great importance to achieving effective breeding for durable resistance to stem rust [30,31].
According to Nzuve et al. [30], the available resistance genes in these materials overcame the stem rust virulence in the field and led to statistically low disease severities despite the compatible host-pathogen reactions. Previously, Ali et al., Li et al., Safavi, Tabassum [32-35] also used final rust severity to assess slow rusting behavior of wheat lines. On the other hand, cultivars Tuse and Abolla had disease severities more than 50% with MR-MS field responses and were regarded as susceptible to the disease. The susceptible check, Morocco, displayed the highest disease severity of 85% with completely susceptible (S) responses indicating that an acceptable epidemic pressure was established over the season for field experiment. There was no complete resistance (zero infection type) observed in all tested wheat cultivars at adult plant stage in the season.
Coefficient of infection
The data on disease severity and host reaction were combined to calculate CI (Table 2). According to Ali et al. [36], varieties with CI values of 0-20, 21-40, 41-60 were regarded as possessing high, moderate and low levels of slow rusting resistance, respectively. In the present study 13 cultivars (Shorima, Hulluka, Hoggana, Galil, Senkegna, Hidase, Tay, Dinkinesh, Gassay, Millenium, Densa, Guna and Shina) showed CI values between 0 and 20 and were designated as having high level of slow rusting. Cultivars Pavon-76, Kakaba, Danda’a, Madawalabu, Tuse, Kingbird, Abolla, Alidoro and Ogolcho had CI values between 21 and 40, designated as having moderate levels of slow rusting resistance.
Cultivars Bonny, Africa Mayo and the susceptible check had a CI value of more than 40 showing low levels of slow rusting resistance. Many earlier researchers such as Draz et al. [10], Pathan et al. [37], Patil et al. [38] also appraised slow rusting resistance to wheat stem rust using coefficient of infection and reported the presence of different partial resistance conferring genes in wheat lines.
MR=moderately resistant; MR-MS=moderately resistant to moderately susceptible; MS=moderately susceptible; MS-S=moderately susceptible to susceptible; S=Susceptible, CI=Coefficient of infection.
Area under disease progress curve
According to Wang et al. [39] area under disease progress curve is a good indicator of partial resistance under field condition. It is directly related with yield loss [40] and provides critical information for designing effective disease management practices [41]. Cultivars which had low AUDPC and terminal severity values may have good level of adult plant resistance. Therefore, selection of cultivars having low AUDPC and terminal disease scores is normally accepted for practical purposes where the aim is to utilize slow rusting resistance as one of the disease management strategy [42]. Based on the AUDPC values in this study, the 25 varieties were categorized in to two distinct groups. The first group comprised varieties exhibiting relative AUDPC values up to 30% of the check, while showing relative AUDPC values up to 70% of check were placed in second group. All those cultivars of group 1 were ranked as better slow rusting and that of group 2 were marked as moderately slow rusting because rust develops slowly at this stage exhibiting high infection types [29].
In the present study, varieties Shorima, Hulluka, Hoggana, Madawalabu, Galil, Tuse, Senkegna, Hidase, Tay, Dinkinesh, Gassay, Millenium, Ogolcho, Densa, Guna and Shina were having lowest rAUDPC values of 30% and less. Of these, Huluka, Madawalabu, Tuse, Senkegna, Dinkinesh and Shina had compatible seedling and field reactions, and were regarded as having high level of partial resistance. Group 2 included varieties Pavon-76, Kakaba, Danda’a, Kingbird, Bonny, Africa Mayo, Abolla and Alidoro. Except variety Alidoro, cultivars under group 2 had susceptible infection types at seedling stage (2+3- to 3-) and compatible field responses (MR-MS to MS-S). Wheat cultivars with slow rusting genes are often susceptible at the seedling stage but may be moderately to highly resistant to all races at the adult stage [11,43]. Singh et al. [12], Brown et al. [44], Kaur et al. [45] also stated that cultivars which had MS or MR infection types may carry genes for durable resistance. Despite the MS infection types exhibited on the cultivars, the disease progression remained slower and highly retarded among these cultivars. Such partially resistant varieties could highly delay evolution of new virulent races of the pathogen because multiple point mutations are extremely rare in normal circumstances [46-48].
Infection rate
A high disease progress rate (Inf-rate=0.054) was observed on susceptible check Morocco and the lowest (Inf-rate=0.007) was recorded from Millenium (Table 3). In the present study, infection rate showed more variation among the tested cultivars than the other slow rusting parameters. Some of the cultivars belonging to group 1 in terms of other slow rusting parameters (such as Galil, Dinkinesh and Shina) had higher infection rate values than some cultivars in group 2 (such as Danda’a, Africa Mayo and Pavon-76). Similarly, the results of previous studies conducted by Ali et al. [46], Sandoval-Islas et al. [49], Safavi [50] indicated that infection rate did not mark some cultivars as having moderate and low level of slow rusting and it was an unreliable estimate of slow rusting resistance when compared with TRS, CI, and rAUDPC. Moreover, more variation in infection rate among the tested lines than the disease severity and AUDPC, is partly because apparent infection rate is a regression coefficient with larger error variance. Ali et al. [32], Ali et al. [46] also found similar results for stem rust and leaf rust of wheat.
| Varieties | AUDPC | rAUDPC | Inf-Rate |
|---|---|---|---|
| Pavon-76 | 1642.7 | 49.4 | 0.017 |
| Kakaba | 1360.0 | 40.9 | 0.019 |
| Danda’a | 1434.7 | 43.2 | 0.009 |
| Shorima | 620.0 | 18.7 | 0.020 |
| Hulluka | 1052.0 | 31.7 | 0.012 |
| Hoggana | 544.0 | 16.4 | 0.019 |
| Madawalabu | 1002.0 | 30.2 | 0.026 |
| Galil | 756.0 | 22.7 | 0.018 |
| Tuse | 844.0 | 25.4 | 0.029 |
| Senkegna | 656.0 | 19.7 | 0.032 |
| Kingbird | 1294.0 | 38.9 | 0.030 |
| Hidase | 600.0 | 18.1 | 0.035 |
| Tay | 668.0 | 20.1 | 0.038 |
| Bonny | 1929.0 | 58 | 0.023 |
| Africa Mayo | 1914.0 | 57.6 | 0.013 |
| Dinkinesh | 644.0 | 19.4 | 0.025 |
| Gassay | 688.0 | 20.7 | 0.023 |
| Abolla | 1446.0 | 43.5 | 0.029 |
| Millenium | 488.0 | 14.7 | 0.007 |
| Alidoro | 1440.0 | 43.3 | 0.039 |
| Ogolcho | 1070.0 | 32.2 | 0.022 |
| Densa | 548.0 | 16.5 | 0.023 |
| Guna | 478.7 | 14.4 | 0.026 |
| Shina | 538.7 | 16.2 | 0.028 |
| Morocco | 3323.3 | 100 | 0.054 |
Table 3: Area under disease progress curve and infection rates of stem rust on the cultivars tested. AUDPC=Area under disease progress curve; rAUDPC=Relative area under disease progress curve; Inf-Rate=Infection rate.
Correlation between slow rusting parameters
A positive and highly significant correlation of TRS with CI (r=0.872) and AUDPC (r=0.860) was observed during 2016 main cropping season (Table 4). The high correlation coefficient (r=0.990) was also observed between AUDPC and CI in the season. These strong correlations agreed with the results of Ali et al. [46]. Although positive correlations were observed between infection rate and other disease parameters, the relationship between the variables was weak. This indicates that although severity or the area under the disease progress curve was increasing, the rate of infection reduced as epidemic progressed because less healthy plant tissue was available for additional infections [51].
| Parameters | TRS | CI | AUDPC |
|---|---|---|---|
| TRS | 1 | ||
| CI | 0.872** | 1 | |
| AUDPC | 0.860** | 0.990** | 1 |
| Inf-rate | 0.309 | 0.292 | 0.332 |
Table 4: Pearson’s correlation coefficient for the disease parameters among the wheat cultivars at Adet, 2016 main cropping season. **Large positive relationship between the variables at p = 0.05; TRS=Terminal rust severity; CI=Coefficient of infection; AUDPC=Area under the disease progress curve; Inf-rate=Infection rate.
Since TRS, CI and AUDPC had strong positive correlations in the present study, selection of lines having terminal disease score less than or equal to 30%, CI between 0 and 20 and rAUDPC less than 30% with compatible seedling and adult plant responses is normally accepted for practical purposes. Feasibility of measuring slow rusting resistance preferably by low final ratings and CI has been reported previously by Hei et al. [29], Safavi et al. [50], Singh et al. [52]. Singh et al. [52] also reported that field selection of the slow rusting trait preferably by low rAUDPC and terminal ratings along with CI is feasible.
In the present study varieties Galil, Dinkinesh, Millenium and Shina had TRS of 30% with MR field responses, CI<20 and rAUDPC<30%. However, Millenium and Galil showed resistant infection types (1+) at seedling stage implying the presence of seedling or major gene resistance towards the disease. Varieties Dinkinesh and Shina had susceptible infection types at seedling stages and were identified as having high slow rusting resistance. On the other hand, Pavon 76, Kakaba, Danda’a, Madawalabu, Kingbird, Bonny, Africa Mayo and Alidoro had TRS 31-50% with compatible field responses (MR-MS to MS-S), CI values ranging from 21-45 (Cultivars Bonny and Africa Mayo which had slightly higher CI values of 45) and rAUDPC 31-70%. These varieties also had susceptible infection types at seedling stage and were regarded as moderately slow rusting. The slow rusting wheat cultivars identified in the current research were supposed to be having genes for durable resistance and may be used for further genetic manipulation in wheat improvement programs. Singh et al. [53] also reported that genotypes under group 1 and 2 could have durable resistance controlled by more than one gene which can serve as good parents for breeding.
Grain yield
There was a highly significant difference (P<0.01) between cultivars for grain yield (Table 5). The highest grain yield, 5.51 t ha-1, was obtained from cultivar Hidase whereas the lowest, 2.79 t ha-1, was displayed from cultivar Africa Mayo. Although varieties Hidase, Densa, Shina, and Guna had grain yields more than 5 t ha-1, the yield obtained from Hidase was significantly different from the others. Since the disease severity recorded in this study was high, it might have caused yield differences. However, disease severity difference alone could not cause variation in yield among the cultivars. Cultivars might also differ in their genetic yield potential. For examples, the disease severity recorded on cultivar Hidase was 35% during the season but the yield obtained from the Hidase was higher than some cultivars that had low disease severities, such as Millenium (30%) and Galil (30%). The yield obtained from Africa Mayo was below the yield of the susceptible cultivar Morocco. This would also be due to the lower genetic potential of Africa Mayo for grain yield.
| Varieties | GY | TKW |
|---|---|---|
| Hidase | 5.50667a‡ | 63.33a |
| Densa | 5.32000b | 53.33ab |
| Shina | 5.24000b | 31.00de |
| Guna | 5.16667b | 22.67efg |
| Kakaba | 4.95667c | 50.33abc |
| Madawalabu | 4.65333d | 40.67bcd |
| Senkegna | 4.58333ed | 30.00def |
| Millenium | 4.57333ed | 48.67bc |
| Tay | 4.56000ed | 50.00abc |
| Tuse | 4.48333ef | 30.00def |
| Pavon-76 | 4.42667ef | 31.00de |
| Alidoro | 4.34333f | 39.67cd |
| Shorima | 4.16333g | 40.33bcd |
| Galil | 4.06667g | 50.00abc |
| Hulluka | 3.82667h | 30.00def |
| Bonny | 3.79333h | 50.33abc |
| Dinkinesh | 3.79333h | 49.33bc |
| Danda’a | 3.75333h | 38.00cd |
| Hoggana | 3.75000h | 40.00bcd |
| Ogolcho | 3.73333hi | 21.33efg |
| Kingbird | 3.71333hi | 38.00cd |
| Abolla | 3.56667ij | 50.00abc |
| Gassay | 3.52333j | 51.00abc |
| Morocco | 2.80667k | 17.00fg |
| Africa Mayo | 2.79000k | 40.00bcd |
| CV | 2.422745 | 21.2359 |
| LSD | 0.1672 | 13.471 |
Table 5: Grain yield and thousand kernel weight of the tested cultivars. ‡Means within column followed by the same letter are not significantly different.
In general, the yields obtained from the majority of the cultivars were more than 3 t ha-1. Among the slow rusting cultivars identified, Shina, Kakaba, Madawalabu, Pavon 76 and Alidoro had the highest yields (>4.0 t ha-1) in the season. Their comparatively better yields make them good candidates as donor parent for the incorporation of durable resistance in the bread wheat improvement programmes. Although there were variations in grain yields among the cultivars, there was no protected check plot established for each genotype to calculate yield loss.
Thousand kernel weight (TKW)
The wheat cultivars also showed variation in TKW (P<0.01). The highest TKW was recorded from variety Hidase (63.33 g). The lowest was obtained from Morocco (17 g). Among the slow rusting genotypes identified, Kakaba and Bonny had high TKW values in the season. Thousand kernel weight is an important component of yield mostly affected by stem rust. The reduction in TKW for Morocco might be due to the effect of the disease on the size and mass of the seed. Infection on wheat stem and leaf sheaths by stem rust affects the transport of assimilates to the developing kernel and results in shriveled kernel [54].
Correlation between yield data and disease data
The correlation coefficients considered between pairs of the respective disease parameters with yield data were highly negatively correlated (Table 6). The negative relationship between disease parameters and yield variables involving thousand kernel weight and grain yield showed harmful effects of stem rust on plant characteristics. Relatively higher negative correlations were observed between yield parameters and terminal stem rust severity. These were -0.64 and -0.66, respectively for grain yield and thousand kernels weight. This indicated that as the stem rust severities increased the negative effects of the disease on the TKW and GY increased.
| Parameters | GY | TKW |
|---|---|---|
| TRS | -0.64** | -0.66** |
| CI | -0.63** | -0.51** |
| AUDPC | -0.62** | -0.61** |
Table 6: Correlation between yield data and stem rust disease data on wheat cultivars at Adet, 2016 main cropping season. **significant at p=0.01 probability level; TRS=terminal rust severity; AUDPC=area under disease progress curve; CI=coefficients of infection.
The large negative correlation between TKW and TRS can be attributed to the fact that the fungus damages the vascular system of the susceptible host plant extensively limiting transportation of water and nutrients from the soil to the developing kernel and other organs as well as interfering with translocation of photosynthesis, which leads to shriveled grains [55]. Similar results have been reported by numerous previous research groups [56-58].
Conclusion
The results of the current study showed that most of the tested bread wheat varieties had susceptible infection types for the TTKSK race at seedling stage. All evaluated cultivars exhibited moderately resistant to susceptible field responses under field condition whereby the susceptible check showed the highest disease severity with susceptible reaction. Varieties Galil, Dinkinesh, Millenium and Shina showed moderately resistance reaction to the disease at adult plant stage, with minimum AUDPC and terminal severities. Of these Galil and Millenium had resistant type of reaction at seedling stage indicating that they possess major gene resistance. Therefore, they can be used as sources of stem rust resistance when the aim of the breeding program is for major gene. Whereas Dinkinesh and Shina with the susceptible seedling infection types and low disease parameters in the field were identified as highly slow rusters. On the other hand, cultivar Pavon 76, Kakaba, Danda’a, Madawalabu, Kingbird, Bonny, Africa Mayo and Alidoro had TRS 31-50% with compatible field responses (MR-MS to MS-S), CI values ranging from 21-45 and rAUDPC 31-70% were regarded as moderately slow rusting. The principal effect of slow rusting resistance in the studied cultivars highlighted the value of having them as sources of durable resistance in breeding programmes.
References
- Minot N, Warner J, Lemma S, Kasa, L, Gashaw A, et al. (2015) The wheat supply chain in Ethiopia: Patterns, trends, and policy options. Addis Ababa, Ethiopia.
- Hailu D, Fininsa C (2007) Relationship between stripe rust (Puccinia striiformis) and grain quality of bread wheat (Triticum aestivum) in the highlands of Bale, southeastern Ethiopia. International Journal of Food, Agriculture and Environment 5: 24-30.
- Zelleke G, Agegnehu G, Abera D, Rashid S (2010) Fertilizer and soil fertility potential in Ethiopia: Constraints and opportunities for enhancing the system. International Food Policy Research Institute (IFPRI), Washington, DC, USA.
- Mann M, Warner J (2015) Ethiopian wheat yield and yield gap estimation: A small area integrated data approach. Research for Ethiopia’s Agricultural Policy, Addis Ababa, Ethiopia.
- Dessalegn T, Girma B, Payne TS, Van Deventer CS, Labuschagne MT (2000). Sources of variation for grain yield performance of bread wheat in northwestern Ethiopia. In the Eleventh Regional Wheat Workshop For Eastern, Central and Southern Africa, p: 16.
- Admassu B, Friedt W, Ordon F (2012) Stem rust seedling resistance genes in Ethiopian wheat cultivars and breeding lines. African Crop Science Journal 20: 149-162.
- Denbel W, Badebo A, Alemu T (2013) Evaluation of Ethiopian commercial wheat cultivars for resistance to stem rust of wheat race UG99. International Journal of Agronomy and Plant Production 4: 15-24..
- Singh RP, Huerta-Espino J, Roelfs AP (2002) The Wheat Rusts. In: Curtis BC, Rajaram S, Gomez Macpherson H (eds.). Bread Wheat Improvement and Production. Food and Agriculture Organization (FAO) of the United Nations. Rome, Italy, P: 554.
- Draz IS, Abou-Elseoud MS, Kamara AEM, Alaa-Eldein OAE, El-Bebany AF (2015) Screening of wheat genotypes for leaf rust resistance along with grain yield. Annals of Agricultural Sciences 60: 29-39.
- Kolmer JA (1996) Genetics of resistance to wheat leaf rust. Annual Review of Phytopathology 34: 435-455.
- Singh RP, Huerta-Espino J, William HM (2005) Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turkish Journal of Agriculture and Forestry 29: 121-127.
- Johnson R (1984) A critical analysis of durable resistance. Annual Review of Phytopathology 22: 309-330.
- Parlevliet JT, Van Ommeren A (1975) Partial resistance of barley to leaf rust, Puccinia hordei. II. Relationship between field trials, micro plot tests and latent period. Euphytica 24: 293-303.
- Ke Y, Deng H, Wang S (2017) Advances in understanding broadâ€spectrum resistance to pathogens in rice. The Plant Journal 90: 738-748.
- Bansal U, Bariana H, Wong D, Randhawa M, Wicker T, et al. (2014) Molecular mapping of an adult plant stem rust resistance gene Sr56 in winter wheat cultivar Arina. Theoretical and Applied Genetics 127: 1441-1448.
- Herrera-Foessel SA, Lagudah ES, Huerta-Espino J, Hayden MJ, Bariana HS, et al. (2011) New slow-rusting leaf rust and stripe rust resistance genes Lr67 and Yr46 in wheat are pleiotropic or closely linked. Theoretical and Applied Genetics 122: 239-249.
- Herrera-Foessel SA, Singh RP, Huerta-Espino J, Rosewarne GM, Periyannan SK, et al. (2012) Lr68: a new gene conferring slow rusting resistance to leaf rust in wheat. Theoretical and Applied Genetics 124: 1475-1486.
- Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, et al. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323: 1360-1363.
- Stakman EC, Stewart DM, Loeggering WQ (1962) Identification of physiological races of Puccinia graminis var. tritici. Washington, USA: US Department of Agriculture, Agricultural Research Services E617 (revised)
- Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Canadian Journal of Research 26: 496-500.
- Roelfs AP, Singh RP, Saari EE (1992) Rust diseases of wheat: concepts and methods of disease management. Mexico City, Mexico: CIMMYT.
- Roelfs AP (1985) Epidemiology in North America. In Roelfs AP and Bushnell WR. (eds.). The Cereal Rusts Vol: II; Diseases, Distribution, Epidemiology and Control. Academic Press, Orlando, pp: 403-434.
- Wilcoxson RD, Skovmand B, Atif AH (1975) Evaluation of wheat cultivars for ability to retard development of stem rust. Annals of Applied Biology 80: 275-281.
- Plank J (1968)Â Disease resistance in plants. Academic Press, New York and London.
- SPSS Institute (2005) Statistical package for social sciences-Users guide, Chicago.
- Gomez KA, Gomez KA, Gomez AA (1984)Â Statistical procedures for agricultural research. John Wiley & Sons.
- Parlevliet JE, Van Ommeren A (1988) Accumulation of partial resistance in barley to barley leaf rust and powdery mildew through recurrent selection against susceptibility. Euphytica, 37: 261-274.
- Hei N, Shimelis HA, Laing M, Admassu B (2015) Assessment of E thiopian Wheat Lines for Slow Rusting Resistance to Stem Rust of Wheat Caused by P uccinia graminis f. sp. tritici. Journal of Phytopathology 163: 353-363.
- Nzuve FM, Bhavani S, Tusiime G, Njau PWR (2012) Evaluation of bread wheat for both seedling and adult plant resistance to stem rust. African Journal of Plant Science 6:426-432.
- Parlevliet JE (1988) Strategies for the utilization of partial resistance for the control of cereal rusts. In Breeding Strategies for Resistance to the Rusts of Wheat, El Batan, Mexico (Mexico). CIMMYT.
- Ali S, Shah SJ, Ibrahim M (2007) Assessment of wheat breeding lines for slow yellow rusting (Puccinia striiformis West. tritici). Pakistan Journal of Biological Sciences 10: 3440-3444.
- Li ZF, Xia XC, He ZH, Li X, Zhang LJ, et al. (2010) Seedling and slow rusting resistance to leaf rust in Chinese wheat cultivars. Plant Disease 94: 45-53.
- Safavi SA (2012) Evaluation of slow rusting parameters in thirty seven promising wheat lines to yellow rust. Tech J Eng Appl Sci 2: 324-329.
- Tabassum S (2011) Evaluation of advance wheat lines for slow yellow rusting (Puccinia striiformis f. sp. tritici). Journal of Agricultural Science 3: 239.
- Ali S, Shah SJA, Raman IKH, Maqbool K, Ullah W (2009) Partial resistance to yellow rust in introduced winter wheat germplasm at the north of Pakistan. Australian Journal of Crop Science 3: 37.
- Pathan AK, Park RF (2006) Evaluation of seedling and adult plant resistance to leaf rust in European wheat cultivars. Euphytica 149: 327-342.
- Patil VS, Hasabnis SN, Narute TK, Khot GG, Kumbhar CT (2005) Rusting behaviour of some wheat cultivars against leaf rust under artificial epiphytotic uyconditions. Indian Phytopathology 58: 221-223.
- Wang ZL, Li LH, He ZH, Duan XY, Zhou YL, et al. (2005) Seedling and adult plant resistance to powdery mildew in Chinese bread wheat cultivars and lines. Plant Disease 89: 457-463.
- Subba Rao KV, Snow JP, Berggren GT (1989) Effect of growth stage and initial inoculum level on leaf rust development and yield loss caused by Puccinia recondita f. sp. tritici. Journal of Phytopathology 127: 200-210.
- Jeger MJ (2004) Analysis of disease progress as a basis for evaluating disease management practices. Annual Review of Phytopathology 42: 61-82.
- Hei NB (2017) Evaluation of wheat cultivars for slow rusting resistance to leaf rust (Puccinia trticina Eriks) in Ethiopia. African Journal of Plant Science 11: 23-29.
- Tsilo TJ, Kolmer JA, Anderson JA (2014) Molecular mapping and improvement of leaf rust resistance in wheat breeding lines. Phytopathology 104: 865-870.
- Brown Jr WM, Hill JP, Velasco VR (2001) Barley yellow rust in North America. Annual Review of Phytopathology 39: 367-384.
- Kaur J, Bariana HS (2010) Inheritance of adult plant stripe rust resistance in wheat cultivars Kukri and Sunco. Journal of Plant Pathology 92: 391-394.
- Ali S, Shah SJA, Maqbool K (2008) Field-based assessment of partial resistance to yellow rust in wheat germplasm. Journal of Agriculture and Rural Development 6: 99-106.
- Schafer JF, Roelfs AP (1985) Estimated relation between numbers of urediniospores of Puccinia graminis f. sp. tritici and rates of occurrence of virulence. Phytopathology 75: 749-750.
- Tsilo TJ, Jin Y, Anderson JA (2010) Identification of flanking markers for the stem rust resistance gene Sr6 in wheat. Crop Science 50: 1967-1970.
- Sandoval-Islas JS, Broers LHM, Mora-Aguilera G, Parlevliet JE, Osada-Kawasoe S, et al. (2007) Quantitative resistance and its components in 16 barley cultivars to yellow rust, Puccinia striiformis f. sp. hordei. Euphytica 153: 295-308.
- Safavi SA, Ahari AB, Afshari F, Arzanlou M (2013) Slow rusting resistance in Iranian barley cultivars to Puccinia striiformis f. sp. hordei. Journal of Plant Protection Research 53: 5-11.
- Freedman J, Mackenzie DR (1992) Disease progress curves, their mathematical description and analysis to formulate predictors for loss equations. In. Teng PS (ed.). Crop loss assessment and pest management. International Book Distributing Co., Lucknow, India, pp: 37-48.
- Singh D, Park RF, McIntosh RA (2007) Characterisation of wheat leaf rust resistance gene Lr34 in Australian wheats using components of resistance and the linked molecular marker csLV34. Australian Journal of Agricultural Research 58: 1106-1114.
- Singh RP, William HM, Huerta-Espino J, Rosewarne G (2004) Wheat rust in Asia: meeting the challenges with old and new technologies. In Proceedings of the 4th international crop science congress. Gosford, Australia: The Regional Institute Ltd.
- Calpouzos L, Roelfs AP, Madson ME, Martin FB, Welsh JR, et al. (1976)Â A new model to measure yield losses caused by stem rust [Puccinnia graminis tritici] in spring wheat. Minnesota. Agricultural Experiment Station.
- Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, et al. (2006) Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 1: 1-13.
- Afzal SN, Haque MI, Ahmedani MS, Rauf A, Munir M, et al. (2008) Impact of stripe rust on kernel weight of wheat varieties sown in rainfed areas of Pakistan. Pakistan Journal of Botany 40: 923-929.
- Tadesse K, Ayalew A, Badebo A (2010) Effect of fungicide on the development of wheat stem rust and yield of wheat varieties in highlands of Ethiopia. African Crop Science Journal 18: 23-33.
- CSA (Central Statistical Agency) (2016) Agricultural sample survey: Report on area and production of major crops (Private peasant holdings, Meher Season). Volume III Statistical Bulletins, Addis Ababa, Ethiopia.
Citation: Mitiku M, Hei NB, Abera M (2018) Characterization of Slow Rusting Resistance Against Stem Rust (Puccinia graminis f. sp. tritici) in Selected Bread Wheat Cultivars of Ethiopia. Adv Crop Sci Tech 6: 389. DOI: 10.4172/2329-8863.1000389
Copyright: © 2018 Mitiku M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5288
- [From(publication date): 0-2018 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 4281
- PDF downloads: 1007
