Comparative Phytochemical Profiling in Swertia Species Collected from Different Geographical Region of India Using High Performance Liquid Chromatography Coupled with Photodiode Array Detector and Mass Spectrometry (HPLC-PDA-MS) Followed by Antioxidant Potential
Received: 12-Jan-2018 / Accepted Date: 29-Jan-2018 / Published Date: 05-Feb-2018
Abstract
The aim of present study is comparative evaluation of iridoid glucosides and flavonoids phytochemicals in different species of Swertia collected from different geographical region in India using HPLC-PDA-MS analytical method and their antioxidant potential. Total seven species of Swertia viz. S. nervosa (SW-1), S. bimaculata (SW- 2) and S. chirata (SW-3), S. ciliata (SW-4), S. paniculata (SW-5), S. cordata (SW-6), and S. alata (SW-7) and six phytochemicals/biological active compounds viz. Swertiamarin, Sweroside, Swertisin, Amarogentin, Luteolin and Genistein have been selected for study. All compounds were separated on C-18 column using mobile phase A (0.5% acetic acid in water) and phase B (100% acetonitrile) in a gradient mode. Total six peaks from six compounds of seven species were unequivocally detected by comparing their UV spectra, retention time, TIC data, and in MS spectra under positive electrospray ionization mode (ESI-MS), fragmentation pattern of all targeted compounds with those of standard compounds. All targeted species were differentiated by variation in relative concentrations of the six compounds. Antioxidant potentials of all selected compounds were also evaluated. Higher total polyphenolic contents is reported in S. chirata and simultaneously validated through antioxidant activity assay such as β-carotene oxidation inhibition, DPPH radicals scavenging activity, and reducing potential in comparison to other species. Our developed and validated analytical methods HPLC-PDA-MS not only facilitate qualitative and quantitative study of targeted compounds in all species of Swertia but have important application in systematic evaluation of distribution of studied iridoids and flavonoid compounds. The developed method can be well employed in the detection and quantitative evaluation of targeted compounds in the bulk samples and different species of Swertia at industrial level.
Keywords: HPLC-PDA-MS; Iridoid compounds; Flavonoid compounds; Swertia species; Antioxidant potential
Introduction
Swertia herbs belongs to family Gentianaceae, is an annual herb, mostly grown in temperate-alpine of Himalayas at altitudinal range 1500-2400 meter, have been used in traditional medicine as antipyretic, analgesic, gastro and liver tonic [1]. In Ayurveda, this herb is commonly addressed by name chirata. Phytochemical screening has already been shown the presence of potent bioactive metabolites such as iridoids (Swertiamarin, Sweroside and Amarogentin), flavonoids (Swertisin and Luteolin), isoflavone (Genistein) xanthones, xanthone glycosides, and triterpenoids [2-4]. These phytochemicals possess wide spectrum of pharmacological activities such as antihyperlipidaemic, hepatoprotective, anti-oxidant, anti-tumor, antiinflammatory, anti-microbial, anti-leishmanial, immunomodulatory, and anti-angiogenesis Suryawanshi [5-7]. Due to high demand of S. chirata in the herbal drug industries, several other species (adulterants) belonging to the same genus are traded under the name of chirata. Reported adulterants are S. dilatata, S. bimaculata, S. paniculata, S. alata, S. minor, S. ciliata, S. cordata, S. nervosa, and S. angustifolia with true chirata [3]. Therefore, it is of urgent need to develop reliable chromatographic screening which could accurately evaluate the Swertia species is highly desirable in turn to reduce adulteration at the commercial level. Few studies have been reported on detection and quantification of Amarogentin, and Swertiamarin in S. chirata collected from west Himalayan using high performance thin layer chromatography and polyphenolic C-glycosides like mangiferin, isomangiferin, and 1,6,7 trihydroxyl-2-C-glucosexanthone and three flavones C-glycosides such as isoorientin, isovitexin, and Swertisin in S. franchetiana using LC–ESI-MS/MS method [8]. There is also report on characterization of Swertiamarin, mangiferin, Swertisin, oleanolic acid, 1,5,8-trihydroxy-3-methoxyxanthone,1,8-dihydroxy-3,7-dimethoxy xanthone and 1,8-dihydroxy-3,5-dimethoxy xanthone in comparison with high-altitude and low altitude populations of S. mussotii using HPLC [9]. From extensive literature survey we have found that there is no report available on comparative phytochemical profiling of targeted active phytochemicals in seven species of Swertia by HPLC-PDAMS detection and followed by their antioxidant potential evaluation. Antioxidant potential of polyphenolic compounds has already been established in the other plants [10-13]. In the present work we have validated positive correlation of polyphenolic/flavonoid compounds contents in the plant Swertia and its species with antioxidant activity. Polyphenolic compounds of Swertia species are of high demand because of their antioxidant property [14]. Higher concentration of these compounds has been reported in the aerial part of the plant Swertia [15]. Therefore, the plant Swertia and species may be served as an excellent dietary source of natural antioxidants with a wide range of biological activities for disease prevention and health promotion. In recent studies, anti-cancer, anti-diabetic and hepatoprotective activities have been reported in the plant Swertia [13,14,16]. Biological activity depends on active metabolites contents present in the herbs. In the herbaceous plant Swertia, the contents of active metabolites significantly vary on varying geographic, environmental, climatic and other factors. Because of extensive application of the herbaceous plant Swertia, it is of urgent need to develop quality standard methods to identify the raw materials. Therefore, we have developed analytical HPLC method coupled with PDA and ESI-MS detector for separation, identification and quantification of targeted six active phyto-compounds in seven major species of Swertia viz. S. chirata, S. bimaculata, S. ciliata, S. cordata, S. alata, S. nervosa, and S. paniculata collected from different phytogeographical locations in India. The purpose of targeting six active metabolites is that single compound in the herb could not be able to produce overall pharmacological activity and synergistic effect of different metabolites simultaneously produce significant action. Here, in development of analytical method various wavelength have been accessed to obtain maximum absorption wavelength and each targeted metabolites in the chromatographic profile were detected based on results of on-line HPLC-(ESI)-MS. On the basis of high sensitivity, selectivity and wide application, the powerful analytical HPLC-ESI-MS technique is selected in the analysis of complex mixture of metabolites in the present study. With the help of mass spectrometer, important structural information of metabolites can be gathered from collisioninduced dissociation (CID). That’s why in the present work, we used HPLC coupled with PDA-(ESI)-MS detection to ensure the structure of targeted metabolites of Swertia and its species and found that it is useful analytical method for qualitative and quantitative standardization of the herb Swertia.
Materials And Methods
Reference compounds, chemicals and solvents
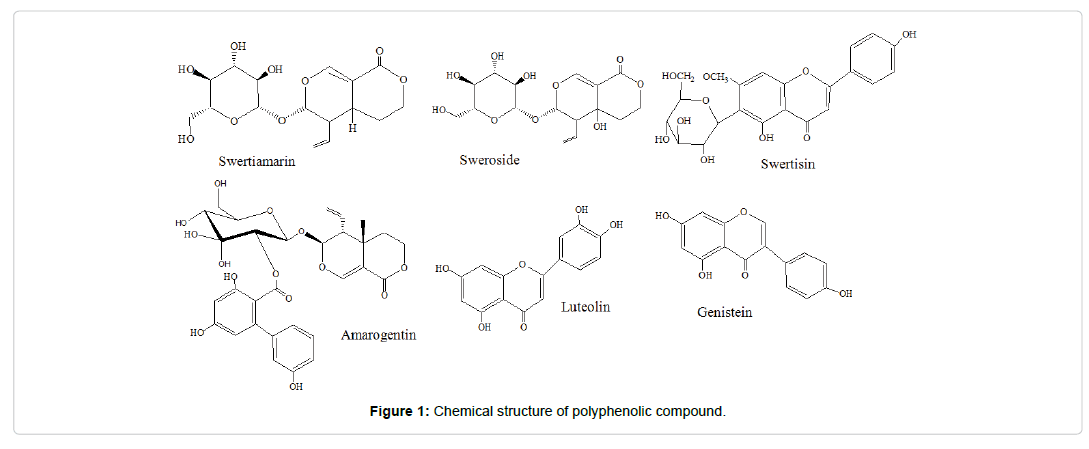
Standard biomarkers viz. Swertiamarin (ASB-00019440-005), Sweroside (ASB-00019435-010), Swertisin (ASB-00019441-010), Amarogentin (ASB-00001650-010), Luteolin (ASB-00012461-025), and Genistein (ASB-00007098-010) (Figure 1) of the Swertia herbs were procured from Chromadex, USA. Antioxidant assay reagent 2, 2-diphenyl-1-picrylhydrazyl (DPPH), β-carotene, tween-40 and linoleic acid were procured from Sigma-Aldrich (USA), and MP Biomedicals, India respectively. All solvents and chemicals were of LC-MS grade purchased from Burdik and Jackson (USA) and SD fine Chemicals (India).
Collection of plant materials
Total seven species of Swertia herbs viz. S. nervosa (SW-1), S. bimaculata (SW-2), S. chirata (SW-3), S. ciliata (SW-4), S. paniculata (SW-5), S. cordata (SW-6), and S. alata (SW-7) were collected from different phytogeographical regions in India. Details of herb collection are presented in the Table 1. The collected species were authenticated by Dr. AKS Rawat, Head, Pharmacognosy and Ethnopharmacolgy Division and Dr. Bhaskar Dutt, Senior Technical Officer, Herbarium Section, CSIR-National Botanical Research Institute, Lucknow, India. All voucher specimens have been deposited in the herbarium of institute.
| Plant | State | Region explored | Material |
|---|---|---|---|
| S. nervosa | D. & WB | India | Aerial part |
| S.bimaculata | D. & WB | India | Aerial part |
| S. chirata | D. & WB | India | Aerial part |
| S. ciliata | Uttarakhand | India | Aerial part |
| S. paniculata | Uttarakhand | India | Aerial part |
| S. cordata | Uttarakhand | India | Aerial part |
| S. alata | Uttarakhand | India | Aerial part |
Table 1: Details of plant collections.
Preparation of stock solution
A stock solution of selected six standard biomarker Swertiamarin, Sweroside, Swertisin, Amarogentin, Luteolin, and Genistein were prepared by dissolving 1 mg of accurately weighed each standard in 1 ml of LC-MS grade methanol. In addition, stock solution (1 mg ml-1) was further diluted upto 0.1 mg ml-1 by adding methanol and stored at 4°C until needed. All diluted standards were passed through 0.45 mm membrane filter and the analyzed by HPLC.
Preparation of crude extracts
The collected plant samples were dried, crushed and grinded into powder form. Each sample powder (2.0 g) placed in glass Stoppard Erlenmeyer flask (250 ml) was extracted on sonicator with 50% water in acetonitrile (4 × 25 ml). After that, the flask was placed in the ultrasonic bath of 20 KHz/1000 Watts (Model CD 4820 Comp lab, India) for 4 × 15 min at 28°C. The extracts were filtered and concentrated on rotary evaporator (Buchi, USA) followed by lyophilized (Labconco, USA). For analysis, 10 mg of crude extracts of each sample were dissolved into 1 ml of methanol. Before placing into auto-sampler, all extracts solutions were filtered through 0.22 μm Millipore filter membrane (Bedford, MA, USA).
Instrumentation and chromatographic conditions
Chromatographic analysis, UV spectra, mass spectra and 3D-plot were acquired using Waters HPLC-PDA-2996 coupled with 3100 Mass detector (Waters Corporation,Milford, MA, USA) in the qualitative and quantitative analysis of all collected samples. Chromatographic separation were achieved using Chromatopak C-18 column AQ (4.6 × 250 mm, 5.0 μm,) provided with C-18 guard column and gradient mobile phase composition A (0.5% acetic acid in water) and B (acetonitrile). All solvents were duly filtered through 0.22 μm millipore filter and degassed ultrasonically for 15 min before delivered to column for separation. Total run time for all analytes was programmed up to 60 minutes. Targeted metabolites peak were detected by mass spectrometry and quantification were accomplished in ESI positive mode by selecting single ion monitoring (SIR) and total ion current (TIC) (scanning at 2000 Da/sec). Mass spectrometry parameters such as source temperature (120°C), disolvation temperature (300°C), capillary voltage (3.50 KV), cone voltage (35 V), extractor voltage (3.0 V), Rf of lence (0.1 V), nitrogen gas used in disolvation and cone gas flow (700 l/hr and 50 l/hr) were programmed based on the nature of analytes accordingly. Peaks of all targeted analytes were identified by comparing retention times, UV-Vis spectra, and LC-MS spectra with those of authentic standards. Data acquisitions were performed using Empower software version 2 (Waters, Milford, MA 01757, USA).
Characterization of targeted metabolites in Swertia herb using mass spectra
Results from mass spectrometry can be employed to obtain data related to nature of compounds like aglycone or glycones and other structurally diverse group of secondary metabolites present in plants. In the present study, mass spectra of all sample extracts have been presented with specific fragmentation pattern which is required in structure determination of targeted flavonoids and iridoids metabolites. Identified structures were compared with structures available in literatures [8,9,17].
Method validation
The developed analytical HPLC-PDA-MS method was validated as per ICH norms. To obtain calibration curve, mixture of all standard solution were prepared and injected in different dilution. Linearity of calibration curve of each standard was determined by the slope, y-intercept and correlation coefficient (R2). Stability of mixture of all standard solution and extracts were determined by analyzing them repeatedly over a period of 24 hrs. All obtained values were given in terms of relative standard deviation (% RSD). Intra and inter-day precision of developed method was performed for each sample and standard compounds. In intra-day precision, the samples were analyzed five times in a day and for inter-day precision samples were analyzed five times up to three days. Accuracy of the method was evaluated by spiking known concentration of each standard into the samples. Three different concentration of each standard was used and analyzed by HPLC and LC-MS.
Evaluation of antioxidant potential
Determination of total phenolic and total flavonoid contents (TPC and TFC): Total phenolic contents (TPC) of each samples was determined using method described by Singh et al. [10] and results were expressed in terms of microgram (μg) gallic acid equivalents (GAE) mg-1 dry extract. The reaction mixture which contain sample extract (1 mg ml-1), Folin–Ciocalteau’s reagent (1N) and 20% sodium carbonate was mixed properly and left at room temperature for 20 min. The volume of mixture was maintained up to 25 ml by adding deionized water (DW) and then absorbance was recorded at A720 nm (Thermo Scientific, USA). On the other hand, Total flavonoids content (TFC) was determined using method described by Srivastava et al. [13] and expressed in terms of microgram (μg) quercetin equivalents (QE) mg-1 dry extract. Here, 2% of AlCl3 solution was added into reaction mixture and after 10 min. absorption was recorded at A415 nm against a blank sample.
β-carotene bleaching assay: Total antioxidant potential/activity (AOA) was determined using method “autoxidation of β-carotene and linoleic acid coupled reaction” described by Singh et al. [10]. In this method, 3 ml of β-carotene solution of concentration (3 mg/20 ml chloroform) was added into 40 mg of linoleic acid and 400 mg of tween-40 emulsion. After mixing, chloroform was removed using vacuum filter system, followed by the addition of 100 ml DW and mixing vigorously. Now the 3 ml of this solution was mixed with the extract and incubated at 50°C for 60 min. Later on, absorbance for AOA was recorded at A470 nm and the results were expressed in % inhibition relative to the control.
Free radical scavenging activity (FRSA) assay: FRSA of the extracts was measured by using DPPH• stable radical [18]. Briefly, each 0.1 ml extract was added to freshly prepared 2.9 ml DPPH• solution (6 × 10-5M) and mixed vigorously. The reduction of the DPPH• radical was measured by monitoring absorbance continuously at A517 nm until stable values was obtained. The percentage of remaining DPPH• (DPPH• rem) was calculated as %DPPH• rems = DPPH•t = 60/DPPH•t = 0 and plotted against the sample concentration. Results were expressed in the terms of percent inhibition and efficiency concentration (EC50).
Ferric reducing power assay: Reducing potential of all sample extract was examined using a slightly modified ferric reducing power assay. In the method, the reaction mixture was prepared by mixing 0.1M phosphate buffer (pH 6.6; 2.5 ml) and 1% potassium ferricyanide (2.5 ml) in the sample extract. The mixture was incubated at 50°C for 30 min followed by addition of 10% trichloroacetic acid (2.5 ml) to stop the reaction. Further, the reaction mixture was centrifuged at 5000 rpm for 20 min and upper layer was diluted with DW (2.5 ml). Finally, 0.1% FeCl3 (0.5 ml) was added into reaction mixture and incubated at room temperature for 10 min. The absorbance was recorded at A700 nm against the blank sample.
Statistical analysis
Results were reported as means ± relative standard deviation (% RSD) of at least three replicates of the each sample extract. Data were subjected to one-way analysis of variance (ANOVA) and the least significant difference (LSD) between the extracts at P<0.01 was calculated by post hoc comparison test (SPSS 11.5).
Results And Discussion
Optimization of HPLC method for separation and quantification of compounds
Method development began with the optimization of chromatographic conditions including mobile phase composition and column type. Versatility, suitability, and robustness of method were checked with several C-18 columns of various manufacturers. Best chromatographic resolution, selectivity, and sensitivity were obtained by Chromatopak C-18 AQ column (4.6 × 250 mm; 5.0 μm). Feasibility of various solvents and their mixtures such as ammonium acetate, acetic acid, and formic acid with variable pH range of 3.0 to 7.0 and altered flow rates (0.3-0.9 ml min-1) was tested. Among three acidified aqueous phases studied, 0.5% acetic acid provided the best separation of targeted compounds. Methanol was also subsequently screened as organic modifier after adjusting the gradient to accommodate the differences in solvent strength between methanol and acetonitrile. Acetonitrile was found to provide better separation than methanol. Therefore, the Chromatopak C-18 AQ column and acetonitrile were selected for further analysis.
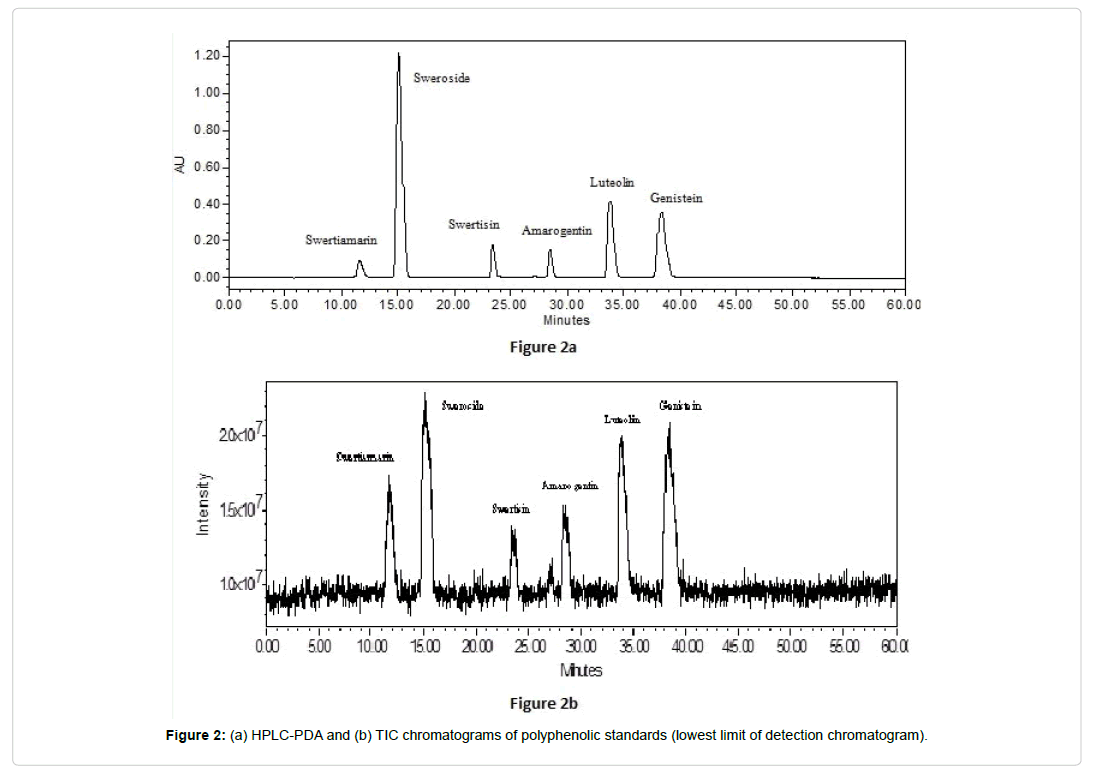
For separation of compounds, gradient mobile phase system 0-5 min (25-30% B) at 0.90 ml min-1, 5 to 40 min (30-40% B) at 0.80 ml min-1, and 40 to 60 min (40-30% B) at 0.80 ml min-1 flow rate was used. A scouting gradient changing from 10% to 40% acetonitrile was employed to remove the smaller steps reported in available methods [19,20]. This approach spread out the polymeric hump which was observed in previous analysis, while maintaining maximum separation of the major compounds and simplifying integration. Compounds were detected on PDA detector at λmax 280 nm and elution/retention time (RT) for each analytes viz. Swertiamarin, Sweroside, Swertisin, Amarogentin, Luteolin, and Genistein were recorded at 11.58, 15.10, 23.41, 28.44, 33.81 and 38.36 min respectively (Figure 2a and 2b). Using gradient times longer than 60 min did not improve separation in the chromatogram of Swertia species; therefore 60 min gradient was selected as optimal for the separation of the major compounds in plant samples. Moreover, the temperature 30°C provided the lowest degree of co-elution for all the phyto-compounds in Swertia species.
Optimization of LC-MS methods for separation of targeted compounds
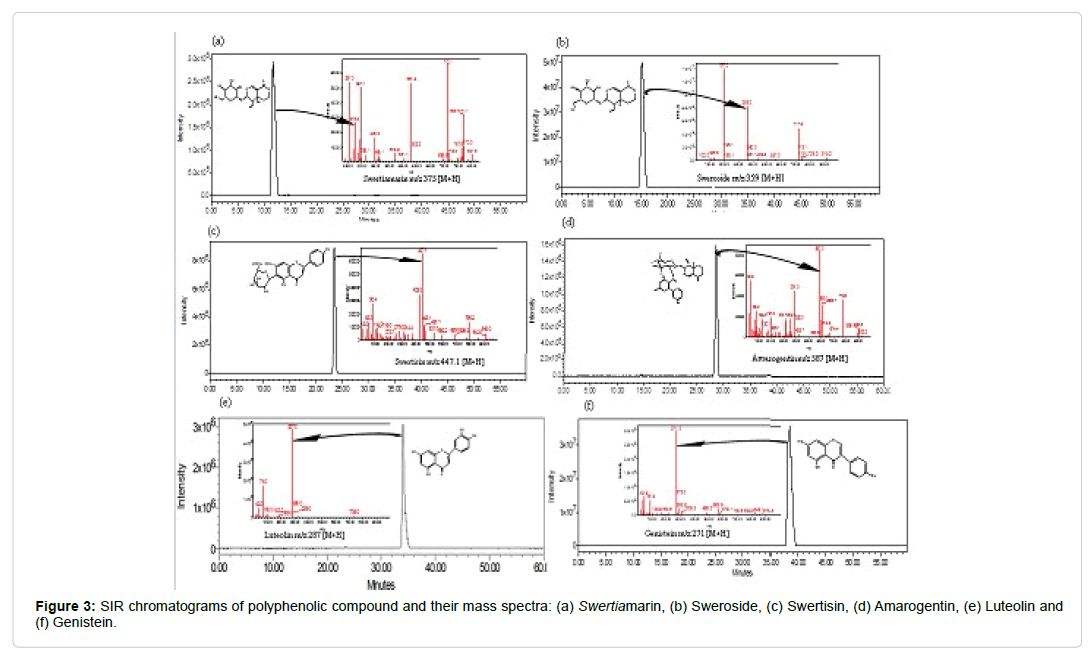
To optimize ESI condition, quadrupole full scan was carried out in both negative and positive ion detection modes. Positive ion mode showed good resolution of targeted compounds on both SIR and TIC scanning. Detection of each targeted compounds were performed on TIC and SIR mode in electro spray positive ion [M+H] scanning by MS detector. On the basis of findings, only SIR values were used for quantification of targeted compounds in sample of Swertia and its species. All targeted compounds were separated using LC-MS analytical method and confirmed by retention times (rt), molecular ion at positive ion mode (M+H) with their base peak and fragment ion(s), molecular formula, and chemical category (Table 2). LC-MS profiling of each compounds viz. Swertiamarin m/z 375 [M+H], Sweroside m/z 359 [M+H], Swertisin m/z 447 [M+H], Amarogentin m/z 587 [M+H], Luteolin m/z 287 [M+H], and Genistein m/z 271 [M+H] were presented in the Figure 3.
| Identification | Retention time (RT) | M+H | Base Peak | Fragment ions | Molecular formula | Chemical category |
|---|---|---|---|---|---|---|
| Swertiamarin | 12.152± 0.10 | 359 | 197 | 126.8 | C16H22O10 | Iridoid |
| Sweroside | 14.818± 0.13 | 375 | 713.1 | 357 | C16H22O9 | Iridoid |
| Swertisin | 14.818± 0.14 | 447.1 | 447.1 | 429.3, 90.4 | C22H22O10 | Flavonoid glycoside |
| Amarogentin | 14.818± 0.15 | 587 | 587 | 391, 197 | C29H30O13 | Iridoid |
| Luteolin | 14.818± 0.16 | 287 | 287 | 185, 74 | C15H10O6 | Flavonoid |
| Genistein | 14.818± 0.17 | 271 | 271 | 87.9, 42.9 | C15H10O5 | Flavonoid |
Table 2: Identification of polyphenolic compound by LC-ESI-MS.
System suitability test
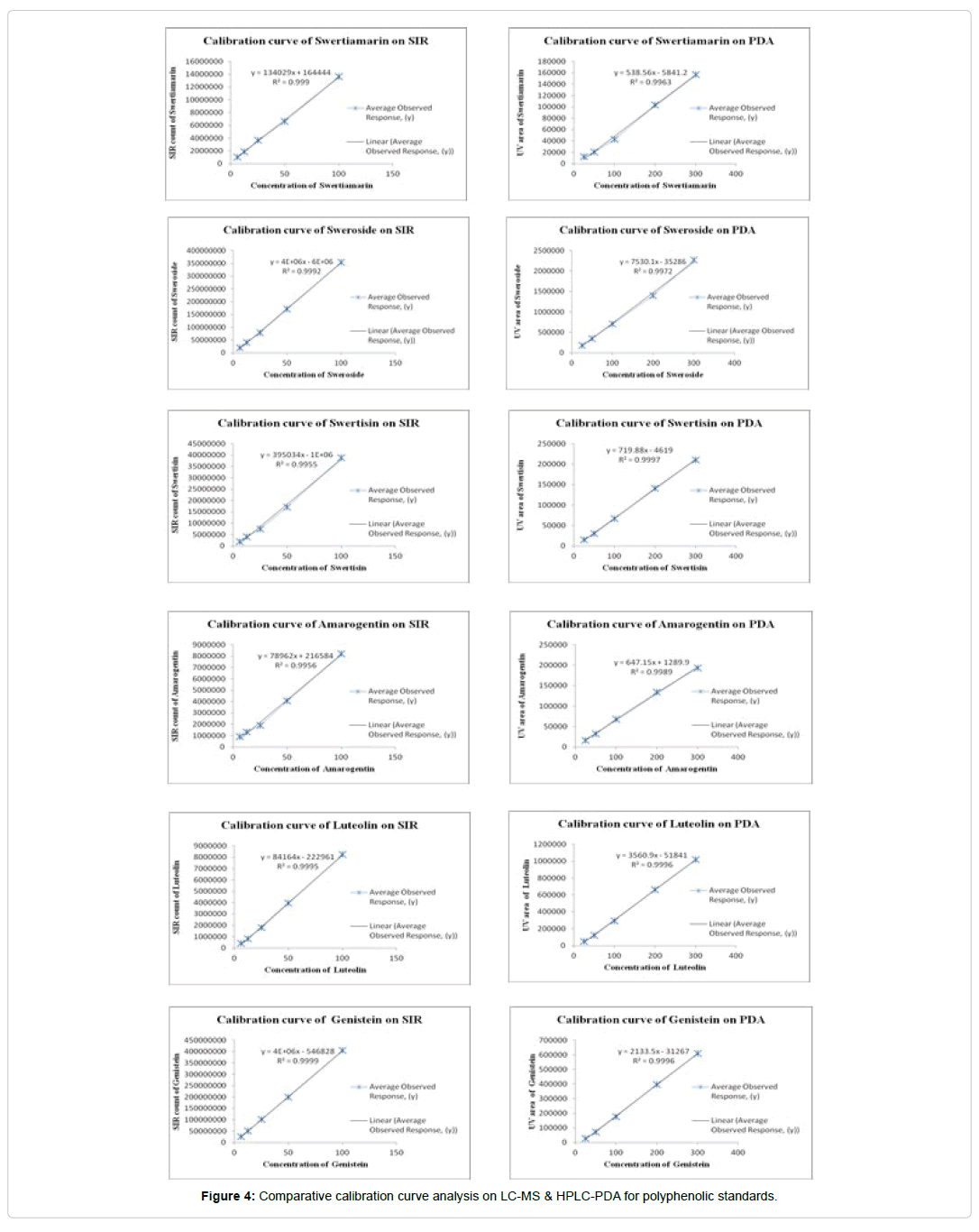
Linearity: In the present analysis, all analytical standards Swertiamarin, Sweroside, Swertisin, Amarogentin, Luteolin and Genistein were injected into column through auto-sampler in the minimum five concentration range (25 to 300 ng) for linearity/ calibration study. Calibration curves were constructed for each analyte by plotting the peak-area ratios from known concentrations of analyte injected into the column (Figure 4). The correlation coefficient (r) for flavonoids, flavonoid glycoside and iridoids compounds were observed in the range of 0.9996 to 0.9997 and 0.9963 to 0.9989 by HPLC-PDA detection (Table 3a). However in LC-MS analysis, concentration range from 6.25 to 100 ng of standards was used and subsequently observed good regression coefficient R2=0.9999 to 0.9991. Results of regression analysis on the calibration curve and quantification range are shown in Table 3b.
| Analytes | Interday precision (n=5), % RSD |
Intraday precision (n=5), % RSD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day one | Day two | Day three | ||||||||||
| TRa | PAb | ACc | TR1 | PA1 | AC1 | TR2 | PA2 | AC2 | TR3 | PA3 | AC3 | |
| Swertiamarin | 0.15 | 0.15 | 0.13 | 0.14 | 0.22 | 0.18 | 0.15 | 0.29 | 0.33 | 0.15 | 0.46 | 0.58 |
| Sweroside | 0.16 | 0.16 | 0.13 | 0.18 | 0.26 | 0.19 | 0.17 | 0.29 | 0.45 | 0.19 | 0.41 | 0.64 |
| Swertisin | 0.14 | 0.18 | 0.12 | 0.17 | 0.25 | 0.18 | 0.12 | 0.29 | 0.45 | 0.12 | 0.38 | 0.61 |
| Amarogentin | 0.15 | 0.22 | 0.16 | 0.15 | 0.3 | 0.19 | 0.2 | 0.38 | 0.39 | 0.11 | 0.4 | 0.58 |
| Luteolin | 0.12 | 0.15 | 0.1 | 0.14 | 0.23 | 0.15 | 0.14 | 0.41 | 0.48 | 0.1 | 0.39 | 0.62 |
| Genistein | 0.17 | 0.13 | 0.12 | 0.18 | 0.27 | 0.18 | 0.18 | 0.3 | 0.48 | 0.17 | 0.36 | 0.59 |
| 1Day one, 2Day two, 3Day three TR; Retention time of analyte, PA; Peak area response, AC; Analyte concentration |
||||||||||||
Table 3a: Inter-day and intra-day precision data of polyphenolic compound on LC-ESI-MS.
| Analytes | Interday precision (n=5), % RSD |
Intraday precision (n=5), % RSD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day one | Day two | Day three | ||||||||||
| TRa | PAb | ACc | TR1 | PA1 | AC1 | TR2 | PA2 | AC2 | TR3 | PA3 | AC3 | |
| Swertiamarin | 0.14 | 0.12 | 0.15 | 0.25 | 0.14 | 0.26 | 0.15 | 0.42 | 0.73 | 0.29 | 0.76 | 0.88 |
| Sweroside | 0.16 | 0.14 | 0.13 | 0.27 | 0.15 | 0.26 | 0.17 | 0.45 | 0.75 | 0.27 | 0.81 | 0.84 |
| Swertisin | 0.13 | 0.15 | 0.16 | 0.21 | 0.17 | 0.23 | 0.12 | 0.49 | 0.65 | 0.26 | 0.68 | 0.85 |
| Amarogentin | 0.19 | 0.2 | 0.14 | 0.23 | 0.19 | 0.25 | 0.2 | 0.48 | 0.69 | 0.25 | 0.7 | 0.86 |
| Luteolin | 0.11 | 0.16 | 0.12 | 0.22 | 0.2 | 0.24 | 0.14 | 0.51 | 0.68 | 0.24 | 0.69 | 0.82 |
| Genistein | 0.12 | 0.19 | 0.11 | 0.2 | 0.19 | 0.23 | 0.18 | 0.61 | 0.62 | 0.27 | 0.66 | 0.79 |
| 1Day one, 2Day two, 3Day three TR; Retention time of analyte, PA; Peak area response, AC; Analyte concentration |
||||||||||||
Table 3b: Inter-day and intra-day precision data of polyphenolic compound on HPLC-PDA.
Precision: Instrumental precision was achieved by repeated measurements of peak obtained from each standard with different concentrations viz. 25, 50, 100, 200 and 300 ng injection-1 using SIR detection. The repeatability of the sample application and measurements of peak area was expressed in terms of percent relative standard deviation (% RSD). Intra- and inter-day precision study were done at different concentrations levels of each targeted standard was injected in three times within 24 hrs and over a period of 5 days and expressed in terms of percent relative standard deviation % RSD. In this method precision met the acceptance criteria, because the relative standard deviation (RSD %) of single values from mean value was less than 5% within the same day (intraday, n=5) and not more than 10% on three consecutive days (interday, n=3) as shown in Table 4a and 4b.
| Standard | Calibration curve | R2 | Linearity range (ng) | LOD (ng) | LOQ (ng) |
|---|---|---|---|---|---|
| Swertiamarin | y = 134029x + 164444 | 0.9994 | 6.25-100 | 4.2 | 14.72 |
| Sweroside | y = 4E+06x - 6E+06 | 0.9992 | 6.25-100 | 3.6 | 12.07 |
| Swertisin | y = 395163x - 1E+06 | 0.9994 | 6.25-100 | 1.79 | 5.99 |
| Amarogentin | y = 74919x + 333250 | 0.9991 | 6.25-100 | 3.8 | 12.94 |
| Luteolin | y = 84164x - 222961 | 0.9995 | 6.25-100 | 2.88 | 9.61 |
| Genistein | y = 4E+06x - 546828 | 0.9999 | 6.25-100 | 1.05 | 3.5 |
Table 4a: Calibration curve, regression (R2), LOD and LOQ studies on LC-ESIMS.
| Standard | Calibration curve | R2 | Linearity range (ng) | LOD (ng) | LOQ (ng) |
|---|---|---|---|---|---|
| Swertiamarin | y = 134029x + 164444 | 0.9994 | 6.25-100 | 4.2 | 14.72 |
| Sweroside | y = 4E+06x - 6E+06 | 0.9992 | 6.25-100 | 3.6 | 12.07 |
| Swertisin | y = 395163x - 1E+06 | 0.9994 | 6.25-100 | 1.79 | 5.99 |
| Amarogentin | y = 74919x + 333250 | 0.9991 | 6.25-100 | 3.8 | 12.94 |
| Luteolin | y = 84164x - 222961 | 0.9995 | 6.25-100 | 2.88 | 9.61 |
| Genistein | y = 4E+06x - 546828 | 0.9999 | 6.25-100 | 1.05 | 3.5 |
Table 4b: Calibration curve, regression (R2), LOD and LOQ studies on HPLCPDA.
Sensitivity and selectivity: In HPLC and LC-MS analysis, limit of detection (LOD) for Swertiamarin, Sweroside, Swertisin, Amarogentin, Luteolin and Genistein were found at a signal-to-noise ratio (S/N) of 3:1 and lower limit of quantification (LOQ) was found for all standards with a signal to-noise (S/N) ratio of greater than 5:1. In comparison of both methods, LOD, LOQ values of LC-MS method given lower values as compared to HPLC values.
Accuracy and recovery: The accuracy of the methods was determined by analyzing the percentage recovery of targeted standard compounds in the all samples. To obtain it, three sets were prepared from Swertia and its species. All samples were spiked with three different concentrations of all standards viz. Swertiamarin, Sweroside, Swertisin, Amarogentin, Luteolin and Genistein and recovered in triplicate and analyzed by proposed HPLC and LC-MS methods. Accuracy corresponded to the acceptance criteria, because the deviations of mean values from true values were within 5% for all levels and less than 10% for the lower limit of quantification (LOQ) expressed in Table 5a and 5b in % RSD.
| Standard | Calibration curve | R2 | Linearity range (ng) | LOD (ng) | LOQ (ng) |
|---|---|---|---|---|---|
| Swertiamarin | y = 538.56x - 5841.2 | 0.9963 | 25-300 | 24.12 | 80.42 |
| Sweroside | y =7530.1x - 35286 | 0.9972 | 25-300 | 20.96 | 69.86 |
| Swertisin | y = 719.88x - 4619 | 0.9997 | 25-300 | 7.14 | 23.81 |
| Amarogentin | y = 647.15x + 1289.9 | 0.9989 | 25-300 | 13.2 | 44 |
| Luteolin | y = 3560.9x - 51841 | 0.9996 | 25-300 | 7.77 | 25.9 |
| Genistein | y = 2133.5x - 31267 | 0.9996 | 25-300 | 7.41 | 24.72 |
Table 5a: Recovery of polyphenolic compound in seven species of swertia on LCESI- MS.
| Species | Recovery studies on LC-MS (% RSD) | |||||
|---|---|---|---|---|---|---|
| Swertiamarin | Sweroside | Swertisin | Amarogentin | Luteolin | Genistein | |
| S. chirayita | 1.474049 | 1.793807 | 1.420835 | 1.495504 | 1.522367 | 1.612755 |
| S. bimaculata | 1.629153 | 1.528872 | 1.088707 | 1.737166 | 1.860616 | 1.818574 |
| S. ciliata | 1.420393 | 1.527014 | 1.054491 | 0.960219 | 1.945054 | 1.509931 |
| S. cordata | 1.849851 | 1.635674 | 1.571988 | 0.527838 | 1.321117 | 1.811556 |
| S. alata | 1.310806 | 1.641167 | 1.063333 | 1.007918 | 0.722452 | 0.334579 |
| S. nervosa | 1.300133 | 1.870555 | 1.03327 | 1.060863 | 1.319395 | 1.531659 |
| S. paniculata | 1.442539 | 1.441937 | 1.090802 | 0.594214 | 0.501137 | 0.582751 |
Table 5b: Recovery of polyphenolic compound in seven species of Swertia on HPLC-PDA.
Robustness: Robustness is a measure of the method to remain unaltered by small but deliberate variations in the method conditions and is indicate of the reliability of the method. For robustness study different mobile phase composition, pH, developing retention time and different mobile phase ratio lots were assessed. On the basis of finding it is concluded that the developed method was robust at gradient elution regardless of small variations within the same mobile phase and to small changes revels with changes in the pH, temperature, flow-rate and concentration of reagents on mobile phase.
Stability study: For short term chemical stability study, pure analyzed standards were kept in the auto-sampler at room temperature (20°C) for 24 hrs followed by 95 to 99% recovery which indicated that there was no chemical decomposition of the standard compounds. While in long term chemical stability study, standard and sample solutions were kept in refrigerator at temperature (-20°C) for 4 weeks followed by recovery which was showed that samples and standards could be stored for a month without degradation in the frozen state.
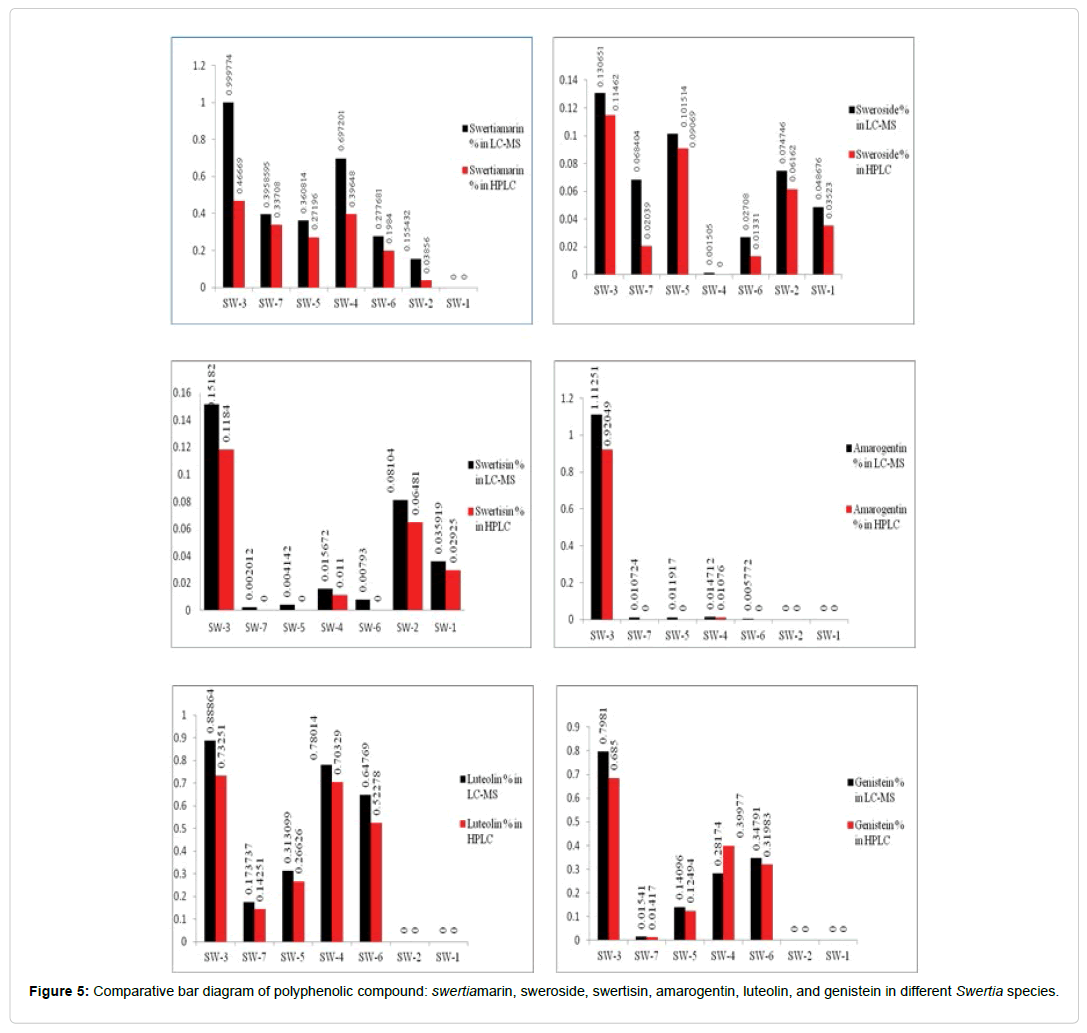
Quantification of targeted compounds in Swertia and its species: For quantification, solutions of six targeted standard compounds were automatically injected to the column via autosampler. The amount of all injected standards was calculated using calibration curve of each standard compounds. The concentrations of targeted compounds viz. Swertiamarin, Sweroside, Swertisin, Amarogentin, Luteolin, and Genistein were quantified in all samples of Swertia species. From the findings, it is observed that highest percentage content of Swertiamarin 0.999%, Sweroside 0.130%, Swertisin 0.151%, Amarogentin 1.112%, Luteolin 0.888% and Genistein 0.798% present in Swertia chirata (SW- 3) compared to other species. While in the other species of Swertia (SW- 4) contain Swertiamarin 0.697%, Amarogentin 0.014% and Luteolin (0.780%) respectively. Percentage contents of Sweroside 0.101% in SW-5, Swertisin 0.081% in SW-2, Genistein 0.347% in SW-6 were also reported and presented in Figure 5.
Mass spectrometry analysis
The electrospray-mass spectrometric data of compounds helped in substantiate the structure and molecular weight of respective compounds. With the help of molecular ion peak and base ion peak the molecular weight of the compound can be obtained. Here, in case of Swertiamain which is detected at RT 11.5, molecular ion peak m/z 375.0 and base ion peak m/z 713.10 together confirming the molecular weight of Swertiamarin as m/z [M+H]+ 951. Further analysis revealed the mass ion peak at m/z 357 (375-H-OH) as a result from loss of hydrogen and hydroxyl group from molecular ion peak. Similarly, Sweroside which was detected at RT 11.58, has shown molecular ion peak m/z 359.0 and base peak m/z 197 confirming the molecular weight of Sweroside as m/z [M+H]+ 359. Further fragmentation led to produce mass ion peak m/z 197 (375-H-Glc) as a result from loss of hydrogen and sugar molecules from compound ion. Another fragment at m/z 126.8 and m/z 162 was result from loss of hydrogen, CO2, CO, H, OH and H2O molecule from mass ion peak m/z 197. Likewise, compound Swertisin eluted at RT 23.41 in LC-MS confirmed the molecular ion peak m/z 447.10 and base ion peak m/z 429.3 with loss of water. Subsequent, fragmentation m/z 314.4, m/z 429.3 and m/z 279 were obtained from loss of 4-OH, 1-CH2OH, 1-OH and 1-H2O molecules. While investigating irodoid Amarogentin which was detected at RT 29.03 minute with molecular ion peak m/z 587 have shown fragments m/z 391 by the loss of H, Sugar and 1-CH3OH molecule from parent moiety. Further fragments m/z 359 and m/z 313 were appeared in the MS profile by the loss of 1-H2O, 1-CO and H2O molecules. Similarly, fragmentation pattern of flavonoid Luteolin which was detected at RT 34.5, have been evaluated with molecular ion peak m/z 287 with base ion peak. In continuation of evaluation, fragments m/z 185, m/z 74, m/z 102, m/z 42 and m/z 72 ion were obtained by the loss of [H-4OH-H2O-CH3], 1-CO and 2-CH3OH molecules. Standard compound Genistein which was eluted at RT 39.5 minute in LC-MS have shown molecular ion peak m/z 271 and base ion peak in ESI spectra. Fragments m/z 163.6, m/z 135.9 and m/z 87.9 were appeared in the chromatogram by the loss of p-hydroxy toluene, 1-CO, 1-H2O and 2-CH3 molecules from the parent Genistein molecule.
Antioxidant activity of Swertia species
Polyphenolic contents: Nowadays Folin–Ciocalteu method is used to determine TPC. TPC in methanolic extract of Swertia species are presented in Table 6. Significant differences (p<0.01) in TPC values were observed among all species, except SW-1 vs. SW-5 and SW-7 vs. SW-6. Results were expressed as μg of GAE mg-1 of dry extract and ranged from 40.28 ± 1.71 to 216.20 ± 5.73 μg/mg. The lowest value was found in SW-2, whereas the highest TPC was found in SW-3, indicating that S. chirata (SW-3) rich with antioxidant compounds and the results were matched as reported by Yang et al. This might be due to the differences between species of Swertia and TPC evaluation method used in these studies. Polyphenols are considered as a major group of chemicals that contribute the antioxidant potential of medicinal plants. Next, we sought to determine whether Swertia species could also accumulate TFC. Interestingly, SW-1 and SW-3 were only found which are accumulated TFC (Table 6). TFC in SW-3 was the highest 44.86 ± 2.55 μg QE mg-1 dry extract, while in SW-1 was contained 17.3 ± 1.13 μg QE mg-1. This difference was statistically significant (***P<0.001). The TPC and TFC can be used as important indicators of the antioxidant capacity for any product that is intended to be considered as a natural source of antioxidants in nutraceuticals and functional foods.
| Species | ATPC | ATFC |
|---|---|---|
| SW-1 | 113.35 ± 0.4.32cd | 17.3 ± 1.13b |
| SW-2 | 40.28 ± 1.71g | ND |
| SW-3 | 216.20 ± 5.73a | 44.86 ± 2.55a |
| SW-4 | 145.9 ± 8.91b | ND |
| SW-5 | 116.08 ± 4.93c | ND |
| SW-6 | 68.74 ± 3.17e | ND |
| SW-7 | 66.69 ± 2.19ef | ND |
| P ANOVA | ** | *** |
TPC, total phenolic content µg gallic acid equivalents (GAE) mg-1 extract; TFC, total flavonoid content µg quercetin equivalents (QE) mg-1 extract; ND, not detected.
SW-1: S. chirayita, SW-2: S. bimaculata, SW-3: S. nervosa, SW-4: S. ciliate, SW-5: S. paniculata, SW-6: S. cordata, SW-7: S. alata
AEach value is expressed as means ± SD (n=3). Data were analysed by ANOVA (**P<0.01 & ***P<0.001) and within each column different letters indicate statistically different values according to post hoc comparison (LSD-test) at P<0.01.
Table 6: Total polyphenolic content of seven species of Swertia.
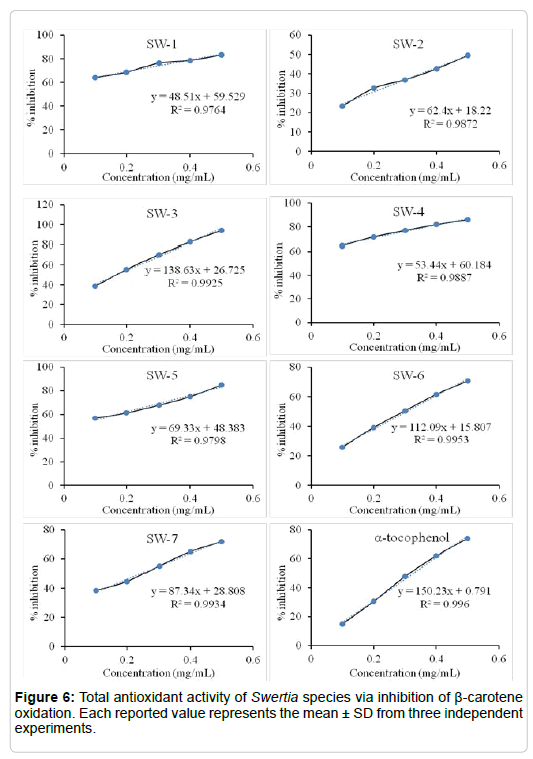
AOA of Swertia species: AOA of the extracts was determined using β-carotene bleaching method, which is based on the decolorization of the yellow color of β-carotene due to its reaction with linolate FRs which are formed by linoleic acid oxidation in an emulsion. The β-carotene oxidation can be attenuated in the presence of antioxidants. This fact is used in AOA evaluation of the different species of Swertia in comparison with, well known, natural antioxidant, namely α-tocopherol. The order of effectiveness of the extracts as antioxidants was SW-3 (94%) > SW-4 (86%) > SW-5 (85%) > SW-1 (83%) > α-tocopherol (74%) > SW-7 (72%) > SW-6 (70%) > SW-2 (49%) (Figure 6). The 50% inhibitions were accomplished with 95 μg ml-1 of SW-3 and 327 μg ml-1 of α-tocopherol. The SW-3 was the most potent antioxidant, suggesting that SW-3 contains high antioxidants, which were confirmed by HPLC- ESI-MS analysis.
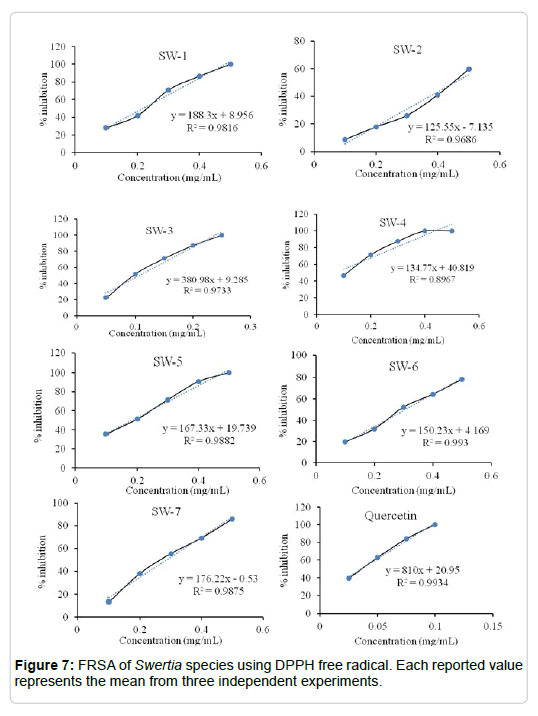
DPPH radical scavenging activity: DPPH method evaluates the potential of polyphenols present in extracts of Swertia species to reduce the DPPH radical. The method is based on the reduction of alcoholic DPPH radical solution in the presence of a hydrogen donating substance(s). DPPH radical solutions show a λ max at 517 nm appearing a deep violet color. The absorption disappears and the resulting decolorization is stoichiometric with respect to degree of reduction. The remaining DPPH radical, measured after a certain time, corresponds inversely to the FRSA of the antioxidant. Figure 7 shows the FRSA of seven species of Swertia. The FRSA in terms of percent inhibition was decreased in the following order: quercetin > SW-3 > SW-4 > SW-5 > SW-1 > SW-6 > SW-7 > SW-2. As indicated in the methods section, FRSA by DPPH radical method was also expressed by the parameters such as IC50, EC50 and antiradical power. In examining the results, SW-3 showed highest FRSA, as evident from their low values of IC50 (0.10 mg ml-1) and EC50 (4.1 mg/mgDPPH-1) and high value of ARP (Table 7). While, SW-2 exhibited lowest FRSA (IC50, 0.5 mg ml-1; EC50, 21.7 mg/mgDPPH-1 and ARP, 5) followed by SW-7 (IC50, 0.3 mg ml-1; EC50, 13.5 mg/mgDPPH-1 and ARP, 7). This difference is strongly related to the polyphenolic content and also to the type of the bioactive compounds present in each species. Significant correlations were also observed between polyphenolic contents and DPPH scavenging activity indicating the role of phenolic compounds in donating electron or hydrogen to DPPH radicals. These results are consistent with those of Singh et al. [10], who reported a positive correlation between TPC of Acacia nilotica fruit extract and FRSA measured by the DPPH method. According to Leung et al. [21], Luteolin, the major flavonoid in medicinal plants, showed a strong FRSA in experiments with the DPPH radical, which may explain the similarities.
| Species | AIC50 | AEC50 | AARP |
|---|---|---|---|
| SW-1 | 0.22±0.048cd | 9.57±0.48 cd | 10.0±0.63cd |
| SW-2 | 0.5±0.021a | 21.74±1.17a | 5.0±0.17fg |
| SW-3 | 0.10±0.013fg | 4.13±0.59efg | 24.0±1.39a |
| SW-4 | 0.18±0.011de | 7.83±0.051cde | 13.0±0.49c |
| SW-5 | 0.11±0.039ef | 4.78±0.061ef | 21.0±0.94b |
| SW-6 | 0.29±0.046bc | 12.61±0.94bc | 8.0±0.85de |
| SW-7 | 0.31±0.019b | 13.48±0.77b | 7.0±0.27ef |
| P ANOVA | ** | ** | ** |
IC50, inhibitory concentration (mg ml-1); EC50, effective concentration (mg/mgDPPH); ARP, anti-radical power. SW-1: S. chirayita, SW-2: S. bimaculata, SW-3: S. nervosa, SW-4: S. ciliate, SW-5: S. paniculata, SW-6: S. cordata, SW-7: S. alata
AEach value is expressed as means ± SD (n=3). Data were analyzed by ANOVA (**P<0.01) and within each column different letters indicate statistically different values according to post hoc comparison (LSD-test) at P<0.01.
Table 7: FRSA of seven species of Swertia.
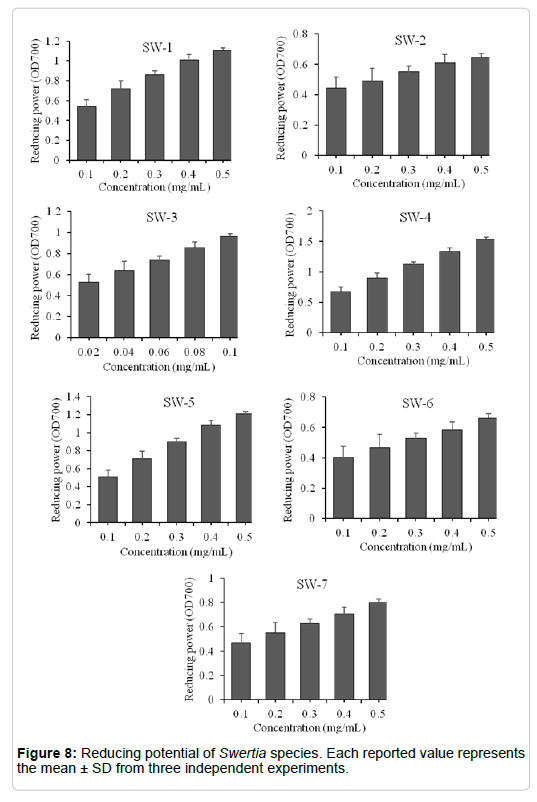
Reducing power: The inactivation of oxidants by antioxidants can be described as redox a reaction in which oxidant is reduced at the expense of the oxidation of another antioxidant. The ferric-reducing assay measures the antioxidant effect of any substance in the reaction medium as reducing ability. Reducing power of the Swertia species extracts was presented in Figure 8 and the order of reducing potential was SW-3 > SW-4 > SW-5 > SW-1 > SW-7 > SW-6 > SW-2. The highest reducing power, expressed in optical density at A700 nm was found in SW-3. As expected, SW-2, which had weak polyphenolic content and FRSA and also showed the weak ferric-reducing power. Several studies reported good correlations for the plant extracts when free radical scavenging activities were compared with its TPC [22].
Correlations between methods used to evaluate antioxidant activity of Swertia species
A higher significant correlation (p<0.001) was found between the TPC and the antioxidant activity (β-carotene oxidation, r=0.963, FRSA, r=0.908; and reducing power, r=0.931) (Table 8). Polyphenols are secondary metabolites of plants and can act as antioxidants via many promising mechanisms such as by donating hydrogen atom or an electron, by chelating metal catalysts, by activating antioxidant enzymes, and by inhibiting oxidases [23,24]. In general, our data indicate that the polyphenols of Swertia species are major source of natural antioxidants. Thus, the antioxidant capacity of Swertia species appears to be largely influenced by the total polyphenolic contents.
| Analyte | β-carotene oxidation | FRSA | Reducing power | TPC |
|---|---|---|---|---|
| β-carotene oxidation | 1 | 0.879** | 0.916*** | 0.963*** |
| FRSA | - | 1 | 0.857** | 0.908*** |
| Reducing power | - | - | 1 | 0.931*** |
| TPC | - | - | - | 1 |
FRSA; free radical scavenging activity using DPPH radicals; TPC; total phenolic content
Table 8: Correlation coefficients between β-carotene bleaching, FRAS, reducing power and TPC.
Conclusion
The whole study is designed to understand variation in phytochemical profiling in Swertia species collected from different geographical region of India using high performance liquid chromatography coupled with photodiode array detector and mass spectrometry (HPLC-PDA-MS) followed by antioxidant potential. To achieve main objective of the present study, the reliable and sensitive analytical HPLC-PDA and LCESI- MS methods for simultaneously quantification of targeted iridoids, flavonoids and flavonoid glycoside compounds in seven species of Swertia were developed and validated as per ICH norms. All targeted compounds were undergoing separation, identification, quantification and characterization by comparing retention time (RT), PDA, TIC and SIR detection, UV and mass spectral analysis. Based on validation results of PDA and ESI-MS methods, it can be concluded that PDA detector is useful only in detection and quantification of flavonoids (Luteolin and Genistein) and flavonoid glycoside (Swertisin) and not for iridoids compounds at their low level concentration. However, MS detection exhibited higher sensitivity for all targeted iridoids, flavonoids and flavonoid glycoside compounds even at their low level concentration (up to 6.25 ng). In MS detection, SIR is in positive mode exhibited higher sensitivity for all targeted analytes and helps in proper identification and quantification of compounds in seven species of Swertia. Both the developed methods are validated as per ICH norms and found reliable method for the study of targeted compounds in the Swertia and its species. The developed LC-ESI-MS method can be employed in quality control of Swertia samples and batch to batch consistency of crude drug in herbal formulation(s). Among all selected species of Swertia significant phytochemical variations were observed and this variation may be explained by genetic make-up, since all species were collected from phytogeographical regions of India. On varying phytochemical composition, medicinal property of herbal formulations will also vary and this imposes pressure on pharmaceutical company to control quality of collected materials even though different species of same genus from different geographical regions in India. Among all analyzed species of Swertia, highest contents of phenolic compounds were obtained in S. chirata and this finding was correlated with evaluation of its antioxidant potential. Positive correlation was found between phenolic contents and antioxidant potential.
Acknowledgements
Authors are thankful to the Director, CSIR-National Botanical Research Institute, Lucknow, India to provide all the research facilities.
References
- Pandey DK, Basu S, Jha TB (2012) Screening of different east himalayan species and populations of Swertia L. based on exomorphology and mangiferin content. Asian Pac J Trop Biomed 2: S1450-S1456.
- Khan AU, Rahim A, Iqbal Z, Gilani AH (2012) Insights into mechanisms underlying the gut and airways modulatory effects of Swertia chirata. J Nat Med 66: 140-148.
- Suryawanshi S, Asthana RK, Gupta RC (2009) Assessment of systemic interaction between swertia chirata extract and its bioactive constituents in rabbits. Phytotherapy Res 23: 1036-1038.
- Ghosal S, Sharma PV, Chaudhuri RK, Bhattacharya SK (1973) Chemical constituents of the gentianaceae v: Tetraoxygenated xanthones of Swertia chirata. J Pharma Sci 62: 926-930.
- Verma H, Patil PR, Kolhapure RM, Gopalkrishna V (2008) Antiviral activity of the indian medicinal plant extract, swertia chirata against herpes simplex viruses: A study by in-vitro and molecular approach. Indian J Medical Microbiol 26: 322-326.
- Srivastava S, Srivastava A, Rawat AKS (2011) Flavonoids: A dietary bioactive natural product. Mandal SC (ed) Herbal Drug, 1st edn. New Central Book Agency, Kolkata (India): 336-355.
- Alam KD, Ali MS, Parvin S, Mahjabeen S, Akbar MA, Ahamed R (2009) In vitro antimicrobial activities of different fractions of swertia chirata ethanolic extract. Pak J Biological Sci 12: 1334-1337.
- Wu W, Yan C, Li L, Liu Z, Liu S (2004) Studies on the flavones using liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatography A 1047: 213-220.
- Yang H, Ding C, Duan Y, Liu J (2005) Variation of active constituents of an important tibet folk medicine swertia mussotii franch. (gentianaceae) between artificially cultivated and naturally distributed. J Ethnopharmacol 98: 31-35.
- Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, et al. (2009) Oxidative DNA damage protective activity, antioxidant and antiquorum sensing potentials of moringa oleifera. Food Chem Toxicol 47: 1109-1116.
- Rawat AKS, Srivastava A, Tiwari SS, Srivastava S (2013) Quantification of protodioscin and prototribestin in fruits of Tribulus terrestris L. collected from different phyto-geographical zones of india. J Liq Chromatogr Relatd Techno 36: 1810-1821.
- Srivastava N, Singh BN, Srivastava A, Khan AR, Srivastava S, et al. (2015) Evaluation of phenolic content recoveries in hydrolyzed extracts of bergenia ciliata using RP-HPLC, GC–MS after silylation, and validation through antioxidant potential. J Liq Chromatogr Reltd Techno 38: 1722-1730.
- Srivastava N, Srivastava A, Srivastava S, Rawat AKS, Khan AR (2014) HPTLC-densitometric determination and kinetic studies on antioxidant potential of monomeric phenolic acids (MPAs) from Bergenia species. RSC Advances 4: 52647-52657.
- Chen Y, Huang B, He J, Han L, Zhan Y, et al. (2011) In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J Ethnopharmacol 136: 309-315.
- Sun Y, Zhang X, Xue X, Zhang Y, Xiao H, et al. (2009) Rapid identification of polyphenol c-glycosides from Swertia franchetiana by HPLC-ESI-MS-MS. J Chromatographic Sci 47: 190-196.
- Handa KL (1952) A modified method for the assay of Swertia chirata. Indian J Pharma Sci 14: 87-88.
- Suryawanshi S, Mehrotra N, Asthana RK, Gupta RC (2006) Liquid chromatography/tandem mass spectrometric study and analysis of xanthone and secoiridoid glycoside composition of swertia chirata, a potent antidiabetic. Rapid Comm Mass Spectro 20: 3761-3768.
- Yen GC, Duh PD (1994) Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agri Food Chem 42: 629-632.
- Liu Y, Xu Q, Xue X, Zhang F, Liang X (2008) Two-dimensional LC-MS analysis of components in swertia franchetiana smith. J Sep Sci 31: 935-944.
- Beer D, Joubert E (2010) Development of HPLC method for Cyclopia subternata phenolic compound analysis and application to other cyclopia spp. J Food Compos Anal 23: 289-297.
- Leung HW, Kuo CL,Yang WH, Lin CH, Lee HZ (2006) Antioxidant enzymes activity involvement in luteolin-induced human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol 534: 12-18.
- Zhou K, Laux JJ, Yu L (2004) Comparison of swiss red wheat grain and fractions for their antioxidant properties J Agri Food Chem 52: 1118-1123.
- Prakash D, Suri S, Upadhyay G, Singh BN (2007) Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int J Food Sci Nut 58: 18-28.
- Tiwari SS, Srivastava A, Srivastava S, Rawat AKS (2012) Isolation and quantification of vanillin through flash & hptlc chromatographic techniques from decalepis hamiltonii wight &arn root and their antioxidant activity. J Liquid Chromat Reltd Technologies 35: 2396-2407.
Citation: Srivastava A, Srivastava N, Tiwari S, Srivastava S, Dutt B, et al. (2018) Comparative Phytochemical Profiling in Swertia Species Collected from Different Geographical Region of India Using High Performance Liquid Chromatography Coupled with Photodiode Array Detector and Mass Spectrometry (HPLC-PDA-MS) Followed by Antioxidant Potential. J Phytochemistry Biochem 2: 105.
Copyright: © 2018 Srivastava A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5966
- [From(publication date): 0-2018 - Nov 15, 2025]
- Breakdown by view type
- HTML page views: 4921
- PDF downloads: 1045