Cochlear Implantation in Advanced Otosclerosis-The Surgeon's Perspective
Received: 11-Sep-2018 / Accepted Date: 08-Oct-2018 / Published Date: 15-Oct-2018 DOI: 10.4172/2161-119X.1000353
Keywords: Cochlear otosclerosis; Advanced and far advanced otosclerosis; Ossification; Cochlear implant.
Introduction
Otosclerosis is per-se a disease of the otic capsule and can arise from different sites in the otic bone, the commonest sites being fissula ante fenestrum (40%) and fossula post fenestrum (25%). Otospongiosis can progress from here inwards into the cochlea to develop various grades of retro-fenestral or cochlear otosclerosis, when this phenomenon comes to be known as advanced otosclerosis which is known to affect approximately 10% of patients with otosclerosis in general. A small percentage of these (5-10%) can also primarily arise within the cochlear lumen without involving the labyrinthine windows [1].
Ossification of the cochlea may increase with the course of the disease resulting in progressive sensorineural or mixed hearing loss. This happens due to reduction of hair cells and spiral ganglion cells without loss of auditory nerve fibers. Ossification starts from the lateral wall of the cochlea, resulting in hyalinization of spiral ligament and atrophy of stria vascularis. It secondarily affects the Organ of Corti by release of proteolytic enzymes into the cochlea and the Otosclerotic foci undergo re-mineralization in the late phase [1,2]. CT scan shows a distinctive pericochlear hypodense double ring appearance due to demineralization of the bone around the cochlea while an MRI reflects a ring of intermediate signal in the pericochlear and perilabyrinthine regions on T1W images, with gadolinium enhancement and T2 W images may show increased signals [3]. As the disease progresses into advanced otosclerosis, the Speech Discrimination Score (SDS) falls <50% and when a patient’s SDS is <30% and SN loss is >85 dBHL, this condition is termed as Far Advanced Otosclerosis (FAO) [4].
Powerful hearing aids (H.aids), stapedotomy followed by H.aids and cochlear implants are the various management options in these patients. In advanced otosclerosis with severe mixed hearing loss and SDS>50% stapedotomy can help in correction of the conductive component with acceptable hearing levels available for conventional amplification. In patients with FAO, effective and safe hearing rehabilitation can be accomplished only with CI [4]. However, extensive cochlear ossification can impede complete electrode insertion in these cases and Facial nerve stimulation can pose programming challenges. Therefore a stringent selection criterion should be individualized and based on a thorough evaluation for offering the right management to patients with advanced otosclerosis, wherein the onus is on implanting prior to manifestation of a full-blown FAO with complete cochlear ossification.
This study aims to analyze the surgeon’s perspective of choosing the right candidate with advanced otosclerosis who has failed conventional amplification either as a primary modality or post-stapedotomy, for offering CI as a safe choice with minimal morbidity in order to achieve successful hearing related outcomes.
Study Method
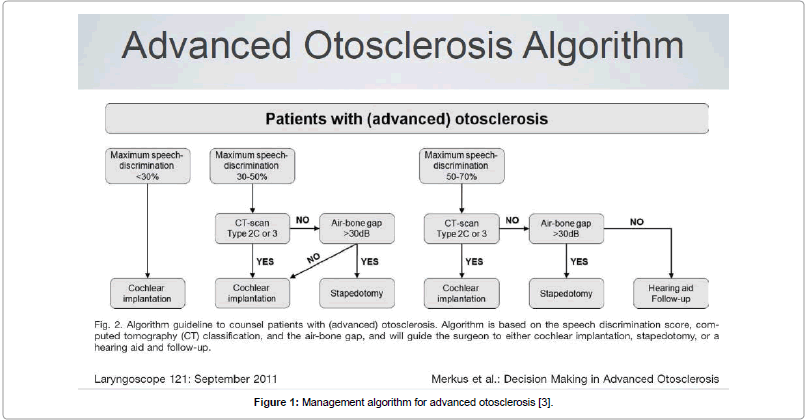
This retrospective study was performed at a premier CI centre in South India between 1997-2017, including 43 post-lingual adults with advanced otosclerosis who received CI. The first few candidates had been offered CI primarily since they had no serviceable hearing due to progressive disease, while a more rational approach for candidacy was followed subsequent to the publication by Merkus et al. [3], whereby the eligible patients could be clearly chosen based on this management algorithm for CI candidacy using SDS scores, SN thresholds and relevant imaging (Figure 1). Institutional Ethical Review Board approval for this retrospective study was obtained on 5th January 2018, prior to commencement of the data collection and all subjects included in this study were intimated by post regarding their participation. All candidates consented while their anonymity and confidentiality was being protected as was recommended by the ethical committee.
Figure 1: Management algorithm for advanced otosclerosis [3].
The current study highlights the surgical issues related to advanced otosclerosis where CI has been performed either as a primary modality or following stapedotomy and it analyzes the hearing related outcomes of surgery in this cohort. There were twenty one men and twenty two women in this study and their age ranged from 35-78 years (mean age was 49 years). All patients were meticulously evaluated clinically where a negative Rinne test result with a 256 Hz tuning fork was found useful to differentiate advanced otosclerosis from SNHL of other causes. Investigations included audiometric test battery (PTA, Tymps, OAE, ABR), speech tests and high resolution CT/MRI imaging to assess the severity of cochlear otosclerosis. Exclusion criteria included patients with profound hearing loss due to causes other than otosclerosis, which also included congenital and post-meningitic labyrinthitis ossificans. The images were graded based on the popular Symons and Fanning grading system of 2005 (Table 1) [5], which was a developed as a follow up to the staging of advanced otosclerosis as first defined by Rotteveel et al. [6].
| CT Grading | N=43 |
|---|---|
| Grade 1-solely fenestral | 31 (72%) |
| Grade 2-patchy localized cochlear disease (with or without fenestral involvement) • Basal cochlear turn (Grade 2A) • Middle/apical turns (Grade 2B) •Both basal turn & middle/apical turns (Grade 2C) |
8 (18.8%) 2 (4.6%) 1 (2.3%) |
| Grade 3-diffuse confluent cochlear involvement (with or without fenestral involvement) | 1 (2.3%) |
Table 1: CT Grading [5].
All candidates underwent cochlear implantation uneventfully as per standard protocol, implanted by the same senior surgeon using standard techniques of round window insertion or cochleostomy. All implants were manufactured by MedEL company (Innsbruck, Austria). Facial Nerve monitor NIM pulse II was used to monitor intra-operative facial nerve stimulation after cochlear implantation, by performing ESRT as a stimulus trigger for current dissipation into the otospongiotic cochlea. This helped identify the at risk electrodes for facial stimulation later on at programming and the audiologist marked out these electrodes for setting appropriate levels, which will not cause non-auditory stimulation later on.
All patients underwent regular auditory verbal rehabilitation on a one to one basis of weekly two sessions for 3 months, biweekly for next 3 months and one a month till one year post-implantation, during which their maps were also programmed with special care to look out for non-auditory stimulation. Evaluation of hearing related quality of life was done based on improvements in Category of Auditory Performances (CAP) scores and also on a validated Glasgow Benefit Inventory questionnaire (GBI-HRQOL) specific to their outcomes. Their pre-implant scores were compared with post-implant scores at 6 months and at the end of habilitation of 1 year and results measured using two-tailed paired t-test for statistical significance.
Among the 43 candidates, 21 (48.8%) had bilateral progressive otosclerosis among whom the poorly deafened ear was chosen for CI, with the hope that they can benefit from binaural bimodal stimulation while using powerful H.aid in their better ear after implantation. Among them 7 (33.3%) had previously undergone bilateral stapedotomy and were currently unaidable with H.Aid in the worse ear which had to be implanted. The rest 22 candidates (51.2%) with unilateral advanced otosclerosis but with bilateral asymmetrical SNHL (50 dB or more in non-otosclerotic ear), were primarily chosen to have CI. All these candidates were aided with H.Aid in their better ear to make use of bimodal hearing as possible.
Observations and Results
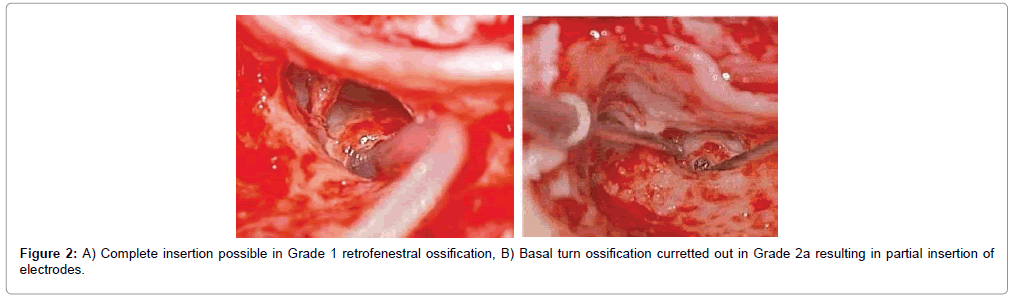
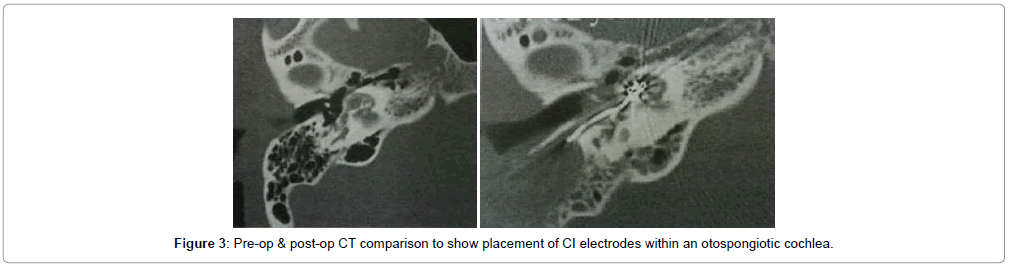
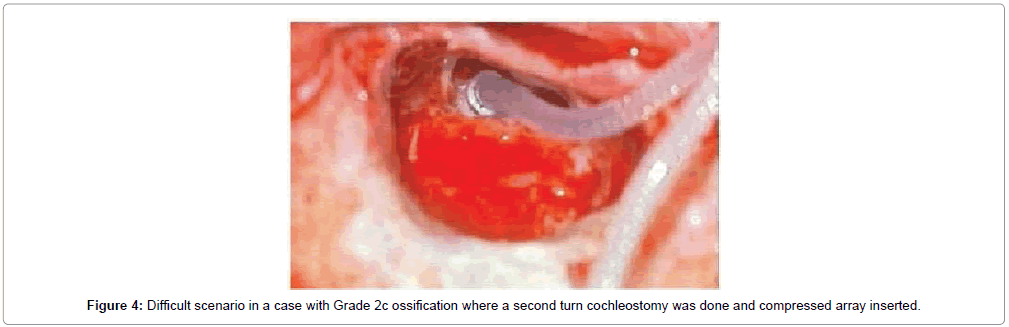
Overall 43 patients received cochlear implants, among whom complete electrode insertion was possible in 39 patients (90.6%) who were in Symons and Fanning Grades 1 to 2a (Figures 2 and 3) and partial insertion was done in 4 patients (9.4%) who were in Symons and Fanning Grades 2b/c to 3 (Figure 4), due to cochlear ossification in the basal and mid turns (Table 1). In one patient the electrode was inserted in the second turn (2%). None of the patients had a scala vestibuli insertion. Occlusion of the round window niche by otospongiotic foci was not seen in any of the patients. Misplacement of the electrode array did not occur in any patient and this was confirmed by intra-operative telemetry and a post-operative X-ray. CSF gusher was not encountered intra-operatively in any patient.
Facial nerve stimulation was seen triggered by Electrodes 7, 8 and 9 (corresponding to labyrinthine segment of nerve) in 5 patients (11.7%) for which appropriate program was reset by the audiologist to negate stimulation. The surgical outcomes were satisfactory in keeping with literature with a complication rate of <5% (including wound infection, chorda tympani injury and facial nerve paresis) and no persistent morbidity was seen.
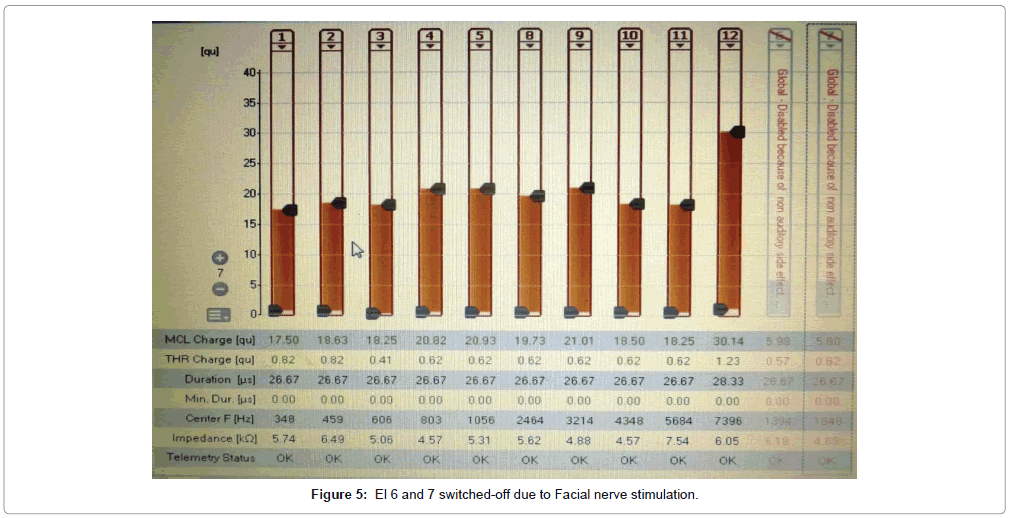
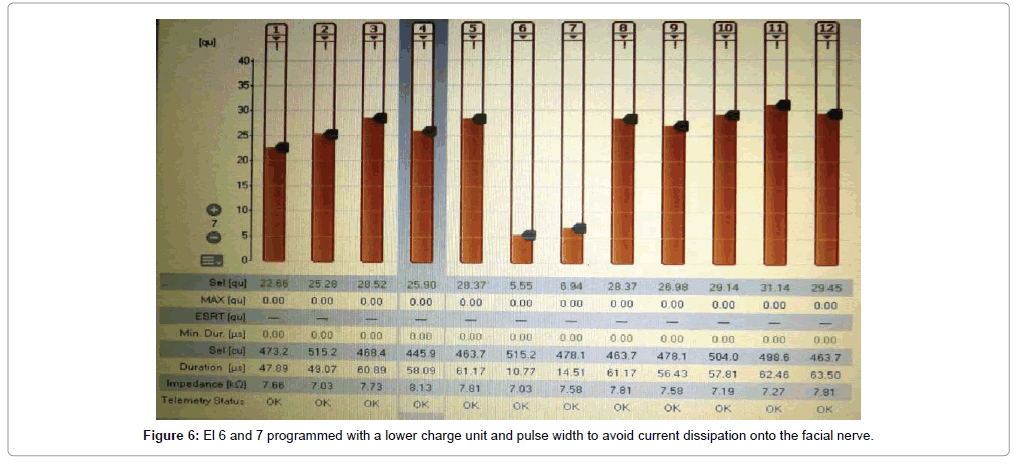
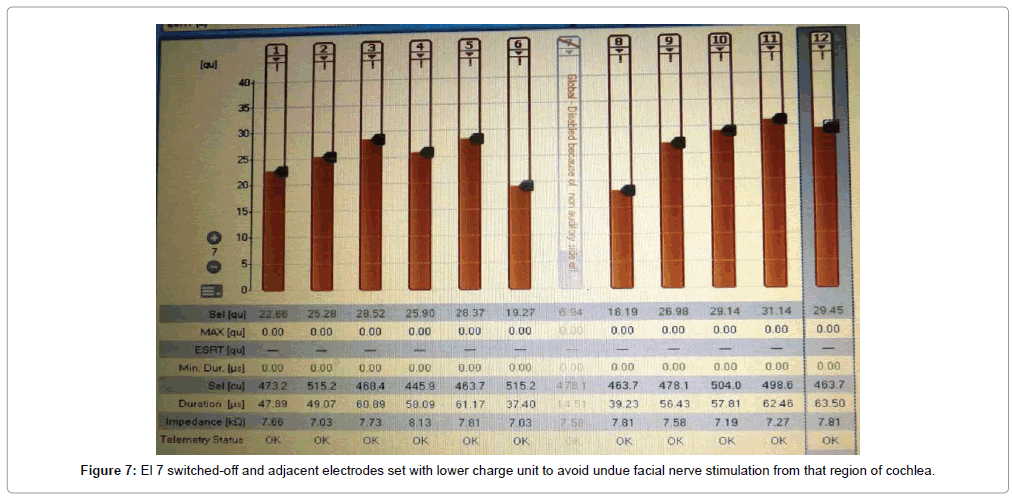
Of the 43 implantees, post-operative facial nerve stimulation was seen in 5 patients (11.8%). In two patients, the concerned electrodes were switched-off. In two patients (4.6%), programming was done with a lower charge unit and pulse width to avoid excess current dissipation onto the facial nerve. In one patient (1.3%), one electrode was switchedoff and adjacent electrodes were set with a lower charge unit to avoid undue facial nerve stimulation from that region of the cochlea (Figures 5-7). Statistically significant improvements in audiological and hearing related quality of life measurements were recorded in this cohort when compared to their pre-operative scores. The average pre-operative pure tone audiometry level in all our patients was 90 dBHL in the implanted ear. The follow up period of cochlear implantees was until 1 year of rehabilitation. The Categories of Auditory Perception (CAP) score [2] used to assess the audiological benefit of CI showed an average pre-operative CAP score of 1, which improved to a mean post-operative CAP score value of 3 (range 0-3) at 6 months and further improved to 5 (range 2-6) at 12 months, which was statistically significant at p<0.05. Overall 15 patients (34.5%) achieved a CAP score of 4, 17 patients (39.5%) achieving score of 5 and 12 patients (28%) achieving a CAP score of 6 one year after implantation. The 5 candidates with Grade 2b, c and 3 ossification with partial insertion were all able to achieve the CAP score of 4 with frequent mapping and intense habilitation. All facial stimulation could be obviated by the programming techniques as described above in the initial mapping sessions.
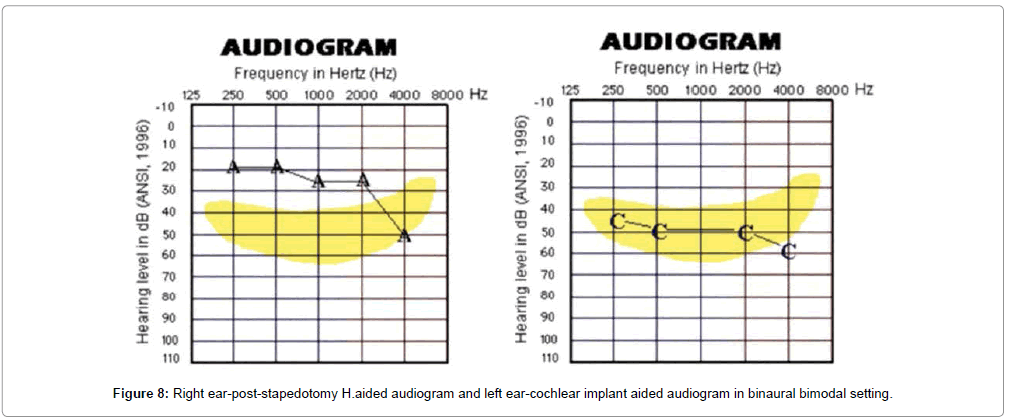
The GBI-HRQOL questionnaire scores were compared using twotailed paired t-test. There was statistically significant improvement in the scores over time from a mean average of 12.5% pre-operatively to 56.2% at 6 months and 97.8% at 1 year of CI use at p<0.05. These results confirm that CI is useful in providing improved quality of communication to advanced otosclerosis candidates. 6 candidates (14%) were also able to benefit from bimodal hearing while using CI in one ear and H.aid (post-stapedotomy) in contralateral ear (Figure 8). Their scores were found to be in the top end of the spectrum and these results were a reflection of their performance at the end of one year post-implantation.
Currently a further study is underway in this cohort to look at the long term audiological and quality of life outcomes and results from this prospective research work is expected to be available by 2020. Such long term results will highlight the benefits of true bimodal binaural hearing in patients with advanced otosclerosis. It is notable that with ageing and progression in hearing loss in the non-implanted H.Aided ear, there is a likely hood that some of these patients may convert to become bilateral cochlear implantees in future.
Discussion
Patients with advanced otosclerosis face an “Acoustic Baffle” wherein higher order sound processing is lost due to cochlear dead regions more common with thresholds above 70 dB HL [7]. They have issues with H.aids like poor inter-aural attenuation, sound localization and music appreciation. This is because their conductive loss precludes over the SN component for sound transmission and high amplification is required to render speech audible which may degrade the acoustic signals. In noisy backgrounds this is not sufficient to allow high levels of speech understanding [8]. The disadvantage with a stapedotomy in advanced otosclerosis is that it has no influence on the sensorineural component of the hearing loss in patients with severe mixed hearing loss, werein H.aid provision post-surgery is necessary. An increase of sensorineural hearing loss, after stapedotomy could result in a totally deaf ear [7].
Patients with severe to profound hearing loss due to advanced otosclerosis are increasingly being considered as CI candidates. Radiology is essential in the pre-surgical evaluation of otosclerosis to evaluate the extent of the lesion and cochlear patency. HRCT (Highresolution Computed Tomography) is considered to be the imaging technique of choice for the diagnosis of otosclerosis. HRCT can detect subtle otosclerotic foci in and around the cochlea and may predict the risk of complications during surgery. The CT grading system of Rotteveel et al. [6] is partially based on location and on the type of lesion: solely fenestral (grade 1), retrofenestral: double ring or halo effect (grade 2A), narrowed basal turn (grade 2B) or both (grade 2C) and diffuse confluent retrofenestral involvement (grade 3). Symons and Fanning [5] in the subsequent year proposed a classification similar to Rotteveel, except where grade 2 is based on anatomic location instead of the type of lesion: basal turn (2A), middle/apical turns (2B), both basal and middle/apical turns (2C). This classification is more practical since it is based on anatomy which will warn the surgeon regarding difficulty in implantation. Hence we have followed this system of grading for our study.
Merkus et al. [3], described an algorithm for management of advanced otosclerosis based on CT grading, speech discrimination and air-bone gap. In patients with Speech Discrimination (SD) scores of <30%, the most effective intervention is CI. Patients with an SD between 30% and 50% may be treated with either CI or stapedotomy. In cases of grade 2C or 3 otosclerosis on HRCT, CI is the better option. If the CT scan shows less cochlear involvement (grade 1, 2A, or 2B), the Air-bone Gap (ABG) will guide the surgeon to either stapedotomy or CI. If the ABG is 30 dB or more, a stapedotomy can result in improvement of hearing. If the ABG is 30 dB or less, patients should be treated with CI. Patients with an SDS of 50% to 70% are candidates for stapedotomy, rehabilitation with hearing aids, or CI. Patients with limited cochlear involvement on HRCT (grade 1, 2A, or 2B) and an ABG of 30 dB or more should be treated with stapedotomy. When the ABG is 30 dB or less and HRCT shows limited cochlear involvement, patients will generally benefit from hearing aids and follow-up. If, HRCT shows extensive retrofenestral otosclerosis (grade 2C or 3), CI should be recommended [3].
CI has been used successfully in patients with cochlear otosclerosis, both as initial treatment and in progressive loss following stapedotomy as noted in studies [9,10]. These authors have enlisted the surgical issues in CI which include extra drilling to overcome cochlear ossification, drilling the round window niche for occlusion of the same by otospongiotic foci and further ossification needing a complete drill out and double array implantation. In such scenarios, there can be misplacement of the electrode array with CI inserted into a false lumen with no neural endings or scala vestibuli insertion. There can be a false passage into eustachian tube, internal auditory meatus or carotid canal and there is also a higher risk of CSF gusher [8,9].
The surgical complication due to ossification of the cochlea has been reported in 10% to 37% of patients wherein more drilling may be required to access the lumen [10]. Occasionally insertion in the second turn or the scala vestibuli may be required. A scala vestibuli electrode insertion may be required in 2% to 25% of the cases. Furthermore, programming of the CI can be challenging because the progression of otosclerosis can cause postoperative failure of the CI.
Apart from the surgical challenge in electrode insertion, the surgeon needs to be aware of the higher risk of facial nerve stimulation which is possible via the otospongiotic bone. This needs to be discussed prior with the patient and an experienced audiologist can follow corrective measures if this happens at device switch-on. Facial Nerve stimulation due to electrodes in vicinity of the geniculate ganglion particularly occurs with non-modiolar hugging electrodes and widely ranges in literature from 0 to 75% (average 20%). Patients with a higher CT classification are significantly more likely to develop facial nerve stimulation. Use of modiolar hugging contour electrodes has been reported to be associated with a reduced incidence of post-operative Facial Nerve stimulation due to less undesired current flow toward the outer rim of the cochlea and Facial Nerve Scala vestibuli insertion is used in 2-25% cases, as it is further from facial nerve segments or reimplantation is a final choice (same side or contralateral side), if programming fails to resolve the facial stimulation [7].
A prelude to this event can be verified by checking ESRT responses intra-operatively in the suspicious electrodes (especially El 7, 8 or 9 in MedEl array corresponding to the geniculate and labyrinthine segment of Facial Nerve). This protocol was followed in our study to identify those 5 patients who had facial stimulation. These patients were optimally managed by the various techniques described in mapping such as reduction in stimulus levels of the cranially located electrodes or deactivating the causative electrodes. Variable mode programming can also solve this while the non-offending electrodes receive normal pulses, the offending electrodes receive wider pulses. Decreased performance of CI occurs if too many electrodes must be inactivated due to Facial Nerve stimulation.
Often there exist other programming challenges for audiologists in advanced otosclerosis since there is reduction of hair and spiral ganglion cells which may need higher charges which result in nonauditory stimulation, otalgia arising from stimulation of tympanic plexus, dizziness from stimulation of vestibule, which rarely may necessitate removal of the device and re-implantation with singlebanded electrodes.
Rotteveel et al. [6] highlighted the 4 factors relating to the success of a CI in advanced otosclerosis-namely early stages of ossification, full insertion of electrodes, absence of facial stimulation and optimal programming techniques with intensive rehabilitation. In Advanced otosclerosis, it is now well established that Stapedotomy with Hearing aid is the option for candidates with SDS >70%, AB Gap >15 dB and HRCT Grades 1 and 2a/b and CI is the right option if SDS <30%, AB Gap <15 dB and HRCT Grades 2c and 3 [3]. But in clinical practice we encounter a range of candidates who may fall in between these two categories. Based on our above experience we infer that it is better to offer CI to such candidates at an earlier stage when lesser ossification, presence of residual hearing and dense bone around facial nerve would make CI safe and effective rather than later on, when outcomes are hampered by extensive otospongiosis with loss of neural elements, narrow or no lumen for insertion, false tract, non-auditory or facial nerve stimulation, programming difficulties etc.
As a result in our institute we meticulously counsel patients with progressive and accelerated forms of otosclerosis who hover in the border of maximal conventional amplification while under long term surveillance, to prepare for CI whenever the situation arises, based on their SN thresholds, SDS scores and scan findings. It will be prudent if the ever expanding CI candidacy defines special rules for this group of individuals, since the situation here is akin to labyrinthitis ossificans, wherein earlier intervention with CI for severe hearing loss is better than waiting for profound hearing loss to manifest by which time optimal implantation may not be possible.
Conclusion
The management of advanced otosclerosis can pose challenges to the otologist. The pre-operative air-bone gap, speech discrimination and extent of involvement on CT scans are the main criteria in deciding on the choice of treatment in these patients. The present study investigates the surgeon’s perspective of choosing the right candidate for CI in advanced otosclerosis, while being prepared for the problems associated with implantation. A rational protocol for surgical management needs to be followed as highlighted in this study with meticulous counseling for patients regarding possibility of complications and realistic expectations is vital.
Limitations of the Study
1. The sample size is small with variability in severity of disease among the cohort
2. Issues with programming while having residual hearing in nonimplanted ear
3. Long term outcomes >1 year need to be studied for influence of CI within a ossified cochlea with depleting spiral ganglion population and its influence on hearing related quality of life.
Future Directions
It is established that CI is effective in the management of advanced otosclerosis among candidates who are beyond the thresholds of conventional amplification but the audiological outcomes between CI and H.aided ears is not directly comparable since modalities of stimulation are different. Currently an ongoing study in the same cohort is comparing the post-stapedotomy H.Aided benefits versus CI aided benefits in the two groups of candidates with progressive otosclerosis. The learning curve for matching both acoustic and electric signals is steep and our audiologists are currently working on the subgroup of candidates who have bilateral otosclerosis with post-stapedotomy H.aid on one ear and CI in the other ear, looking at the summative benefits of binaural bimodal stimulation. This remains the future direction of the present study and the results should be available soon.
References
- Semaan MT, Gehani NC, Tummala N, Coughlan C, Fares SA, et al. (2012) Cochlear implantation outcomes in patients with far advanced otosclerosis. Am J Otolaryngol 33: 608-614.
- Archbold S, Lutman ME, Nikolopoulos T (1998) Categories of auditory performance: Inter-user reliability. Br J Audiol 32: 7-12.
- Merkus P, van Loon MC, Smit CF, Smits C, de Cock AF, et al. (2011) Decision making in advanced otosclerosis: An evidence-based strategy. Laryngoscope 121: 1935-1941.
- Castillo F, Polo R, Gutiérrez A, Reyes P, Royuela A, et al. (2014) Cochlear implantation outcomes in advanced otosclerosis. Am J Otolaryngol 35: 558-564.
- Marshall AH, Fanning N, Symons S, Shipp D, Chen JM, et al. (2005) Cochlear implantation in cochlear otosclerosis. Laryngoscope 115: 1728-1733.
- Burmeister J, Rathgeb S2, Herzog J3 (2017) Cochlear implantation in patients with otosclerosis of the otic capsule. Am J Otolaryngol 38: 556-559.
- Rotteveel LJ, Proops DW, Ramsden RT, Saeed SR, van Olphen AF, et al. (2004) Cochlear implantation in 53 patients with otosclerosis: demographics, computed tomographic scanning, surgery and complications. Otol Neurotol 25: 943-952.
- Calmels MN, Viana C, Wanna G, Marx M, James C, et al. (2007) Very far-advanced otosclerosis: Stapedotomy or cochlear implantation. Acta Otolaryngol 127: 574-578.
- van Loon MC, Merkus P, Smit CF, Smits C, Witte BI, et al. (2014) Stapedotomy in cochlear implant candidates with far advanced otosclerosis: A systematic review of the literature and meta-analysis. Otol Neurotol 35: 1707-1714.
- Psillas G, Kyriafinis G, Constantinidis J, Vital V (2007) Far-advanced otosclerosis and cochlear implantation. B-ENT 3: 67-71.
Citation: Kumar RS, Kameswaran M (2018) Cochlear Implantation in Advanced Otosclerosis-The Surgeon’s Perspective. Otolaryngol (Sunnyvale) 8: 353. DOI: 10.4172/2161-119X.1000353
Copyright: © 2018 Kumar RS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6375
- [From(publication date): 0-2018 - Nov 07, 2025]
- Breakdown by view type
- HTML page views: 5322
- PDF downloads: 1053