Clinical Evaluation of Mucin-1 (MUC1) and P16 in Laryngeal Cancer
Received: 22-Jul-2016 / Accepted Date: 05-Aug-2016 / Published Date: 11-Aug-2016 DOI: 10.4172/2161-119X.1000255
Abstract
Background: Adapted from results in the field of cervical cancer, a direct connection between HPV infection and oropharyngeal carcinoma development could be established. Aim of this study was to evaluate p16 and TA-MUC1 in laryngeal cancer and their correlation to diagnostic, since TA-MUC1 is primarily restricted to malignancies. Methods: Paraffin-embedded laryngeal cancer specimens (n=129) and normal tissue (n=5) were analyzed for TA-MUC1 expression using hPankoMab-GEXTM antibody and evaluated according the immunoreactive score. Survival was assessed via log-rank test and Kaplan-Meier-survival analysis. Results: Significant correlation with tumor grading and staging was exhibited by TA-MUC1staining, while being negative in normal tissues. Expression of p16 significantly increased in T4 compared to T1 tumors. Significant differences in overall survival were found in correlation to TNM-classification, grading and relapse. TA-MUC1 showed a positive trend correlating to p16. Conclusion: Because of this positive trend, we suggest a HPV association in head and neck tumors. Most likely due to an insufficient quantity of HPV-positive patients, no statistical significance could be established. However, targeting TA-MUC1 would improve tumor therapy by linking hPankoMab-GEXTM to the overexpressed galectin. Systematic analysis of HPV-association should be performed generally in laryngeal cancer to gain further information about the interaction of HPV and malignancies.
Keywords: MUC-1; Laryngeal cancer; TA-MUC1; p16; Human papilloma virus (HPV); Head and neck squamous cell carcinoma (HNSCC); hPankoMab-GEXTM
252648Abbreviations
MUC: Mucin-1; TA-MUC1: Tumor-Associated MUC1; HPV: Human Papilloma Virus; RTK: Receptor Tyrosin Kinase; SLeX: Sialyl Lewis x; SLeA: Sialyl Lewis a; LeY: Lewis Y; TF: Thomsen-Friedenreich Antigen; Gal-1: Galectin-1; DAB: Diaminobenzidine; HNSCC: Head and Neck Squamous Cell Carcinoma, pRb: Retinoblastoma Protein; uVIN: Usual-Type Vulvar Intraepithelial Neoplasia; ADCC: Antibody Dependent Cellular Cytotoxicity
Introduction
Malignant neoplasms of the larynx belong to the most frequent cancer entities in the upper aero digestive tract and squamous cell carcinomas are most common. Treatment decisions depend on stage of disease. Surgery or definitive irradiation with a curative intent is performed often in early stages, whereas more advanced disease stages are usually treated with surgery, radio chemotherapy or radio chemotherapy/radio immunotherapy. Accurate determination of tumor size and localization, as well as detailed knowledge of the presence of lymph node metastases is obligatory for an individualized therapy [1].
HPV in head and neck cancer
Human papillomavirus (HPV) is one of the most investigated pathogenic DNA viruses. Primarily HPV was held responsible only for cervical cancer, but in the last two decades it appeared as a major cause for head and neck cancer as well [2,3]. A total of 28% of all laryngeal carcinomas are associated with HPV [4,5]. More affected by HPV is the oropharyngeal cancer. The publicized numbers of entities range between 25% and 60%, sometimes up to 90% [6]. Patients suffering from head and neck cancer associated with HPV feature a 30% better survivability because of younger patients [7], less relapse [8] and better response to therapies [9]. The cell cycle regulation protein p16 is overexpressed in HPV infected epithelial cells and its verification is still the most common proof of a HPV infection [10,11].
TA-MUC1
Mucin 1 (MUC1) is a high molecular weight transmembrane glycoprotein and expressed on the surface of epithelia all over. In addition its intracellular part is an active receptor tyrosine kinase (RTK) and so it is involved in different signaling pathways [12-14]. In malignant processes MUC1 becomes a carrier protein for oncofetal carbohydrates such as sialyl Lewis x (SLeX), sialyl Lewis a (SLeA), Lewis Y (LeY) and the Thomsen-Friedenreich Antigen (TF) [15]. Expression of the described antigens in benign tissue is mainly restricted to epithelial tissue of human reproduction [16]. Laryngeal cancer shows high expression of SLeA, Gal-1 and TF in contrast to normal tissue of tongue, vocal cord, pharynx, epiglottis and larynx [17]. The latest established epitope of MUC1 is the exclusively tumor related TA-MUC1 [18]. This tumor specific epitope stays adherent to the cell membrane. The appropriate matching monoclonal humanized antibody hPankoMab-GEXTM is unrivalled compared to all current MUC1 antibodies due to strongest specificity and greatest binding capacity [19]. It reacts with a great number of different carcinomas [18,20,21]. On the other hand, hPankoMab-GEXTM already provided good results in clinical trials, phase 1 and 2, for patients suffering from ovarian cancer (unpublished information from Glycotope GmbH, Berlin, Germany).
The screening of laryngeal cancer patients for HPV and TA-MUC1 might not only provide better assessment of prognosis, but also new approaches for therapy. Therefore the aim of our study was the evaluation of p16, its foundation for HPV diagnosis and staining of TA-MUC1. Second aim was the correlation of evaluated staining results to TNM-classification, grading and relapse and their influence on overall survival.
Materials and Methods
Study population
Laryngeal carcinoma specimens of 129 patients were taken after undergoing surgery and histological classification including TNM staging. Thereof 31 were classified as G1, 58 as G2 and 40 as G3. Complete histological and follow up data of all patients were available (grading, staging, date of surgery, relapse, last contact, viability). Normal material, such as tongue, vocal cord, larynx, pharynx and epiglottis, was taken from autopsies at legal medicine (n=5). Omission of any kind of cancer is assured. All samples were processed anonymously; the study was approved by the Ethics Committee of University Hospital Erlangen with a declaration of no objection on 10.07.2012 for using retrospective data analysis and was carried out in compliance with the guidelines of the Helsinki Declaration.
Immunohistochemistry
TA-MUC1: The peroxidase-labeled humanized monoclonal PankoMab-GEXTM was used in a concentration of 2.7 μg/ml (Glycotope GmbH, Berlin, Germany). Immediately after surgery or autopsy tissue specimens were formalin-fixed and subsequently embedded in paraffin. Paraffin sections of 3 μm were prepared and provided for immunohistochemistry by heating them at 55°C overnight. Slides were deparaffinized and rehydrated step wisely in ethanol. No antigen retrieval was necessary, but endogenous peroxidase activity was blocked by 3% H2O2 in methanol for 20 min. unspecific binding sites were of no consequence because of the purity of the antibody. The sections were incubated with the peroxidaselabeled humanized PankoMab-GEXTM (2.7 μg/ml) for 90 min at room temperature. Color development was done by DAB (diaminobenzidine) and counterstaining by hematoxylin [20]. At each approach ovarian and breast cancer specimen were taken as positive controls and omission of the specific antibody as well as incubation with bovine serum as negative controls. According to the immunoreactive score of Remmele and Steger (IRS) slides were analyzed by two different investigators. Intensity of staining and the percentage of positive cells were multiplied for evaluation.
p16: CINtecHistology, Roche, Mannheim, German Specimens were automatically stained using Ventana Benchmark XT. The slides were evaluated by a pathologist. The staining intensity was disposed in 1=low, 2=moderate, 3=strong. Negative and positive control slides were carried along.
HPV diagnosis: Strong p16 expression (intensity 3) was considered as HPV positive. In accordance to histological norm moderate expression (intensity 2) was assessed as HPV positive only in event of outspread, not only focal immunohistochemically staining of p16.
Statistics
Data were analyzed employing the SPSS (v19, IBM, Armonk, New York) statistic software for MS windows and visualized using Microsoft Office 7. Spearman coefficients were employed to correlate data, while the Mann-Whitney U was applied to test for differences between groups. Differences in survival were assessed by applying the log-rank test and survival curves were plotted in accordance with Kaplan-Meier survival analysis. Statistical significance for all tests was set as p<0.05 and data were expressed in terms of mean ± standard error (SEM).
Results
Evaluation of the hPankoMabTM specificity and staining of breast and ovarian cancer tissue as positive controls
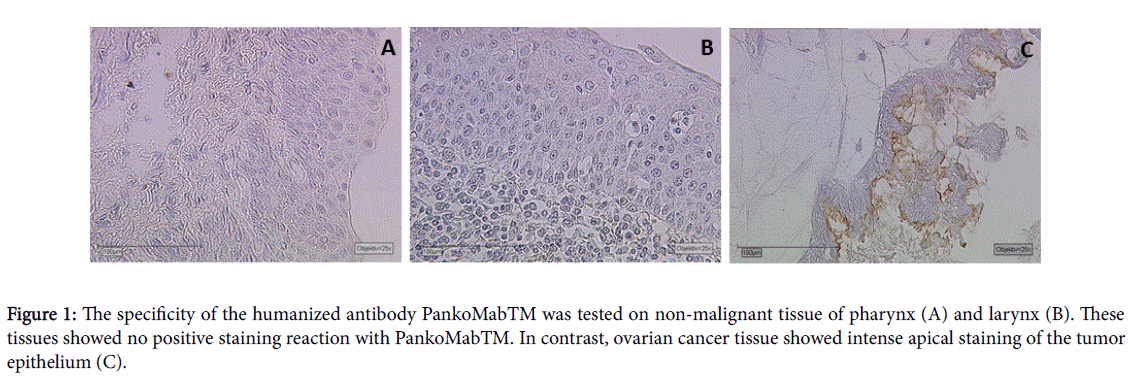
All normal tissues of the upper aero-digestive region, such as vocal cord, pharynx (Figure 1A), larynx (1B), tongue and epiglottis remained completely negative. Human epithelia cancer tissue was used as positive control tissue. We identified an intense staining of TA-MUC1 in breast cancer as well as ovarian cancer tissue (1C). Negative control was performed by omission of hPankoMab-GEXTM and incubation with bovine serum.
The expression of TA-MUC1 is increased in laryngeal tumors related to grading and tumor growth
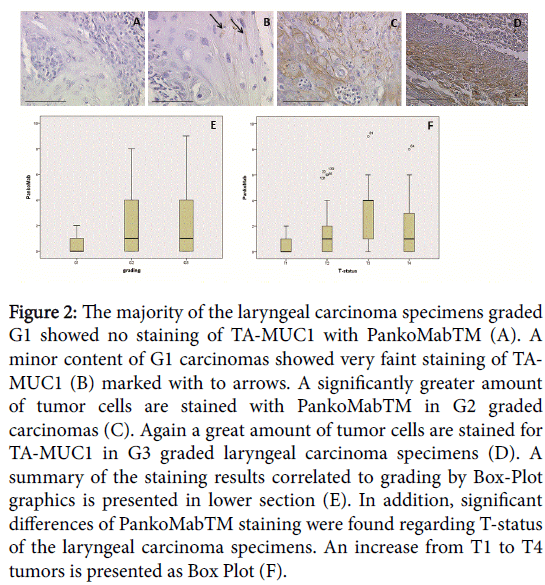
A total of 22 cases of 31 G1 laryngeal tumors (71%) were completely negative for TA-MUC1 with an IRS=0 and the remaining 9 didn`t reach an IRS higher than 2. All of the G1 tumors didn`t score an IRS relevant scope (Figure 2A). Only few cases showed faint expression of TA-MUC1 (2B). In contrast to the former latter, G2 (2C) and G3 tumors (2D) showed an enhanced TA-MUC1 staining. G2/G3 graded tumor specimens did not differ in the range of the immunoreactive score (IRS). In large part they appeared positive with an IRS up to 9. Focusing on the tumor staging the expression of TA-MUC1 increased with the tumor growth. On average T3 and T4 tumors reached a higher IRS. According the expression of TA-MUC1 to tumor grading reaches statistical significance (p=0.001). G1 tumors showed significantly less staining compared to G2 (p<0.001) and G3 (Figure 2E, p=0.001) carcinomas. In correlation to the tumor stadium the expression of TA-MUC 1 showed an significant increase of staining in tumors in correlation to staging (Figure 2F, p<0.001).
Figure 2: The majority of the laryngeal carcinoma specimens graded G1 showed no staining of TA-MUC1 with PankoMabTM (A). A minor content of G1 carcinomas showed very faint staining of TAMUC1 (B) marked with to arrows. A significantly greater amount of tumor cells are stained with PankoMabTM in G2 graded carcinomas (C). Again a great amount of tumor cells are stained for TA-MUC1 in G3 graded laryngeal carcinoma specimens (D). A summary of the staining results correlated to grading by Box-Plot graphics is presented in lower section (E). In addition, significant differences of PankoMabTM staining were found regarding T-status of the laryngeal carcinoma specimens. An increase from T1 to T4 tumors is presented as Box Plot (F).
The expression of the HPV-related protein P16 is increased in laryngeal tumors related to tumor growth but not to grading
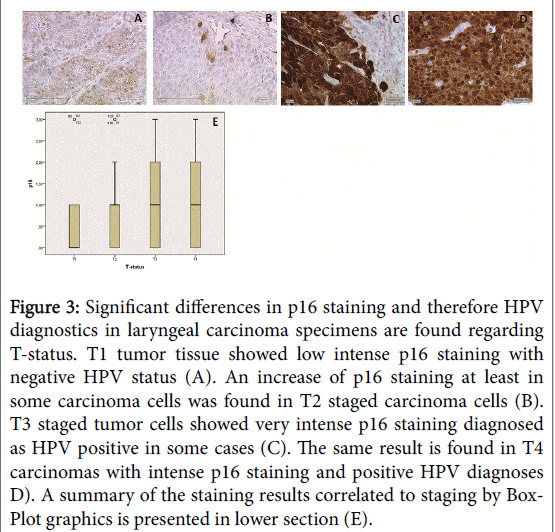
All G1 laryngeal carcinomas revealed no or only a low expression (intensity 1). G2 and G3 tumors presented mostly a moderate till strong staining level (intensity 2 and 3) in addition of a few cases without an expression (41%). Concerning the tumor staging T1 staged tumor tissue showed faint and non-intense p16 staining (Figure 3A), T2 staged tumors showed more intense but focal p16 expression (3B) an increasing staining intensity can be observed in T3 staged tumor tissue (3C) and a very intense p16 expression was found in T4 carcinomas (3D), which are already spreading and not restricted to the larynx anymore. Summarized 46% of all reviewed paraffin sections remained completely negative. According the expression of p16 to tumor grading doesn`t reach statistical significance. In correlation to the tumor stadium the expression of p16 showed a significant increase of staining in T4 in comparison to T1 tumors (Figure 3F, p=0.034).
Figure 3:Significant differences in p16 staining and therefore HPV diagnostics in laryngeal carcinoma specimens are found regarding T-status. T1 tumor tissue showed low intense p16 staining with negative HPV status (A). An increase of p16 staining at least in some carcinoma cells was found in T2 staged carcinoma cells (B). T3 staged tumor cells showed very intense p16 staining diagnosed as HPV positive in some cases (C). The same result is found in T4 carcinomas with intense p16 staining and positive HPV diagnoses D). A summary of the staining results correlated to staging by Box- Plot graphics is presented in lower section (E).
Evaluation in laryngeal cancer specimen on the basis of HPV detection
Laryngeal carcinoma tissue slides assessed with staining intensity 3 were rated as HPV positive as well as cases with an outspread moderate stain (intensity 2). Summarized only 23 tumors of a total of 129 (18%) were considered HPV positive.
Correlation analysis of p16, hPankoMabTM and TNM classification
TA-MUC1 showed a strong correlation to the tumor grading (rho=0.247; p=0.002), tumor growth (rho=0.326; p<0.001) and a trend to the p16 staining (rho=0.146, p=0.098). The HPV related protein p16 showed a trend to a positive correlation with grading (rho=0.156; p=0.077) and a significant correlation to tumor growth (rho=199; p=0.023). The T status showed a significant correlation to tumor grading in our group of patients (rho=0.539; p<0.001). A summary is presented in Table 1.
| Correlation analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| p16 | HPV | PankoMab | T-Status | relapse | grading | |||
| Spearman-Rho | p16 | coefficient of correlation | 1,000 | ,627** | ,146 | ,199* | ,129 | ,156 |
| Sig. (2-tail) | . | ,000 | ,098 | ,223 | ,145 | ,077 | ||
| N | 129 | 129 | 129 | 129 | 129 | 129 | ||
| HPV | coefficient of correlation | ,627** | 1,000 | ,082 | ,085 | ,031 | ,122 | |
| Sig. (2-tail) | ,000 | . | ,353 | ,339 | ,725 | ,169 | ||
| N | 129 | 129 | 129 | 129 | 129 | 129 | ||
| PankoMab | coefficient of correlation | ,146 | ,082 | 1,000 | ,326** | ,051 | ,274** | |
| Sig. (2-tail) | ,098 | ,353 | . | ,000 | ,565 | ,002 | ||
| N | 129 | 129 | 129 | 129 | 129 | 129 | ||
| T-Status | coefficient of correlation | ,199* | ,085 | ,326** | 1,000 | ,072 | ,539** | |
| Sig. (2-tail) | ,023 | ,339 | ,000 | . | ,417 | ,000 | ||
| N | 129 | 129 | 129 | 129 | 129 | 129 | ||
| relapse | coefficient of correlation | ,129 | ,031 | ,051 | ,072 | 1,000 | ,096 | |
| Sig. (2-tail) | ,145 | ,725 | ,565 | ,417 | . | ,278 | ||
| N | 129 | 129 | 129 | 129 | 129 | 129 | ||
| grading | coefficient of correlation | ,156 | ,122 | ,274** | ,539** | ,096 | 1,000 | |
| Sig. (2-tail) | ,077 | ,169 | ,002 | ,000 | ,278 | . | ||
| N | 129 | 129 | 129 | 129 | 129 | 134 | ||
Table 1: Spearman Rho analysis revealed positive correlation between p16 staining and T-status (rho=0.199; p=0.023), a trend for a positive correlation was found between p16 and grading (p=0.077) and PankoMabTM staining (p=0.098), PankoMabTM staining is highly significant correlated to both, grading (rho=0.247; p=0.002) and T-status (rho=0.326; p<0.001).
Survival analysis
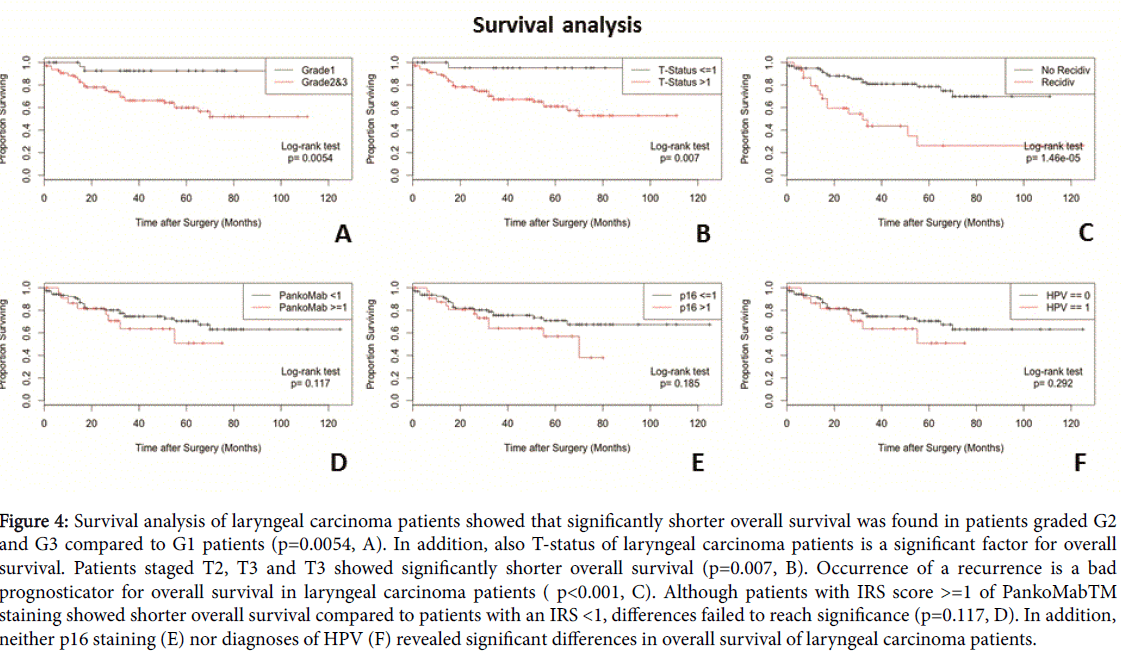
p=0.034 Kaplan-Meier analysis revealed significant difference in prognosis of laryngeal tumor patients whose tumors showed a higher grading (G2 and 3 compared to G1; p=0.0054). In addition, significant differences in prognosis of laryngeal tumor patients were found in correlation to TNM classification (T<=1 compared to T>1; p=0.007). A third significant parameter in prognosis of laryngeal tumor patients was relapse (no relapse compared to relapse; p<0.001). Kaplan-Meier analysis of TA-MUC1 staining revealed no significant differences (hPankoMab-GEXTM_ IRS<1 compared to hPankoMab-GEXTM IRS >=1; p=0.117). In addition, also p16 staining (p16 IRS <=1 compared to p16 IRS >1; p=0.185) and evaluation of HPV status (HPV negative compared to HPV positive; p=0.292) showed no significant correlation to patient survival. A summary of the survival analysis is presented in Figure 4.
Discussion
Within this study we could show that TA-MUC1 exhibited a strong and significant correlation with tumor grading and staging in head and neck squamous cell carcinomas (HNSCC), but was negative in normal tissues. Expression of p16 as a tool for HPV diagnosis in this type of carcinoma revealed a significant increase of staining in staged T4 tumors in comparison to T1 tumors. TA-MUC1 showed a positive trend in the correlation to p16 staining and therefore HPV involvement. Significant differences in analyses of overall survival were found in correlation to TNM-classification, grading and relapse. The main threat of an ongoing HPV infection is cervical cancer, which is already the third most common type of cancer and also the fourth leading cause of death in women (Symposium “HPV and Cervical Cancer” 2014 DKFZ, Heidelberg, Germany). The number of new cases fell from 9.410 in 1980 to 4.660 in 2010 (Robert-Koch-Institute, Berlin, Germany). The main reason for this decline is undoubtedly the discovery of the interaction of cervical cancer and Human Papilloma Virus (HPV) infection. In addition, the decreasing number of new cases may also be associated with increasing research and better detection methods [22,23]. Based on newly developed methods, improved screening [24] and preventive vaccination [25] were employed.
Figure 4:Survival analysis of laryngeal carcinoma patients showed that significantly shorter overall survival was found in patients graded G2 and G3 compared to G1 patients (p=0.0054, A). In addition, also T-status of laryngeal carcinoma patients is a significant factor for overall survival. Patients staged T2, T3 and T3 showed significantly shorter overall survival (p=0.007, B). Occurrence of a recurrence is a bad prognosticator for overall survival in laryngeal carcinoma patients ( p<0.001, C). Although patients with IRS score >=1 of PankoMabTM staining showed shorter overall survival compared to patients with an IRS <1, differences failed to reach significance (p=0.117, D). In addition, neither p16 staining (E) nor diagnoses of HPV (F) revealed significant differences in overall survival of laryngeal carcinoma patients.
Adapted from the results in the field of cervical cancer a direct connection between HPV infection and oropharyngeal carcinoma development could be established as well. The virus can be spread through direct skin-to-skin-contact during vaginal, anal and oral sexual intercourse. Therefore, women suffering from cervical cancer carry an increased risk to develop a malignancy also in the upper aerodigestive tract as well as their sexual partner [26,27].
Head and neck squamous cell carcinomas (HNSCC) are the sixth most common cancer worldwide [28]. Formerly tobacco and alcohol abuse used to be the most supposed causes for all tumors of the oral cavity, oropharynx and larynx [29,30]. In 1999, a subset of oropharyngeal cancer was considered HPV associated [31]. Today HPV DNA prevalence for oropharyngeal cancer is found in approximately 50% of all cases. In addition, HPV involvement is estimated in about 25% of the cases of laryngeal and oral cavity cancer [32]. Because there is a strong correlation between HPV positive oropharyngeal cancer and sexual behavior nowadays comprehensive sex education and more information on the benefits of vaccination is indispensable [3,33,34]. Vaccination is most effective, when given before sexual activity starts. Therefore it should be considered for boys as well [35].
Oropharyngeal tumors which are related to HPV infection and p16 overexpression gain better prognosis and overall survival [36,37]. HPV positive patients with an oropharyngeal cancer are on average younger and this cohort shows a distinct reduction in death rate and progression, furthermore response to radio/chemotherapy is enhanced [38]. HPV associated tumors perpetually express the viral E6 /E7 proteins suggesting that these proteins are required for continued growth of the tumor cells [39,40]. E6 oncoprotein complexes with p53 and as a consequence p53`s growth arrest and DNA repair is disposed. E7 inactivates retinoblastoma tumor suppressor (pRb) pathway. [40,41]. Therefore, DNA damages caused by cytostatic drugs will not be repaired. In addition toxic anticancer treatment of HPV positive cancer cells leads to a strong repression of the oncogenes E6 and E7 [42,43].
The tumor suppressor p16 is able to influence N- and Oglycosylation and galectin (Gal) expression [44,45]. The total number of stromal cells expressing Gal1, Gal3 and Gal9 was significantly higher in human papillomavirus-induced usual-type vulvar intraepithelial neoplasia (uVIN) than in vulvar tissue from healthy women undergoing labial reduction surgery [46]. Among HPV positive cases of laryngeal cancer a higher percentage of specimen showed an increased Gal3 expression than among the HPV negative group [47]. Moreover Gal1 and Gal3 have been proposed as biological markers of aggressiveness in several types of head and neck tumors [17,48,49].
Mucin1 (MUC1) is a receptor for Gal1 and Gal3. MUC1 is a large membrane bound glycoprotein which is expressed on the surface of epithelia [50]. During genesis of malignancies the glycosylation pattern of tumor cells changes [51] while MUC1 acts as a carrier for oncofetal carbohydrates like the Thomsen-Friedenreich antigen and supports invasive growth [52,53]. The recently described MUC1 epitope TAMUC1 is almost exclusively limited to malign tumors while being overexpressed and remaining adherent to the cell membrane. The newly established antibody hPankoMab-GEXTM recognizes specifically and exclusively the tumor-associated TA-MUC1 [18,19]. Beside its antibody dependent cellular cytotoxicity (ADCC), hPankoMab-GEXTM is able to influence different cell signaling pathways via binding, because the intracellular part of MUC1 is an active receptor tyrosine kinase (RTK) [12,54].
HNSCC have a huge impact upon quality of life and survival. Despite of innovative new treatment implantation such as Laser Surgery, robotic surgery and EGFR antibodies, the overall survival did not improve substantially [55-58].
Within this study, we could demonstrate universal absence of TAMUC1 in normal tissues of the upper aero digestive tract like larynx, pharynx, vocal-cord, epiglottis and tongue, but overexpression in worse graded laryngeal tumors. The antibody therapy used so far for head and neck carcinoma focuses on inhibition of receptors of the epidermal growth factor family. Side effects like paronychia, abnormalities of hair growth and serious skin irritations are inevitable, because the target is not restricted to malignancies [59]. We were able to show a strong and significant correlation between the TA-MUC1 expression and grading as well as staging. Together with the fact, that TA-MUC1 stays adherent to the cell membrane, this epitope shows great potential being a promising target for an antibody therapy with hPankoMab-GEXTM.
Conclusion
TA-MUC1 and p16 revealed relation by a trend, but we could not show a significant correlation between MUC1 and HPV association. Therefore, we suppose that HPV-associated tumors of head and neck will particularly profit by a TA-MUC1 targeted therapy. This assumption could be due to the fact that after hPankoMab-GEXTM binding the overexpressed Gal1 and GAL3, they cannot act as ligands for MUC1 as a RTK and switch on several signaling pathways. Even though, TA-MUC1 showed a positive trend in correlation to p16 staining a lack of significance is supposedly a problem of an insufficient number of included cases. An analysis of HPV association should be performed generally in laryngeal cancer specimens like in oropharyngeal cancer [60]. Additional data might lead to the conclusion that HPV is also relevant for the onset of laryngeal cancer.
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Competing Interests
The authors declare that they have no financial or non-financial competing interests.
Funding
This work was supported by ‘‘Forschungsstiftung Medizin am Universitätsklinikum Erlangen‘‘, Germany and the ‘‘Deutsche Forschungsgemeinschaft‘‘ (DFG).
Author’s Contribution
IW, CA, KF, DM, CF, AS, PB, MP: Conception and design, performed research, writer, revision and approval of the final version JT, SG, TW, DD, UJ, BK: Conception and design, revision and approval of the final version.
References
- Kau RJ, Alexiou C, Stimmer H, Arnold W (2000) Diagnostic procedures for detection of lymph node metastases in cancer of the larynx.ORL J OtorhinolaryngolRelat Spec 62: 199-203.
- Atula S, Auvinen E, Grenman R, Syrjänen S (1997) Human papillomavirus and Epstein-Barr virus in epithelial carcinomas of the head and neck region.Anticancer Res 17: 4427-4433.
- Lajer CB, von Buchwald C (2010) The role of human papillomavirus in head and neck cancer.APMIS 118: 510-519.
- Â Li XC,Gao Y,Yang F,Zhou M, Li Q,et al. (2014) Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment PharmacolTher 39: 270-281.
- Li X, Gao L, Li H, Gao J, Yang Y, et al. (2013) Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis.J Infect Dis 207: 479-488.
- Wittekindt C, Wagner S, Mayer CS, Klußmann JP (2012) [Basics of tumor development and importance of human papilloma virus (HPV) for head and neck cancer].Laryngorhinootologie 91 Suppl 1: S1-26.
- Khode SR, Dwivedi RC, Rhys-Evans P, Kazi R (2014) Exploring the link between human papilloma virus and oral and oropharyngeal cancers.J Cancer Res Ther 10: 492-498.
- Atighechi S, Ahmadpour Baghdadabad MR, Mirvakili SA, Sheikhha MH,Baradaranfar MH, et al. (2014)Human papilloma virus and nasopharyngeal carcinoma: Pathology, prognosis, recurrence and mortality of the disease. ExpOncol 36: 215-216.
- Chen AM, Zahra T, Daly ME, Farwell DG, Luu Q, et al. (2013) Definitive radiation therapy without chemotherapy for human papillomavirus-positive head and neck cancer. Head Neck 35: 1652-1656.
- Mao C, Balasubramanian A, Yu M, Kiviat N, Ridder R, et al. (2007) Evaluation of a new p16(INK4A) ELISA test and a high-risk HPV DNA test for cervical cancer screening: results from proof-of-concept study. Int J Cancer120: 2435-2438.
- Melkane AE, Mirghani H, Auperin A, Saulnier P, Lacroix L, et al. (2014) HPV-related oropharyngeal squamous cell carcinomas: a comparison between three diagnostic approaches. Am J Otolaryngol 35: 25-32.
- Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA (2003) Cell signaling through membrane mucins.Bioessays 25: 66-71.
- Pochampalli MR, el Bejjani RM, Schroeder JA (2007) MUC1 is a novel regulator of ErbB1 receptor trafficking.Oncogene 26: 1693-1701.
- Singh PK, Hollingsworth MA (2006) Cell surface-associated mucins in signal transduction.Trends Cell Biol 16: 467-476.
- Â Croce MV, Rabassa ME, Price MR, Segal-Eiras A (2001) MUC1 mucin and carbohydrate associated antigens as tumor markers in head and neck squamous cell carcinoma. PatholOncol Res 7: 284-291.
- WiestI, Schulze S, Kuhn C, Seliger, Hausmann R, et al. (2007) Expression of the carbohydrate tumour marker SLeX, SLeA (CA19-9), LeY and Thomsen-Friedenreich (TF) antigen on normal squamous epithelial tissue of the penis and vagina. Anticancer Res27: 1981-1988.
- Wiest IC,Alexiou C,Kuhn S,Schulze S, Kunze D, et al. (2012)Expression of different carbohydrate tumour markers and galectins 1 and 3 in normal squamous and malignant epithelia of the upper aaerodigestive tract. Anticancer Res 32: 2023-2029.
- Â Fan XN, Karste U,Goletz S, Cao Y (2010) Reactivity of a humanized antibody (hPankoMab) towards a tumor-related MUC1 epitope (TA-MUC1) with various human carcinomas. Pathol Res Pract 206: 585-589.
- Danielczyk A, Stahn R, Faulstich D, Löffler A, Märten A, et al. (2006) PankoMab: a potent new generation anti-tumour MUC1 antibody.Cancer ImmunolImmunother 55: 1337-1347.
- Â Dian D, Lenhard M, Mayr D,Heublein S, Karsten U, et al. (2013) Staining of MUC1 in ovarian cancer tissues with PankoMab-GEX detecting the tumour-associated epitope, TA-MUC1, as compared to antibodies HMFG-1 and 115D8.HistolHistopathol 28: 239-244.
- Dian D, Janni W, Kuhn C,Mayr D,Karsten U, et al. (2009) Evaluation of a novel anti-mucin 1 (MUC1) antibody (PankoMab) as a potential diagnostic tool in human ductal breast cancer; comparison with two established antibodies. Onkologie 32: 238-244.
- Â Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T (1998) Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol 153: 1741-1748.
- Griffin NR, Bevan IS, Lewis FA, Wells M, Young LS(1990) Demonstration of multiple HPV types in normal cervix and in cervical squamous cell carcinoma using the polymerase chain reaction on paraffin wax embedded material. J ClinPathol 43: 52-56.
- Bhatla N, Singla S, Awasthi D (2012) Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res ClinObstetGynaecol 26: p. 209-220.
- Sander BB, Rebolj M, Valentiner-Branth P, Lynge E (2012) Introduction of human papillomavirus vaccination in Nordic countries.Vaccine 30: 1425-1433.
- Hemminki K, Dong C, FrischM (2000) Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur J Cancer Prev 9: 433-437.
- Newell GR, Krementz ET, Roberts JD (1975) Excess occurrence of cancer of the oral cavity, lung, and bladder following cancer of the cervix.Cancer 36: 2155-2158.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008.Int J Cancer 127: 2893-2917.
- Andréasson L, Björlin G, Hocherman M, Korsgaard R, Trell E (1987) Laryngeal cancer, aryl hydrocarbon hydroxylase inducibility and smoking. A follow-up study.ORL J OtorhinolaryngolRelat Spec 49: 187-192.
- Â Maier H, Dietz A, Gewelke U, Seitz HK, Heller WD (1990) [Tobacco- and alcohol-associated cancer risk of the upper respiratory and digestive tract]. Laryngorhinootologie69: 505-511.
- zurHausen H (1999) Papillomaviruses in human cancers.ProcAssoc Am Physicians 111: 581-587.
- Ndiaye C, Mena M2, Alemany L3, Arbyn M4, Castellsagué X3, et al. (2014) HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis.Lancet Oncol 15: 1319-1331.
- Rettig E, Kiess AP, Fakhry C (2015) The role of sexual behavior in head and neck cancer: implications for prevention and therapy.Expert Rev Anticancer Ther 15: 35-49.
- MartÃn-Hernán F, Sánchez-Hernández JG, Cano J, Campo J, del Romero J (2013) Oral cancer, HPV infection and evidence of sexual transmission.Med Oral Patol Oral Cir Bucal 18: e439-444.
- Dalianis T (2014) Human papillomavirus (HPV) and oropharyngeal squamous cell carcinoma.Presse Med 43: e429-434.
- Gronhoj Larsen C, Gyldenlove M, Jensen DH,Therkildsen MH, Kiss K, et al. (2014) Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer 110: 1587-1594.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer.N Engl J Med 363: 24-35.
- Shaughnessy JN, Farghaly H, Wilson L, Redman R, Potts K, et al. (2014) HPV: a factor in organ preservation for locally advanced larynx and hypopharynx cancer?Am J Otolaryngol 35: 19-24.
- Androphy EJ, Hubbert NL, Schiller JT, Lowy DR (1987) Identification of the HPV-16 E6 protein from transformed mouse cells and human cervical carcinoma cell lines.EMBO J 6: 989-992.
- Chung CH, Gillison ML (2009) Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications.Clin Cancer Res 15: 6758-6762.
- Kim MJ, MS Ki, K Kim, HJ Shim, JE Hwang, et al.(2014) Different protein expression associated with chemotherapy response in oropharyngeal cancer according to HPV status. BMC Cancer 14: 824.
- Butz K, Geisen C, Ullmann A, Spitkovsky D, Hoppe-Seyler F (1996) Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. Int J Cancer 68: 506-513.
- Munagala R, Kausar H, Munjal C, Gupta RC (2011) Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis 32: 1697-1705.
- Amano M, Eriksson H, Manning JC, Detjen KM, Andre S, et al. (2012) Tumour suppressor p16(INK4a)-anoikis-favouring decrease in N/O-glycan/cell surface sialylation by down-regulation of enzymes in sialic acid biosynthesis in tandem in a pancreatic carcinoma model. FEBS J279: 4062-4080.
- Â Sanchez-RuderischH, Fischer C, Detjen KM, Welzel M, Wimmel A, et al. (2010)Tumor suppressor p16 INK4a: Downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model. FEBS J277: 3552-3563.
- Van Esch EM, Van Poelgeest MI, Kouwenberg S, Osse EM, Trimbos JB, (2015) Expression of coinhibitory receptors on T cells in the microenvironment of usual vulvar intraepithelial neoplasia is related to proinflammatory effector T cells and an increased recurrence-free survival. Int J Cancer136: E95-E106.
- Â Miranda FA, Hassumi MK, Guimaraes MC, Simoes RT, Silva TG, et al. (2009) Galectin-3 overexpression in invasive laryngeal carcinoma, assessed by computer-assisted analysis. JHistochemCytochem57: 665-673.
- Saussez S, Decaestecker C, Mahillon V, Cludts S, Capouillez A, et al. (2008) Galectin-3 upregulation during tumor progression in head and neck cancer.Laryngoscope 118: 1583-1590.
- Saussez S, Decaestecker C, Lorfevre F, Chevalier D, Mortuaire G, (2008) Increased expression and altered intracellular distribution of adhesion/growth-regulatory lectins galectins-1 and -7 during tumour progression in hypopharyngeal and laryngeal squamous cell carcinomas. Histopathology 52: 483-493.
- Mall AS (2008) Analysis of mucins: role in laboratory diagnosis.J ClinPathol 61: 1018-1024.
- Brockhausen I1 (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions.EMBO Rep 7: 599-604.
- Mommers EC, Leonhart AM, von Mensdorff-Pouilly S, Schol DJ, Hilgers J, et al. (1999) Aberrant expression of MUC1 mucin in ductal hyperplasia and ductal carcinoma In situ of the breast.Int J Cancer 84: 466-469.
- Jeschke U, Richter DU, Hammer A, Briese V, FrieseK (2002)Expression of the Thomsen-Friedenreich antigen and of its putative carrier protein mucin 1 in the human placenta and in trophoblast cells in vitro. Histochem Cell Biol 117: 219-226.
- Bitler BG,Menzl I, Huerta CL, Sands B, Knowlton W(2009) Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res 15: 100-109.
- Prince A, Aguirre-Ghizo J, Genden E, Posner M, Sikora A (2010) Head and neck squamous cell carcinoma: new translational therapies.Mt Sinai J Med 77: 684-699.
- Iro H, Waldfahrer F,Altendorf-Hofmann A, Weidenbecher M, Sauer R, et al. (1998)Transoral laser surgery of supraglottic cancer: follow-up of 141 patients. Arch Otolaryngol Head Neck Surg 124: 1245-1250.
- Hans S, Delas B, Gorphe P, Ménard M, Brasnu D (2012) Transoral robotic surgery in head and neck cancer.Eur Ann Otorhinolaryngol Head Neck Dis 129: 32-37.
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck.N Engl J Med 354: 567-578.
- Madke B, Gole P, Kumar P,Khopkar U (2014)Dermatological Side Effects of Epidermal Growth Factor Receptor Inhibitors: 'PRIDE' Complex. Indian J Dermatol 59: 271-274.
- Laskaris S,Sengas I, Maragoudakis P, Tsimplaki E, Argyri E, (2014) Prevalence of human papillomavirus infection in Greek patients with squamous cell carcinoma of the larynx. Anticancer Res34:5749-5753.
Citation: Wiest I, Alexiou C, Friese K, Mayr D, Freier C, et al. (2016) Clinical Evaluation of Mucin-1 (MUC1) and P16 in Laryngeal Cancer. Otolaryngol (Sunnyvale) 6:255. DOI: 10.4172/2161-119X.1000255
Copyright: ©2016 Wiest I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12450
- [From(publication date): 8-2016 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 11496
- PDF downloads: 954