Review Article Open Access
Chemosensors for Hg Ions: A Review of Literature
Sivaprasad Ganesabaskaran* and Karthikeyan Kandasamy
Department of Chemistry, University College of Engineering-Tindivanam, Tamilnadu, India
- Corresponding Author:
- Ganesabaskaran S
University College of Engineering–Tindivanam (Constituent College of Anna University Chennai)
Melpakkam–604001, Villupuram, Tamilnadu, India
Tel: +91-9444656169
E-mail: sibi.gb@gmail.com
Received Date: June 02, 2015 Accepted Date: July 25, 2015 Published Date: July 27, 2015
Citation: Ganesabaskaran S, Kandasamy K (2015) Chemosensors for Hg Ions: A Review of Literature. Biosens J 4:117. doi:10.4172/2090-4967.1000117
Copyright: © 2015 Ganesabaskaran S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biosensors Journal
Abstract
In this review, recently reported Hg ion sensors are briefly discussed. We also summarized selectivity and sensitivity of various chemosensors for mercury ions. The basic principle for emission of radiation, pH limit, suitable solvent, detection limit, mode of binding, ratio between metal and ligand are detailed in this paper.
Keywords
Mercury ion; Naked eye; Quantum yield; Fluorescent sensor; Bathochromic shift; Dosimeter
Introduction
Chemosensors have been placing a crucial role in the analytical chemistry, bio-medicinal science and environmental chemistry [1]. Chemosensors offer an accurate and low-cost finding of anions, enzymes and toxic heavy metal ions with high selectivity and sensitivity [2]. Mercury is considered as highly hazardous, lethal and easily changed into most toxic form like methyl mercury by bacteria and it is extensively scattered in the environment owing to the numerous human deeds and later bio accumulates through the food chain. Hence, the effective and selective detection of Hg ions is of great importance. In this regard, many organic compounds have been synthesized and are being used as a successful chemosensors. Hence we would like to provide the overall summary of the design and application of Hg ion selective chemosensors.
Song et.al utilized an irreversible desulfurization reaction to build up a new fluorescent ratiometric chemosensor (Figure 1) based on the FRET, which would be used to the detection of Hg2+ in aqueous medium [3]. The colorimetric and fluorescent response to Hg2+ can be easily detected even by the naked eye. Chemosensor, compound 1 shown high selectivity and sensitivity for Hg2+ with a wide pH range (1.0-8.0) and can be utilized to set up the fluorescence assay for Hg2+ in living cells.
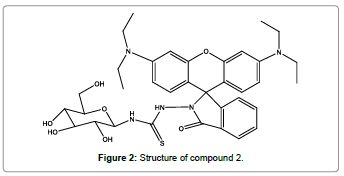
A novel ‘‘turn-on’’ fluorescent sensor (Figure 2) based on glucose and rhodamine B for finding of Hg2+ ions was designed and synthesized by Li et al. [4]. The fluorescent sensor showed a great specificity for Hg2+ ions than for other metal ions in aqueous medium. On the addition of Hg2+ ions to the solution of glucose-based rhodamine B sensor, the absorption and fluorescence signals enhanced remarkably at 567 and 587 nm respectively. Titration of sensor with Hg2+ ions showed 1:1 stoichiometric reaction. Furthermore, glucose-based rhodamine B sensor can be used for the detection of the limited Hg2+ ions in drinking water.
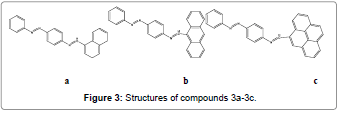
Udhayakumari and Co-workers have designed and synthesized a series of novel azo linked polycyclic aromatic hydrocarbons based sensors (Figures 3a-3c) in single step. Compound 3a and 3b were developed for the selective and sensitive detection of Hg2+ ions over the other transition metal ions. These sensors exhibit fluorescence enhancement with a detectable naked-eye color changes in presence of Hg2+ ions in aqueous solution [5].
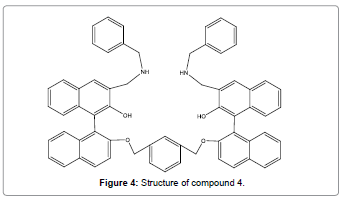
Velmurugan et al. have successfully designed and synthesized a novel BINOL based fluorescent chemosensor (Figure 4) which exhibited a very selective “turn-on” fluorescent chemosensor for Hg2+ ion in the existence of all other metal ions (transition, heavy and alkali metal ions) at neutral pH [6]. The significant enhancement with high emission selectivity of compound 4 toward Hg2+ is due to PET inhibition process. Moreover, the finding limit of receptor compound 4 toward Hg2+ was 4.4 × 10-7 M, which shows that the sensor Figure 4 can be utilized in toxicological, biological, and environmental applications.
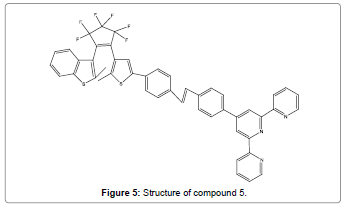
Jing et al. synthesized a highly selective ratiometric fluorescent chemosensor (Figure 5) for Hg2+ ions. It was prepared by joining terpyridine and photochromic perfluoro-diarylethene via a stilbene linkage [7]. When triggered by Hg2+, a 1:2 metal/ligand complex was formed, with the result that its emission intensity enhanced considerably by three fold larger fluorescence quantum yield and the emission peak shown a remarkable bathochromic-shift from 454 nm to 514 nm with clear color change from light blue to bright green.
By intramolecular charge transfer blocking mechanism, Zhang and co-workers have developed a highly selective dual channel chemosensor (Figure 6) for Hg2+ions based on a non-sulfur, easily prepared Schiff base compound [8]. Particularly, the high selectivity experiments showed that the fluorescent sensor is specific for Hg2+ even after interference by high concentrations of other metal ions.
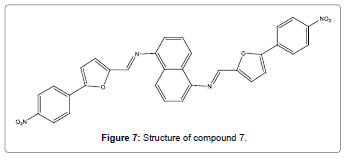
A non-sulfur sensor (Figure 7) of mercury ions has been reported by Wu and co-workers [9]. This sensor has 1, 5-diaminonaphthalene Schiff base derivatives which showed brilliant fluorescent and UVvis responses for Hg2+ ions by the direct cleavage of carbon-nitrogen double bond changing an ICT state mechanism.
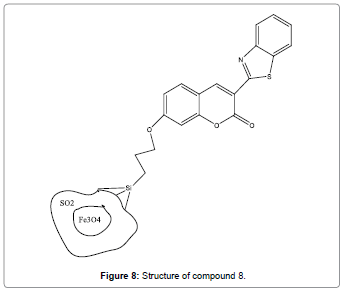
Hosseini et al. have designed and synthesized magnetic core-shell Fe3O4@SiO2 nanoparticles functionalized by BTC which acts as a fluorescent chemosensor (Figure 8) for Hg2+ ion [10]. The enhancement of fluorescence is attributed to the strong covalent binding of Hg2+ ions with the binding constant value of 1.7×105/ M. Sensor compound 8 can be utilized for analysis of Hg2+ ions in environmental sample.
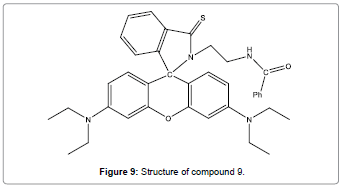
Liu et al. have reported a new rhodamine-based fluorescent probe (Figure 9) for Hg2+ [11]. The colorimetric and fluorescent response to Hg2+ can be easily detected by the naked eye. The selectivity of this system for Hg2+ over other metal ions is admirable.
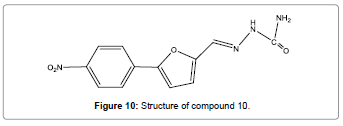
An easy, facile, low-cost and efficient semicarbazone-based chemosensor (Figure 10) with extremely selectivity for Hg2+ has been reported by Qu, and co-workers [12]. Compound 10 gives an instant answer to the Hg2+ ions by fluorescence quenching response. The distinct color changes in UV light and speedy fluorescence extinction can be detected by naked eyes. It is found that this sensor totally free of interference from any other ions. In addition, this sensor acts as a recyclable component in sensing materials. It can be repeated use above 10 times.
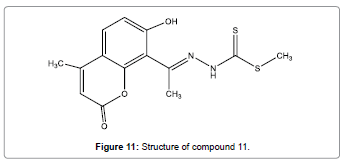
A simple and easy-to-prepare coumarin dithioate derivative has been reported by El-Shekheby et al. as a new, highly selective and sensitive chemosensor (Figure 11) for sensing trace amounts of Hg2+ ions [13]. Compared with some other Hg2+ chemosensors, coumarin dithioate derivative shows advantages such as low finding limits, quick response and high selectivity. It shows effective enhances fluorescence upon complexation with Hg2+ions. The spectral response towards Hg2+ is reversible making it of great potential use in straight recognition.
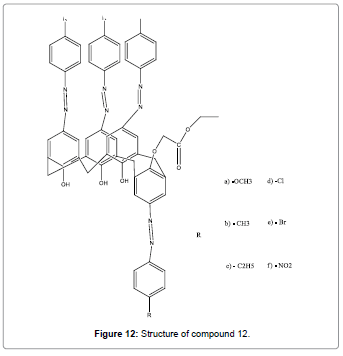
New azocalix[4]arene based chemosensors (Figure 12) that are mainly selective for Hg2+ due to presence of azo groups at the upper rim as the metal binding sites was designed and reported by Elçin et al. [14]. These compounds could be used for the selective extraction of Hg2+ ions from particular samples such as industrial effluents or environmental surface waters.
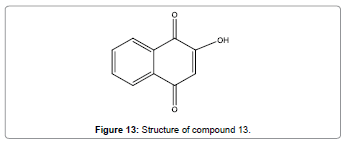
Kavitha et al. have reported a simple, efficient, low price and water soluble chemosensor (Figure 13) that utilizes for exact recognition of Hg2+ ions [15]. High selectivity and sensitivity for toxic Hg2+ ions in neutral aqueous solution was revealed by this chemosensor. This sensor also displays an on-off fluorescent signaling pattern. The emission spectra can be quenched between pH ranging from 6.0 to 12.0. This gives strong confirmation that sensor compound 13 could be used as a fluorescent probe for pH monitoring.
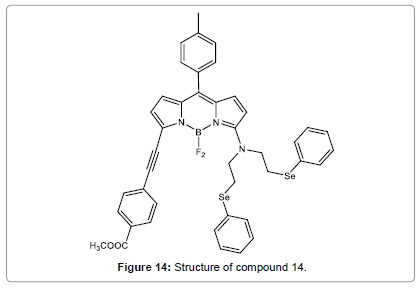
He et al. have designed and synthesized a new chemosensor (Figure 14), having a BODIPY signal moiety and a bis[2-(phenylseleno)ethyl] amine detection site [16]. It was shown remarkable color changes and fluorescence enhancement upon the binding with Hg2+ in CH3CN, which make compound 14 be used as a colorimetric fluorescent sensor. More amusingly, the Hg2+ induced chromogenic process could be reversed by addition of EDTA or cysteine. Based on the “off-on-off” fluorescence of compound 14 induced by Hg2+and cysteine, compound 14 may be engaged to design molecular switches.
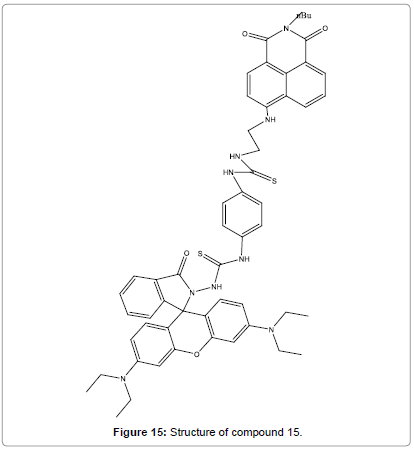
Wang et al. prepared a novel single molecular FRET based fluorescent sensor (Figure 15) for the ratiometric and reversible detection of Hg2+ in aqueous medium [17]. It was shown high sensitivity and selectivity toward Hg2+ and could be suitably identified even by naked eye. Sensor compound 15 was fit in a broad pH range 1.0–10.0 and permitted the ratiometric detection of intracellular Hg2+ levels in live EC 109 cells. Furthermore, sensor compound 15 for the ratiometric fluorescent detection of Hg2+ was speedy and the identifying event could complete in 13 min in practical detection.
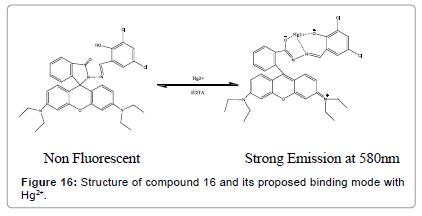
Yang et al. have designed a novel bifunctional colorimetric and fluorescent chemosensor (Figure 16). It shows significant “off–on” fluorescence accompanied with color changes from colorless to red upon binding to Hg2+ ions [18]. The spectral response of compound 16 toward Hg2+ was demonstrated to be reversible and robust against interference from other metal ions.
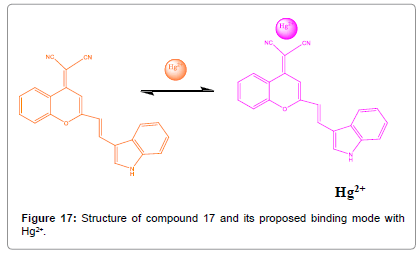
An (E)-2-(2-(2-(1H-indol-3-yl) vinyl)-4H-chromen-4-ylidene) malononitrile (Figure 17) was synthesized by Lee et al. and its cation detecting properties in DMSO were examined by UV–vis spectroscopy [19]. On the addition of Hg2+ to dye compound 17 showed color change and the absorption band indicates a formation of a 1:1 dye compound 17-Hg2+ coordination compound. The dye compound 17 displayed high selectivity for Hg2+ ions as compared with other cations.
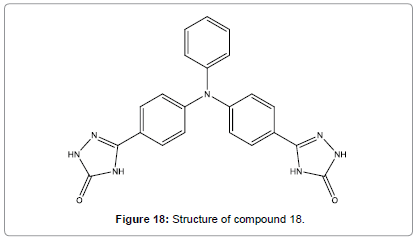
A triphenyl amine semicarbazone derivative (Figure 18) serving as chemosensor for Hg2+ ions using two different discovery modes have been designed by Malkondu et al. [20]. Compound 18 can be utilized as a selective naked eye chemosensor for Hg2+. Besides, TOCAZOL got from the reaction of compound 18 with Cu(ClO4)2 shown a selective fluorescent sensing behavior towards Hg2+ ions. For practical metal ion finding, the investigational conditions were conducted in a binary mixture of MeCN/H2O (9/1).
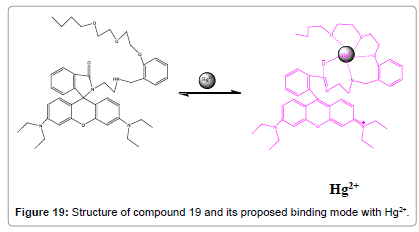
Ghosh and co-workers have reported that rhodamine-labelled receptor (Figure 19) is capable of sensing Hg2+ ions in aq. CH3CN [21]. Concurrent involvement of amide parts of the rhodamines with the polyether chain favours the robust chelation Hg2+ ions. Additionally, the chemosensor compound 19 is found to be effective in reporting the existence of Hg2+ ions inside the cell.
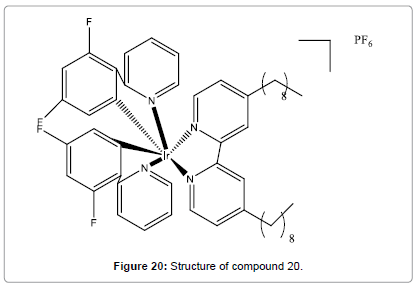
Chan et al. have synthesized and characterized a new non-reaction based luminescent iridium(III) complex (Figure 20) for the quick, selective and direct detection of Hg2+ in aqueous medium [22]. This chemosensor shows a robust luminescence ‘‘switch-on’’ response to Hg2+ with a detection limit in low-micromolar range and is extremely selective for Hg2+ over other metal ions. Besides, the addition of cysteine to the system can return the luminescence signal of compound 20 to the ‘‘off’’ state.
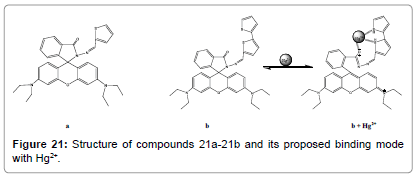
Two new rhodamine hydrazone derivatives bearing one (Figure 21a) and two (Figure 21b) thiophene units have been reported by S. Park and co-workers [23]. The X-ray crystal structure of compound 21a was also got, in which the unique spirolactam ring was clearly shown. compound 21a and compound 21b exhibited highly selective fluorescence changes with Hg2+ among the various metal ions. These selective changes were attributed to the spirolactam ring opening methods and following hydrolysis. The selectivity for Hg2+ ions can be attributed to the affinity of the thiophene group towards Hg2+ and the rigid hydrazone binding site.
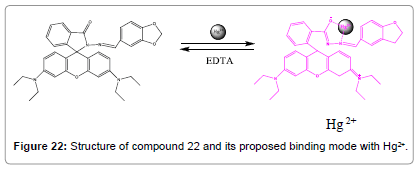
A novel rhodamine B based fluorescent probe (Figure 22) was prepared and characterized by Zhang et al. Probe compound 22 displayed good sensitivity and selectivity recognition to Hg2+ ions over other metal ions and anions [24]. On the addition of Hg2+ ions, compound 22 revealed remarkably improved fluorescent intensity and color alteration from colorless to pink in Tris–HCl (10 mM, pH = 7.2) aqueous buffer medium. The intra cellular fluorescent imaging renders it a capable probe to analyze Hg2+ ions in living cells, highly selective and sensitive detection method for Hg2+ was effectively applied to water samples analysis.
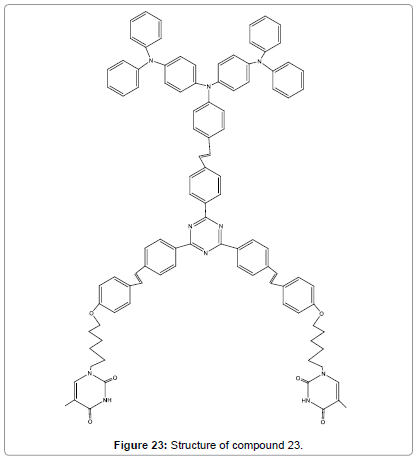
A new red fluorescent sensor (Figure 23) for Hg2+ was designed by using of the specific binding of thymine with Hg2+ ions as well as the AIE feature of triphenylamine–triazines motif [25]. The aggregation was confirmed by TEM analyses, absorption and 1H NMR spectral of compound 23 in the presence of Hg2+ ions. This test revealed that the probe is highly selective and sensitive for Hg2+ ions even in the presence of other metal ions. Additionally, compound 23 showed large two photon absorption cross-section (3328 GM), telling that the targeted two-photon imaging is highly suitable for biological applications.
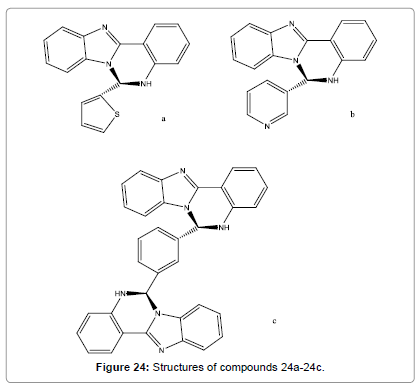
Pandey et al. have designed and synthesized some new quinazolines (Figure 24a-24c). Strong fluorescence at RT was shown by these probes. Absorption, fluorescence, 1H NMR, HRMS and FAB-MS spectral studies have been utilized for the Hg2+ detection. Fluorescence intensity of compound 24a-24c quenches selectively in the presence of Hg2+ ions. Job’s plot study shown that compound 24a and 24b interact with Hg2+ ions in 1:1, while compound 24c in 1:2 (probe/metal) stoichiometries [26].
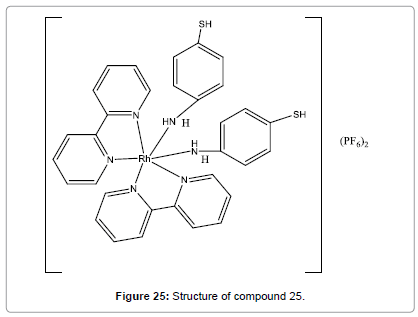
Hamid et al have synthesized dye (Figure 25). This probe works efficiently with amazing high selectivity and sensitivity [27]. The interaction taking place between Hg(II) ions and compound 25 complex (through thiol groups) was responsible for the spectral changes. It can be used for highly selective and sensitive chemosensor for Hg2+ ions in alcoholic–aqueous medium even in the presence of comparatively high concentrations of competing other metal ions. Moreover, and in terms of sensitivity, the limit of quantification of the probe was estimated to be lesser than 0.4 ppm.
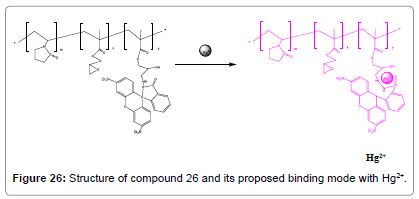
A simple and low-cost post-functionalization policy was used to synthesize a new fluorescent Hg2+ polymeric chemosensor (Figure 26) by covalent coupling of organic fluorescent molecular RhBH to a watersoluble copolymer poly (VP-co-GMA).This RhBH-functionalized polymer poly (VP-co-GMA-g-RhBH) showed outstanding selectivity and high sensitivity towards the finding of Hg2+ in aqueous medium with remarkably better fluorescent intensities and also unblemished color changes from colorless to pink. It can be utilized for many practical applications in environmental, chemical and biological systems [28].
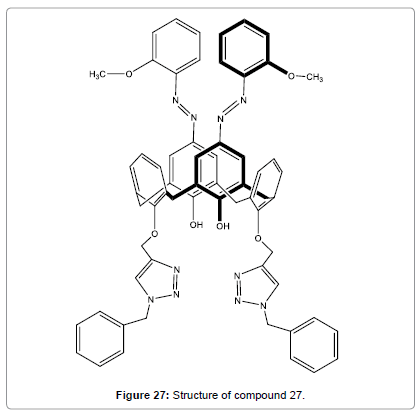
A series of ortho-methoxyphenylazocalix[4]arenes with different lower-rim substituents or different number of the upper-rim azo unit were prepared to probe the ion sensing abilities of the triazole groups and the o-methoxy-phenylazophenol units by Wang et al. Ligand (Figure 27), which has two distal ortho-methoxyphenyl azo groups on the upper-rim and bis-benzyl-1,2,3-triazolylmethoxy groups at the lower rim, was confirmed to be a ratiometric and specific chromogenic sensor for Hg2+ in polar protic solvent MeOH/CHCl3 (v/v, 98:2) [29].
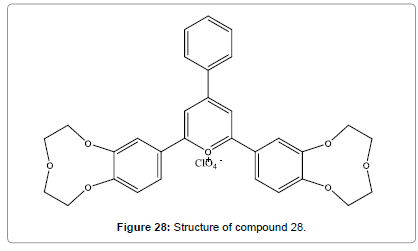
Yari et al reported (Figure 28), the ability of a ligand containing crown sub-units to coordinate Hg2+ ion in the sol–gel derived film [30]. As the ligand having a suitable light sensitive moiety, its changes at the electronic level upon interaction with metal centers. Furthermore, probe has many advantages including outstanding extensive dynamic range, very low detection limit, high selectivity and better reproducibility.
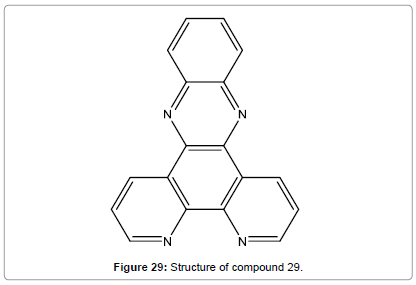
Wang et al. have synthesized Dipyrido[3,2-a:20,30-c]-phenazine(L) which was employed as a selectively fluorescent chemosensor (Figure 29) for Hg2+ in DMF [31]. The fluorescence increasing was attributed to the formation of compound 29 Hg2+ by 1:1 complex ratio. The experiment results also showed that the response behavior of compound 29 to Hg2+ is pH independent in the range of pH 6.0-9.0 and show outstanding sensitivity and selectivity for Hg2+ over other examined metal ions.
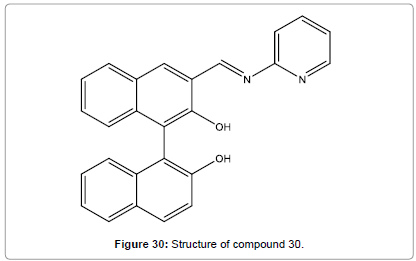
Ma et al. have designed and reported a binaphthyl-based fluorescent chemosensor (Figure 30) which could be used as a highly selective and sensitive chemosensor, and specifically identify Hg2+ ion [32]. The high emission selectivity of compound 30 to Hg2+ was purely driven by the selective creation of 1:2 complex.
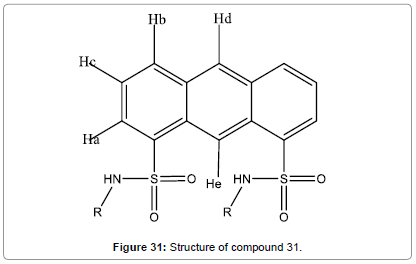
A simple and easy-to-prepare fluorescent chemosensor (Figure 31) for Hg2+ based on 1,8-anthracenedisulfonamide has been designed and reported by Hu et al. [33]. The selectivity of this chemosensor for Hg2+ ions in aqueous medium over other metal ions is high. The spectral response to Hg2+ is reversible.
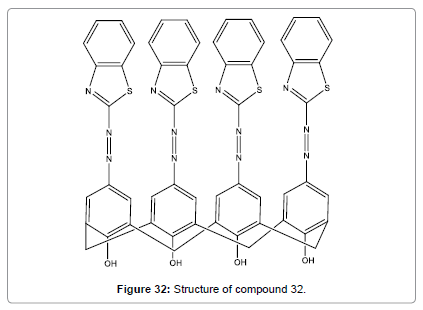
Bingol et al. have designed and synthesized a novel calix[4]arene chemosensor (Figure 32) with benzothiazole azo groups at the upper rim as the metal ion binding sites [34]. It showed high selectivity for Hg2+ in DMF. The Hg2+ ion detection provides a large bathochromic shift in the absorption spectrum (from light orange colour to reddish colour), which is clearly visible to the naked eye. This probe may be very useful for rapid detection of Hg2+ ion.
Ramesh et al. designed, a simple nanocomposite thin film sensor based on silver nanoparticles embedded in poly(vinyl alcohol) fabricated through a facile in situ assembly procedure [35]. It was shown fast, sensitive and selective detection of mercury in all its oxidation states. It can be used in situ and ex situ analysis and also shows Low-cost, linear response over a wide range of concentrations.
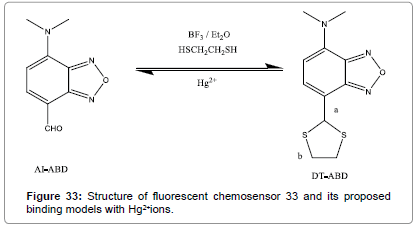
A new Hg2+ chemodosimeter DT-ABD (Figure 33) was designed and synthesized by Chem et al. which displays specific Hg2+-induced emission enhancement over other metal ions [36]. DT-ABD Hg2+ sensing ability via “turn-on” fluorescent response and colorimetric response are obtained through Hg2+ triggered aldehyde recovery from 7-(1`, 3` dithiane) of DT-ABD. ICT effect of AI-ABD is favoured by the electron withdrawing nature of 7-aldehyde that makes emission behavior. It shows high sensitivity, quick response, pH-independent sensing ability. Hence it can be used a practical detector as well as imaging agent for Hg2+.
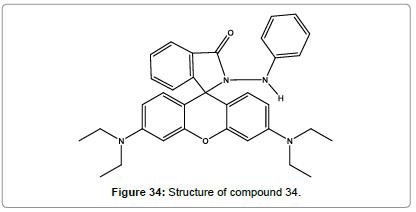
Suresh kumar et al. [37] have synthesized a novel rhodamine based chemosensor (Figure 34). As it displays visible colour change, it can be used a naked eye detection in various industrial samples. It gave selective binding to Hg2+ and spirolactum ring opening. During this process, it results in remarkable fluorescence enhancement at 580 nm emission. Hence it can be used a selective and sensitive chemosensor for Hg2+ ions. They also demonstrated that RBPH is applicable for Hg2+ imaging in cellular media. The RBPH exhibits higher quantum yield when compare to other reported rhodamine florescent probes for Hg2+ ions.
A novel naphthalene diimide-dithiocarbomate based fluorescence probe (Figure 35) was synthesized by Pal et al. [38]. On the addition of various metal ions, the probe gave an irreversible change only for Hg2+ ions. The detection limit of the sensor is 2.1X10-7 M. It can also probe Hg2+ ions in cells by using the dosimeter exploiting its high cell permeability.
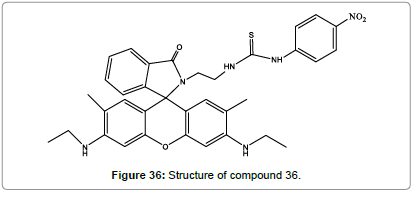
A novel rhodamine-based chemodosimeter (Figure 36) has been prepared for the detection of Hg2+ in aqueous medium by Quy et al. [39]. Compound 36 shows good selectivity and sensitivity to Hg2+ ions over other ions. The solution of compound 37 with Hg2+ can cause OFF-ON fluorescence which is a visible color change from colorless to pink. The low detection limit is 0.04 μM which make it a promising for the determination of Hg2+ ions.
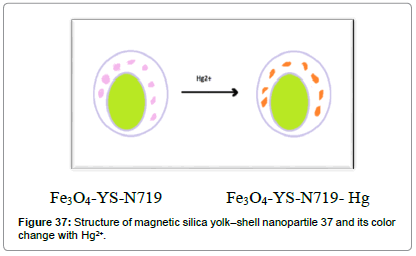
Yao et al. [40] have demonstrated the construction of magnetic silica yolk–shell structure nanoparticles (Figure 37). Filling of N719 endowed the nanocomposites with detecting and eliminating functions towards mercury ions. This probe response to mercury ions within 1 minute and eliminate more than 97% of Hg2+ ions.
References
- Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108: 1517-1549.
- Kim JS, Quang DT (2007) Calixarene-derived fluorescent probes. Chem Rev 107: 3780–3799.
- Song J, Huai M, Wang C, Xu Z, Zhao Y, et al. (2015) A new FRET ratiometric fluorescent chemosensor for Hg2+ and its application in living EC 109 cells. SpectrochimActa AMolBiomolSpectrosc 139: 549-554.
- LI L, Fang Z (2014) A Novel ‘‘Turn On’’ Glucose-Based Rhodamine B Fluorescent Chemosensor for Mercury Ions Recognition in Aqueous Solution. SpectroscLett 48: 578-585.
- UdhayakumariD, Velmathi SA (2015) Linked Polycyclic Aromatic Hydrocarbons Based Dual Chemosensor for Cu2+ and Hg2+ ions. IndEngChem Res 54:3541- 3547
- Velmurugan K, Nandhakumar R (2015) Binol based “turn on” fluorescentchemosensor for mercury ion. JLumin 162: 8-13
- Jing S, Zheng C, Pu S, Fan C, Liu G (2014) A highly selective ratiometric fluorescent chemosensor for Hg2+ based on a new diarylethene with a stilbene-linked terpyridine unit. Dyes Pigm 107: 38-44.
- Zhang YM, Qu WJ, Gao GY, Shi BB, Wu GY, et al. (2014) A highly selective dual-channel chemosensor for mercury ions: utilization of the mechanism of intramolecular charge transfer blocking. New JChem 38: 5075-5080.
- Wu G, Shi B, Hu B, Zhang Y, Lin Q, et al. (2014) A Rational Designed Dual-channel Chemosensor for Mercury Ions Based on Hydrolysis of Schiff Base. Chin J Chem 32: 637-644.
- Hosseini M, Memari Z, Ganjali MR, Khoobi M, Faridbod F, Shafiee A, et al. (2014) A Novel Mercury-Sensitive Fluorescent Nano-chemosensor using new Functionalized Magnetic core-shell Fe3O4@SiO2 Nanoparticles. Int J Environ Res 8:861-870.
- Liu Y, Yang EB, Han R, Zhang D, Ye Y, et al. (2014) A new rhodamine-based fluorescent chemosensor for mercury in aqueous media. Chin ChemLett 25:1065-1068.
- Qu WJ, Gao GY, Shi BB, Wei TB, Zhang YM, et al. (2014) A highly selective and sensitive fluorescent chemosensor for mercury ions based on the mechanism of supramolecular self-assembly.Sens Actuators B Chemical 204: 368-374
- El-Shekheby HA, Mangood AH, Hamza SM, Al-Kady AS, Ebeid EZM (2014) A highly efficient and selective turn-on fluorescent sensor for Hg2+, Ag+ and Ag nanoparticles based on a coumarindithioate derivative. Luminescence29:158-167
- Elçin S, Deligöz H (2014) A versatile approach toward chemosensor for Hg2+ based on para substituted phenylazocalix[4]arene containing mono ethyl ester unit. Dyes Pigm 107:166-173.
- Kavitha R, Stalin T (2014) A highly selective chemosensor for colorimetric detection of Hg2+ and fluorescence detection of pH changes in aqueous solution. J Lumin 149: 12-18.
- He X, Zhang J, Liu X, Dong L, Li D, et al. (2014) A novel BODIPY-based colorimetric and fluorometric dual-mode chemosensor for Hg2+and Cu2+. Sens Actuators B 192: 29-35.
- Wang C, Zhang D, Huang X, Ding P, Wang Z, et al. (2014) A ratiometric fluorescent chemosensor for Hg2+ based on FRET and its application in living cells. Sens Actuators B 198: 33-40.
- Yang Y, Gao C, Li B, Xu L, Duan L (2014) A rhodamine-based colorimetric and reversible fluorescent chemosensor for selectively detection of Cu2+ and Hg2+ ions. Sens Actuators B 199: 121-126.
- Lee EM, Gwon SY, Kim SH (2014) Spectral properties of highly selective chemosensor for Hg2+. Spectrochim. Acta AMolBiomolSpectrosc 120: 646-649.
- Malkondu S, Erdemir S (2014) A triphenylamine based multi-analytechemosensor for Hg2+ and Cu2+ ions in MeCN/H2O. TetrahedronLett70:5494-5498
- Ghosh K, Sarkar T, Majumdar A (2013) Rhodamine-labelled new architecture for dual sensing of Co2+ and Hg2+ ions. Tetrahedron Lett 54: 6464-6468.
- Chan DSH, Fu WC, Wang M, Liu LJ, Leung CH, et al. (2013) A Highly Selective and Non-Reaction Based Chemosensor for the Detection of Hg2+ Ions Using a Luminescent Iridium(III) Complex. Plos One 8: e60114.
- Park S, Kim W, Swamy KMK, Lee HY, Jung JY, et al. (2013) RhodamineHydrazone Derivatives Bearing Thiophene Group as Fluorescent Chemosensors for Hg2+. Dyes Pigm 99:323-328
- Zhang J, Zhou Y, Hu W, Zhang L, Huang Q, et al. (2013) Highly selective fluorescence enhancement chemosensor for Hg2+ based on rhodamine and its application in living cells and aqueous media. Sens Actuators B 183: 290-296.
- Zhang H,Qu Y, Gao Y, Hua J, Li J, et al. (2013) A red fluorescent ‘turn-on’ chemosensor for Hg2+ based on triphenylamine–triazines derivatives with aggregation-induced emission characteristics. Tetrahedron Lett 54: 909-912.
- Pandey R, Yadav M, Shahid M, Misra A, Pandey DS (2012) Design and synthesis of fluorescent 6-aryl[1,2-c]quinazolines serving as selective and sensitive ‘on-off’ chemosensor for Hg2+ in aqueous media. Tetrahedron Lett 53:3550-3555.
- Amer AG, Hamid AA, Al-Khateeb M, Tahat ZA, Qudah M, et al. (2011) A Selective Chemosensor for Mercuric Ions Based on 4-Aminothiophenol-Ruthenium (II) Bis(bipyridine) Complex. Int J InorgChem 2011.
- Luo J, Jiang S, Qin S, Wu H, Wang Y, et al. (2011) Highly sensitive and selective turn-on fluorescent chemosensor for Hg2+ in pure water based on a rhodamine containing water-soluble copolymer. Sens Actuators B 160: 1191-1197.
- Wang NJ, Sun CM, Chung WS (2011) A specific and ratiometricchemosensor for Hg2+ based on triazole coupled ortho-methoxyphenylazocalix[4]arene. Tetrahedron 67: 8131-8139.
- Yari A, Abdoli HA (2010) Sol–gel derived highly selective optical sensor for sensitive determination of the mercury (II) ion in solution. J Hazard Mater 178: 713-717.
- Wang XL, Zheng WY, Liu GC, Lin HY (2010) A turn-on fluorescent chemosensor for Hg2+ based on phenanthrolinefluorophore. JLumin 130:52-55.
- Ma TH, Zhang AJ, Dong M, Dong YM, Peng Y, et al. (2010) A simply and highly selective ‘‘turn-on’’ type fluorescent chemosensor for Hg2+ based on chiral BINOL-Schiff’s base ligand. JLumin 130: 888-892.
- Hu ZQ, Yang XD, Cui CL, Ding L, Lin CS, et al. (2010) 1,8-Anthrancene disulfonamide: A simple but highly sensitive and selective fluorescent chemosensor for Hg2+ in aqueous media. Sens Actuators B 145: 61-65.
- Bingol H, Kocabas E, Zor E, Coskun A (2010) A novel benzothiazole based azocalix[4]arene as a highly selective chromogenic chemosensor for Hg2+ ion: A rapid test application in aqueous environment. Talanta 82:1538-1542.
- Ramesh GV,Radhakrishnan TP (2011) A Universal Sensor for Mercury (Hg, HgI, HgII) Based on Silver Nanoparticle-Embedded Polymer Thin Film. ACS Appl Mater Interfaces 3: 988-994.
- Chen Y, Zhu C, Yang Z, Li J, Jiao Y, et al. (2012) A new ‘‘turn-on’’ chemodosimeter for Hg2+: ICT fluorophore formation via Hg2+-induced carbaldehyde recovery from 1,3-dithiane. ChemCommun 48: 5094-5096.
- Kumar SK,Ramakrishnappa T, Balakrishna RG,Pandurangappa M (2013) A Fluorescent Chemodosimeter for Hg2+ Based on a Spirolactam Ring-Opening Strategy and its Application Towards Mercury Determination in Aqueous and Cellular Media. J Fluoresc 24:67-74
- Pal S, Hatai J, Samanta M, Shaurya A,Bandyopadhyay S (2014) A highly selective chemodosimeter for fast detection and intracellular imaging of Hg2+ ions based on a dithiocarbamate–isothiocyanate conversion in aqueous ethanol. Org BiomolChem 12: 1072-1078.
- Quy PT, Hien NK, Bao NC, Nhan DT, Khanh DV, et al. (2014) A new rhodamine-based fluorescent chemodosimeter for mercuric ions in water media. Luminescence 30:325-329.
- Yao L, Shen B, Cao C, Feng W, Li F (2014) Chemodosimeter functionalized magnetic silica yolk–shell nanocomposite for sensing and removal of Hg2+. RSC Adv 4: 20252-20255.
Relevant Topics
- Amperometric Biosensors
- Biomedical Sensor
- Bioreceptors
- Biosensors Application
- Biosensors Companies and Market Analysis
- Biotransducer
- Chemical Sensors
- Colorimetric Biosensors
- DNA Biosensors
- Electrochemical Biosensors
- Glucose Biosensors
- Graphene Biosensors
- Imaging Sensors
- Microbial Biosensors
- Nucleic Acid Interactions
- Optical Biosensor
- Piezo Electric Sensor
- Potentiometric Biosensors
- Surface Attachment of the Biological Elements
- Surface Plasmon Resonance
- Transducers
Recommended Journals
Article Tools
Article Usage
- Total views: 14874
- [From(publication date):
specialissue-2015 - Aug 25, 2025] - Breakdown by view type
- HTML page views : 13369
- PDF downloads : 1505