Combined Therapy with Transarterial Embolization Utilizing Radiopaque LUMI Beads and Percutaneous Microwave Ablation for Liver Tumors
Received: 20-Mar-2019 / Accepted Date: 08-Apr-2019 / Published Date: 15-Apr-2019 DOI: 10.4172/2167-7964.1000308
Abstract
Transarterial Embolization (TAE) and Microwave Ablation (MWA) are efficacious minimally invasive therapies for loco regional treatment of Hepato-Cellular Carcinoma (HCC) and Colorectal Liver Metastases (CRLM). Newer data suggest that combination therapy with TAE and MWA provides better tumor response and survival than with monotherapy alone, but further investigation is warranted. In addition, contrast agents such as Lipiodol are traditionally used during TAE to improve efficacy by allowing direct visualization of the completeness of the embolization. Newer radiopaque embolic beads, LC LUMI beads, are now available in the United States but have not yet been studied. We aim to discuss two cases performing combined TAE/MWA using LC LUMI beads for the treatment of HCC and CRLM. Compared to lipiodol alone, LC LUMI beads cause a larger degree of ischemic necrosis via particle embolization, thus resulting in a higher potential for complete response and successful thermal ablation.
Keywords: LC LUMI beads; Transarterial embolization; Percutaneous microwave ablation; Hepatocellular carcinoma; Colorectal liver metastases
Introduction
Transarterial embolization (TAE) and thermal ablation are effective minimally invasive techniques for locoregional treatment of hepatocellular carcinoma (HCC) and colorectal liver metastases (CRLM). HCC is the fifth most common tumor worldwide and third most common cause of tumor-related death. Surgery is the treatment of choice but <30% of patients with HCC qualify for surgical resection for various reasons including tumor location, stage of disease, poor liver functional reserve, or high operative risk [1]. In addition, colorectal cancer is the third leading cause of cancer death in the world and 50% of patients with colorectal cancer will develop liver metastases. Surgical resection is the only curative therapy, however only 10-15% of patients with CRLM are suitable for curative resection. The median survival time for untreated CRLM is 6.9 months [2].
Available regional treatment strategies for HCC and CRLM include TAE and local thermal ablation including microwave ablation (MWA) and radiofrequency ablation (RFA). Compared to RFA, MWA has a shorter procedure time, wider ablation area, higher ablation rate, and a lower heat sink effect, so it is often preferred [2]. Some studies have demonstrated better outcomes and increased flexibility with microwave ablation over RFA. A recent study phase 2 clinical trial in LANCET concluded no significant difference between the efficacy of RFA and MWA for HCC lesions 4 cm or smaller [3].
In terms of HCC ablation achieves complete responses in >80% of tumors 5 cm. Since HCC is a hypervascular tumor and it receives most of its blood supply from the hepatic artery, TAE will embolize the arteries that supply the tumor and cause tumor necrosis. However, many HCC tumors, especially those >5 cm in diameter, receive blood from both the hepatic artery and the portal vein, and despite multiple embolization procedures tumor necrosis may still be incomplete [1].
TAE or MWA alone may not be a sufficient treatment for unresectable HCC or CLRM. As mentioned, TAE is not effective for tumors with a poor blood supply and MWA may not lead to complete necrosis if the induced area of necrosis is small. TAE has the advantage of reducing the local blood supply to the tumor, thus resulting in tissue necrosis and inflammatory edema. Therefore, it can decrease the cooling effect of blood flow on the heating action of microwaves, which will thus enhance the coagulation action of microwaves. New studies have shown that MWA combined with TAE provides better tumor response and overall survival rates in patients with small hepatocellular carcinomas, without severe complications [4]. Several clinical studies have examined combined arterial embolization with thermal ablation and shown better overall survival (OS) and less disease progression compared to monotherapy alone [5-8].
In addition, TAE procedural efficacy can be enhanced with the use of contrast mediums, which are used to directly visualize the embolic material in order to assess the completeness of the target tissue embolization, which improves efficacy. Traditionally Lipiodol has been used for embolization procedures because it combines three specific characteristics: drug delivery, embolization, and radiopacity [9]. Newer radiopaque beads provide additional procedural accuracy by improving the visualization of target and non-target embolization compared to soluble contrast medium alone because the beads can be directly visualized in real-time. Many newer imageable beads have not gained clinical adoption. While Lipiodol has traditionally been used, LC LUMI beads are the first commercially available radiopaque embolic bead in the United States and lack extensive clinical studies [10]. Furthermore, compared to lipiodol alone, transarterial particle embolization bringing tumor vessels to stasis allows for the possibility of a more effective response and higher potential for complete response by causing a larger degree of ischemic necrosis which in turn allows for a more efficacious thermal ablation [11]. We aim to discuss our experience performing TAE with LC LUMI beads combined with percutaneous MWA for the treatment HCC and CRLM at our institution.
Case Series
Ethical considerations
The study was granted IRB exemption and was in accordance with the World Medical Association Declaration of Helsinki.
Case 1
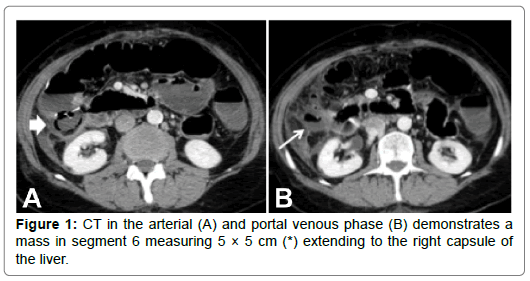
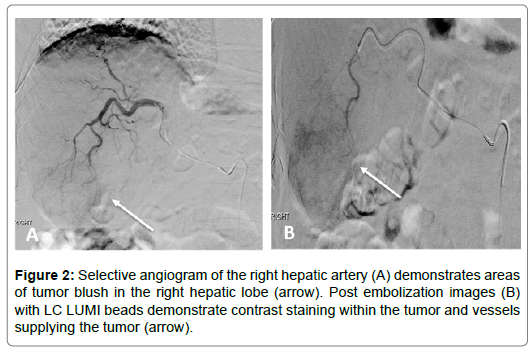
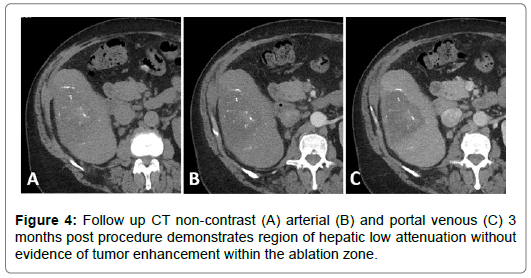
A 59-year-old male with past medical history of hepatitis C, cirrhosis, and HCC and underwent a left hepatectomy. The patient subsequently had no evidence of recurrent disease until 4 years later at which time he was found to have a new 2 cm HCC in the right hepatic lobe. He underwent a two-step staged procedure involving hepatic arterial embolization with radio-opaque particles (LUMI beads) followed by microwave ablation. Both procedures were successful without any complications. Imaging available at 3 months postprocedure demonstrated no evidence of intratumoral enhancement within the zone of ablation (Figures 1-5).
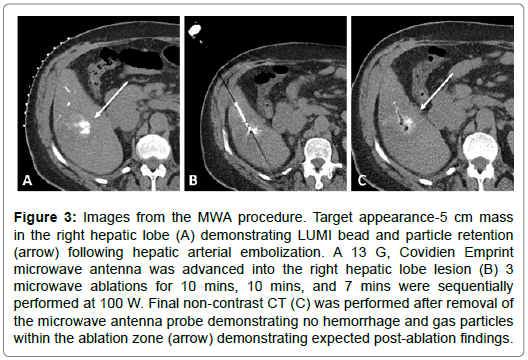
Figure 3: Images from the MWA procedure. Target appearance-5 cm mass in the right hepatic lobe (A) demonstrating LUMI bead and particle retention (arrow) following hepatic arterial embolization. A 13 G, Covidien Emprint microwave antenna was advanced into the right hepatic lobe lesion (B) 3 microwave ablations for 10 mins, 10 mins, and 7 mins were sequentially performed at 100 W. Final non-contrast CT (C) was performed after removal of the microwave antenna probe demonstrating no hemorrhage and gas particles within the ablation zone (arrow) demonstrating expected post-ablation findings.
Case 2
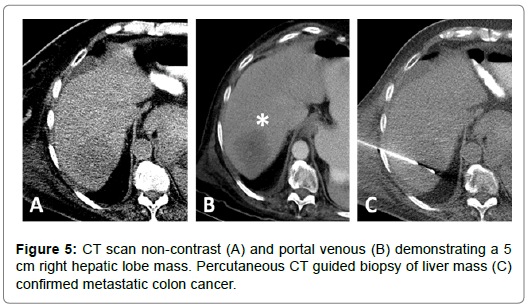
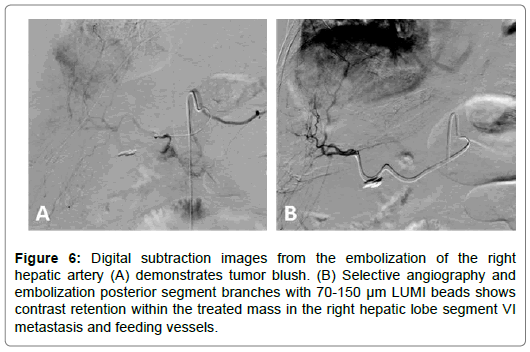
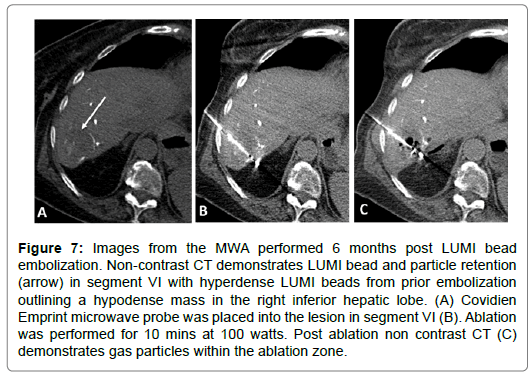
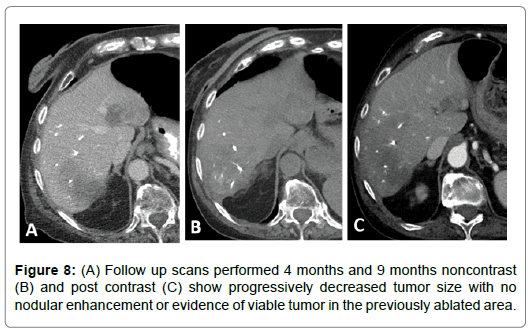
67-year-old female with a T3N1M1 cecal invasive adenocarcinoma with metastatic spread to the liver. The patient underwent a right hemicolectomy and ileocolic anastomosis. The mass in the right hepatic lobe measured 5 cm and was biopsy proven metastatic colorectal cancer. The patient underwent LUMI bead embolization of the right hepatic artery posterior segment branches supplying the tumor. The patient subsequently underwent microwave ablation of the segment VI lesion. Follow up scans performed at 2, 4, 7 and 9 months post ablation show progressively decreased tumor size with no nodular enhancement or evidence of viable tumor in the previously ablated area (Figures 6-8).
Figure 6: Digital subtraction images from the embolization of the right hepatic artery (A) demonstrates tumor blush. (B) Selective angiography and embolization posterior segment branches with 70-150 μm LUMI beads shows contrast retention within the treated mass in the right hepatic lobe segment VI metastasis and feeding vessels.
Figure 7: Images from the MWA performed 6 months post LUMI bead embolization. Non-contrast CT demonstrates LUMI bead and particle retention (arrow) in segment VI with hyperdense LUMI beads from prior embolization outlining a hypodense mass in the right inferior hepatic lobe. (A) Covidien Emprint microwave probe was placed into the lesion in segment VI (B). Ablation was performed for 10 mins at 100 watts. Post ablation non contrast CT (C) demonstrates gas particles within the ablation zone.
Discussion
Recent studies have reported good rates of success treating both HCC and CRLM with TAE combined with MWA. In a study by Yang et al. 41 small HCC nodules were treated with TAE followed by microwave ablation. Complete tumor necrosis was observed in 34 cases and all patients were alive at the end of the follow-up period (6-31 months) [4]. In a study by Wu et al. 43 CRLM were treated with a complete ablation rate of 81.4% with complete response achieved in 26.7% of cases and partial response in 56.7%. Progression-free survival and overall survival were 5.0 months and 11.0 months respectively [2]. In a study limited to lesions smaller than 3 cm, Yang et al. showed a 100% 1-year survival rate and a 5% local recurrence rate in patients treated with TACE-MWA [4]. Tanaka et al. also were able to show near 100% complete response rates after combination TACE-MWA, albeit again for lesions <3 cm [8,9]. In a study done by Smolock et al. comparing TACE monotherapy to combination TACE-MWA for local control of 3-5 cm HCC it was demonstrated that TACE-MWA trended toward both a lower rate of local tumor progression (22.3 months vs. 4.2 months) and a higher complete response rate (65.2% vs. 37.5%) [10].
Furthermore, Maluccio et al. compared survival rates after bland arterial embolization and ablation vs. surgical resection for treating solitary HCC up to 7 cm after a median follow-up of 23 months and showed no statistical difference in overall survival rates. The 1-, 3- and 5-year actuarial overall survival rates were 97%, 77%, and 56% for the embolization/ablation group and 82%, 70%, and 58% for the surgical group [12]. They later followed these patients over a median of 134 months and confirmed their initial findings that there was no difference in survival among patients with solitary HCC treated with resection vs. combined embo-ablation suggesting that there may be a greater role for primary embo-ablation in the treatment of potentially respectable solitary HCC [13].
Theoretically better results can be obtained with the use of contrast mediums during TAE. Lipiodol, an iodinated ethyl ester of poppy seed oil, traditionally has been used as an embolic agent and as a vehicle for carrying chemotherapeutic agents that contains 38% iodine by weight [14]. Normally, hepatocytes can remove Lipiodol in 7 days; however HCC retains it for weeks. Since it is a radiopaque substance it can be visualized on post treatment computed tomography (CT) scans in the target lesion [15]. Treatment with Lipiodol is two-fold: it has a tumor necrosis effect by embolization and helps target the lesion during subsequent ablation procedures [16].
LC LUMI beads are new radiopaque embolic bead, which contain iodine and can be visualized by X-ray, fluoroscopy, and CT scans. They are non-resorbable microspheres with calibrated size ranges. LC LUMI beads stain the tumor and blood vessels supplying the tumor, causing embolization/occlusion. After the vessels recanalize they stay radiopaque. The added benefit of the beads is that visualization of the beads during an embolization procedure enables real-time visible confirmation of bead location. This allows the operator to see precisely where the beads need to be delivered during the procedure and allows real time adjustments/better precision of delivery into the tumor, determination of embolization endpoint, and recognition of regions of undertreatment. Visualizing circumferential uptake within the tumor helps exclude any additional feeding vessels and increases the potential for a complete response. The beads remain visible on follow-up scans, which help improve efficacy of subsequent ablation procedures [17].
An additional benefit of LC LUMI beads is that the small size of the particles allows for good tumor penetration and thus better ischemic necrosis. The beads come in sizes ranging from 70-150 μm and 100-300 μm [18]. On the other hand, Lipiodol is not designed to achieve complete and permanent arterial occlusion unless loaded with chemotherapy, as it eventually washes out from the target organ/ area [19]. Transarterial particle embolization to stasis causes a more robust ischemic necrosis which subsequently primes the tumor and surrounding tissues for a more efficacious response to thermal ablation resulting in larger ablation zones and a greater likelihood of achieving a complete response [11].
Conclusion
Our experience with combined TAE/MWA using LC LUMI beads for the treatment of HCC and CRLM demonstrates good response without evidence of tumor recurrence. We experienced better ease of operation by being able to control the embolization particles and more precise target appearance on follow-up ablation. We suspect that this contributed to the good response our patients experienced.
No studies to this date have investigated the results of combined TAE using LC LUMI beads with MWA. Currently clinical trials are being conducted by the National Institutes of Health Clinical Center: LC Bead LUMI Radio-Opaque Embolic Beads to Detect and Characterize the Vascularity of Hepatic Tumors during Treatment with Transarterial Embolization (TAE) Alone or Combined with Thermal Ablation. Further randomized control studies are necessary to validate efficacy and comparison should be made with other contrast mediums such as Lipiodol.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Gu YK, Luo RG, Huang JH, Tu QJS, Li XX, et al. (2010) Transarterial embolization ablation of hepatocellular carcinoma with a lipiodol-ethanol mixture. World J Gastroenterol 16: 5766.
- Wu ZB, Si ZM, Qian S, Liu LX, Qu XD, et al. (2016) Percutaneous microwave ablation combined with synchronous transcatheter arterial chemoembolization for the treatment of colorectal liver metastases: Results from a follow-up cohort. OncoTargets Ther 9: 3783.
- Violi NV, Duran R, Guiu B, Cercueil JP, Aubé C, et al. (2018) Efficacy of microwave ablation vs. radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: A randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 3: 317-325.
- Yang WZ, Jiang N, Huang N, Huang JY, Zheng QB, et al. (2009) Combined therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation for small hepatocellular carcinoma. World J Gastroenterol 15: 748-752.
- Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, et al. (2012) Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: A prospective randomized trial. J Clin Oncol 31: 426-432.
- Potretzke TA, Ziemlewicz TJ, Hinshaw JL, Lubner MG, Wells SA, et al. (2016) Microwave vs. radiofrequency ablation treatment for hepatocellular carcinoma: A comparison of efficacy at a single center. J Vasc Interven Rad 27: 631-638.
- Ni JY, Liu SS, Xu LF, Sun HL, Chen YT (2013) Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 19: 3872.
- Tanaka T, Isfort P, Braunschweig T, Westphal S, Woitok A, et al. (2013) Superselective particle embolization enhances efficacy of radiofrequency ablation: Effects of particle size and sequence of action. Cardiovasc Interven Rad 36: 773-782.
- Carmi L, Georgiades C (2010) Combination percutaneous and intraarterial therapy for the treatment of hepatocellular carcinoma: A review. In Sem Interven Rad 27: 296-301.
- Smolock AR, Cristescu MM, Hinshaw A, Woo KM, Wells SA, et al. (2018) Combination transarterial chemoembolization and microwave ablation improves local tumor control for 3-to 5 cm hepatocellular carcinoma when compared with transarterial chemoembolization alone. Abd Radiol 43: 2497-2504.
- Knavel EM, Green CM, Gendron-Fitzpatrick A, Brace CL, Laeseke PF (2018) Combination therapies: Quantifying the effects of transarterial embolization on microwave ablation zones. J Vasc Interven Radiol 29: 1050-1056.Â
- Maluccio M, Covey AM, Gandhi R, Gonen M, Getrajdman GI, et al. (2005) Comparison of survival rates after bland arterial embolization and ablation vs. surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interven Radiol 16: 955-961.
- Elnekave E, Erinjeri JP, Brown KT, Thornton RH, Petre EN, et al. (2013) Long-term outcomes comparing surgery to embolization-ablation for treatment of solitary HCC<7 cm. Ann Surg Oncol 20: 2881-2886.
- Idée JM, Guiu B (2013) Use of lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: A review. Critical Rev Oncol 88: 530-549.
- Gabr A (2017) Radioembolization for liver tumors, Blumgart’s surgery of the liver, biliary tract and pancreas. Elsevier, USA.
- Shin SW (2009) The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol 10: 425-434.
- Duran R, Sharma K, Dreher MR, Ashrafi K, Mirpour S, et al. (2016) A novel inherently radiopaque bead for transarterial embolization to treat liver cancer-a pre-clinical study. Theranostics 6: 28-39.
- Wáng YXJ, De-Baere T, Idée JM, Ballet S (2015) Transcatheter embolization therapy in liver cancer: An update of clinical evidences. Chinese J Cancer Res 27: 96-121.
Citation: Cornish N, Igonkin D, Birney A and Sarkar D (2019) Combined Therapy with Transarterial Embolization Utilizing Radiopaque LUMI Beads and Percutaneous Microwave Ablation for Liver Tumors. OMICS J Radiol 8:308. DOI: 10.4172/2167-7964.1000308
Copyright: © 2019 Cornish N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4538
- [From(publication date): 0-2019 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 3505
- PDF downloads: 1033