Cytotoxic Effects of Amorpha Fruticosa Leaf, Stem, and Root Extractions on PC-12 Adrenal Neural Cells From Male Rattus Norvegicus
Received: 06-Dec-2016 / Accepted Date: 17-Jan-2017 / Published Date: 27-Jan-2017 DOI: 10.4172/2472-0429.1000120
Abstract
Cytotoxic properties of leaf, stem, and root extractions of Amorpha fruticosa were measured on PC-12 adrenal neural cells. Plant parts were processed via Soxhlet extraction method using a methanol solvent. Sterilized extractions of each plant component were applied to 48-hour PC-12 cells in increasing concentrations of 0.25 mg/ mL, 0.5 mg/mL, 1.0 mg/mL, 2.0 mg/mL, 5.0 mg/mL, 10.0 mg/mL, and 20.0 mg/mL. MTS assays determined cell viability. Data results demonstrated a dose dependent increase in death of the PC-12 cells with increased extraction concentrations. Cells exposed to leaf and root extractions exhibited similar results when compared to the positive controls produced from the addition of either 10 μg/mL cycloheximide or 3% hydrogen peroxide. The study concluded that leaf and root extractions of Amorpha fruticosa provided the greatest potency in their cytotoxicity against PC-12 adrenal cells. These results encourage further exploration of other segments of Amorpha fruticosa, including the plant’s seeds and fruit, in relation to their anti-carcinogenic effects on the PC-12 adrenal neural cell line
Keywords: Adrenal neural cancer; Pheochromocytoma; Amorpha fruticosa extractions
127468Introduction
Amorpha fruticosa, also known as false indigo or indigo bush, is a perennial species [1] related to the family Fabaceae or “pea family” [2]. This deciduous shrub is located in almst every state in the U.S (Department of Ecology 2014) and can often be found along riverbanks. The leaf structure of the false indigo plant is pinnately compound [2], containing 13 to 25 leaflets at each leaf (Department of Ecology 2014), and as the common name implies, the false indigo’s flowers exhibit vibrant shades of blue and purple, which bloom from mid-spring to early-summer [2]. Likewise, the plant’s fruit is roughly three-eighths of an inch and contains 2 seeds [1].
Previous studies have examined the antimicrobial and antibacterial properties of Amorpha fruticosa [3], especially in regards to wound healing [4]. In comparison other studies have analyzed the plant for its potent antioxidant properties [4]. Amorpha fruticosa has also been shown to demonstrate acetylcholinesterase inhibition, which could provide an alternative to chemotherapy in treating free radical pathologies, particularly those related to neurodegenerative disorders [5].
Based on these properties, the plant may be beneficial in treating PC-12 cells that derive from carcinogenic adrenal tumors from Rattus norvegicus. This study examined the cytotoxic effects of Amorpha fruticosa leaf, stem, and root extractions as administered on a PC-12 cell line.
PC-12 cells arise from pheochromocytomas, neuroendocrine tumors that affect the chromaffin tissue of the adrenal medulla [6]. PC-12 pheochromocytomas may also be located within the chromaffin cells outside the adrenal glands in the sympathetic or parasympathetic nervous tissue, in which case they are known as paraganglioma [7] Pheochromocytomas account for 4% of adrenal incidentalomas, and while most are benign, 10% prove to be malignant [6]. Similarly, 10% of pheochromocytomas may be hereditary, bilateral, or asymptomatic; therefore, this type of tumor is traditionally known as the “tumor of tens” [7]. In more recent years, however, sporadic familial pheochromocytomas now account for 15-24% of hereditary presentations [7]. Multiple endocrine neoplasia type 2 (MEN-2), Carney’s syndrome, and von Hippel-Lindau (VHL) disease are just a few hereditary conditions in which pheochromocytomas occur [6]. One study reported that 50% of patients with MEN-2 had pheochromocytomas [6], while another study stated that mutations of the succinate dehydrogenase B gene (SDHB) accounted for 15-35% of carcinogenic paragangliomas in the abdomen [6].
Patients in their 40’s have had the greatest incidences for pheochromocytomas [5,8] but rarely are the tumors found in children or patients under 20 years of age z [6]. Though uncommon, these malignant pheochromocytomas can cause very serious, life-threatening effects for the patient, as the tumor can alter the chromaffin cells to hypersecrete catecholamines, such as dopamine, epinephrine, and norepinephrine [8]. The result of these excessive secretions may lead the patient to experience the following symptoms: transitory electrocardiographic changes, tremulousness, orthostatic hypotension, anxiety, polydypsia, nausea, vomiting, and more [9]. Unfortunately, pheochromocytomas can be challenging to diagnose, as they can vary in their clinical appearances in patients and their symptoms often mimic indications related to alternate medical conditions [10].
Patients who do not receive treatment for malignant pheochromocytomas have less than 50% at a 5-year survival rate [6]. Eisenhofer [6] discusses in his study some current treatments for pheochromocytomas, with surgical resection being one of the most common means of treatment, as it aids in tumor reduction and patient longevity, but the results are usually temporary and only palliative. Chemotherapy and radiopharmaceutical therapy are also possible treatment options; however, their effectiveness is limited and can result in further debilitating disease [4]. Due to these disastrous effects, scientists are considering plant extractions as a medicinal outlook and a substitute for chemo and radiation therapy, since their extractions have been used to treat various illnesses for centuries due to their natural potency [3].
When testing plant extractions on cancer cell lines, one factor to consider is that some extractions from one section of a plant may be more potent than other sections on a given cancer cell line. A study conducted in 2013 compared the potency of two plant extractions, Phytolacca americana and Amorpha fruticosa, and considered their antioxidant and acetylcholinesterase inhibition properties [5]. The study looked at both the leaves and fruits of each plant and compared the results. In this particular study there were greater amounts of polyphenols and flavonoids in the A. fruticosa leaves than there were for the P. Americana leaves; the same applied to the fruits of both plants, with A. fruticosa containing higher trace amounts of polyphenols and flavonoids [5]. It is understood that polyphenols and flavonoids correlate to antioxidant potency, thereby confirming that A. fruticosa was more potent than P. americana. Interestingly enough, a positive correlation between acetylcholinesterase inhibition and total polyphenolic count was also found. The study ultimately suggested using A. fruticosa for treating free radical pathogens [5].
Materials and Methods
Plant material
The Amorpha fruticosa plants used in this study were purchased on September 2015 from located in Fayetteville, Georgia. One 3-gallon plant and one 1-gallon plant were utilized for extraction purposes and stored at Watkins Teaching Center at Anderson University. Plants were inspected and verified by Dr. Tom Kozel.
Plant preparation
The plant was deconstructed into its constituent parts (leaves, stems, and roots) via dissection scissors and a kitchen knife. The leaves were moderately compacted into an extraction thimble, leaving approximately a one inch margin from the thimble’s edge. The stems and roots were also cut into small pieces and compacted into their own extraction thimbles, leaving a similar margin of space between plant components and the extraction thimble’s edge. The average amount of material compacted per thimble was 1.777 g of leaves, 10.231 g of stems, and 16.969 g of roots.
Plant extractions
Each extraction thimble was placed in a Soxhlet extractor set with a 500 mL flask filled half-way with methanol. Extractions were carried out for a period of 8 to 12 hours under reflux, and afterwards, methanol from the solution was evaporated via rotary evaporator. Soxhlet extraction method and rotary evaporation were conducted multiple times to meet the demands of the study. The extractions used in the second cell treatment trial underwent additional preparation, as the extractions were subjected to lyophilization, after rotary evaporation and refrigeration, to ensure removal of residuals.
Media preparation
Media were prepared according to the American Type Culture Collection (ATCC) [11] protocol with some modifications [9]. Penicillin streptomycin was added aseptically in dosage of one percent of the total medium volume, and 31 μL of nerve growth factor at a 5 ng/ mL concentration was also added. Medium underwent sterile filtration under a tissue culture hood before being administered to PC-12 cells.
Thawing and growing cells
PC-12 adrenal gland cells were purchased from ATCC and were cultured following the ATCC protocol-(American Type Culture Collection) [9]. Cells were maintained in an incubator at 37°C and 5% CO2.
Splitting cells
PC-12 cell adherence and proliferation took place in ATCC collagen coated, Type IV tissue culture flasks. When cells became 75% to 80% confluent within the tissue culture flasks, old medium was removed with an electronic pipette, and the remaining cells were washed with Dulbecco’s phosphate buffer saline (DPBS), which was later discarded, and treated with trypsin. After 5 minutes of incubation, the cells could then detach for sub-culturing. Cells were transferred aseptically from old flasks to a 50 mL conical tube. New flasks contained 0.5 mL of the PC-12 cell line, previously transferred to the 50 mL conical tube, and 4.5 mL of prepared, sterile medium. Flasks were maintained in an incubator at 37ºC and 5% CO2 for 48 hours.
Cell plating
When cells were adequately confluent, approximately 80%, they were washed with DPBS, which was later removed after a minute before adding trypsin. The PC-12 cells then underwent incubation for 5 minutes (but no longer than 10 minutes) in order to allow for the cells to detach from the collagen tissue culture flasks. After incubation 1 mL of fetal bovine serum and 1 mL of prepared medium were added to the flasks before transferring cells to a 50 mL conical tube. Once the cells had been transferred, they were aspirated via a 5 mL electronic pipette to re-suspend the cells. Twenty microliters of the cells, suspended in the 50 mL conical tube, was placed in an Eppendorf tube along with 20 μL of trypan blue. After aspirating the cells and trypan blue, 20 μL of the solution was set on a hemocytometer slide, 10 μL in each of the two hemocytometer wells. Cells were then counted within 5 grids of each well and averaged between the two well counts. In order to calculate cell density, the cell count per hemocytometer well was divided by 5 (to account for the 5 grids), multiplied by 2 (to accommodate the 1:1 trypan blue, cell solution ratio), and multiplied by 1 × 104 (to achieve volume of cells/mL). Once the cell density had been calculated and adjusted accordingly, the amount of medium added to the cell concentration could then be configured in accordance to how many 96 well plates were necessary for experimentation via a dilution calculation (C1V1=C2V2). Cells were plated via a multichannel pipette to the desired cell concentration of 1 × 105 in a collagen coated, 96 well plate, with a total volume of 100 μL of cell solution and media per well [11]. Only columns 4 through 12 of the 96 well plates were plated with PC-12 cells. Plates were left in an incubator for approximately 48 hours at 37°C and 5% CO2.
Cell treatment
Stock solutions of leaf, stem, and root extractions were prepared at a 20.0 mg/mL concentration by re-suspending the extractions in fresh medium. Experimental concentrations were then diluted appropriately by adding more medium. After plated cells had undergone incubation for a minimum of 48 hours, old medium was removed, cells were washed with DPBS, and fresh medium, mixed with the desired experimental concentration of extraction, was added. Extractions were then administered to the cells in increasing concentrations of 0.25 mg/mL, 0.5 mg/mL, 1.0 mg/mL, 2.0 mg/mL, 5.0 mg/mL, 10.0 mg/ mL, and 20.0 mg/mL from columns 5 through 11 of the 96 well plates. Columns 1 through 4 provided for the experimental controls. Column 1 contained nothing; column 2, media; column 3, media and 1 mg/mL of experimental extraction; and column 4, media and PC-12 cells. Cells in column 12 were treated with either 10 μg/mL of cycloheximide or 3% hydrogen peroxide, in order to provide a “total-kill” control. Treated cell plates were left for incubation at 37oC and 5% CO2 for 48 hours.
Experimental readings
After treated cells were left to incubate for 48 hours, old media and experimental extraction concentrations were removed and cells were washed with DPBS. One hundred microliters of fresh medium and 20 μL of MTS assay were then added to each plate well, bringing the total volume to 120 μL per well. Plates underwent further incubation for a minimum of one hour and were removed for absorbency readings. Data were collected via an enzyme-linked immunosorbent assay (ELISA) plate reader under a preset filter of 450 nm [12].
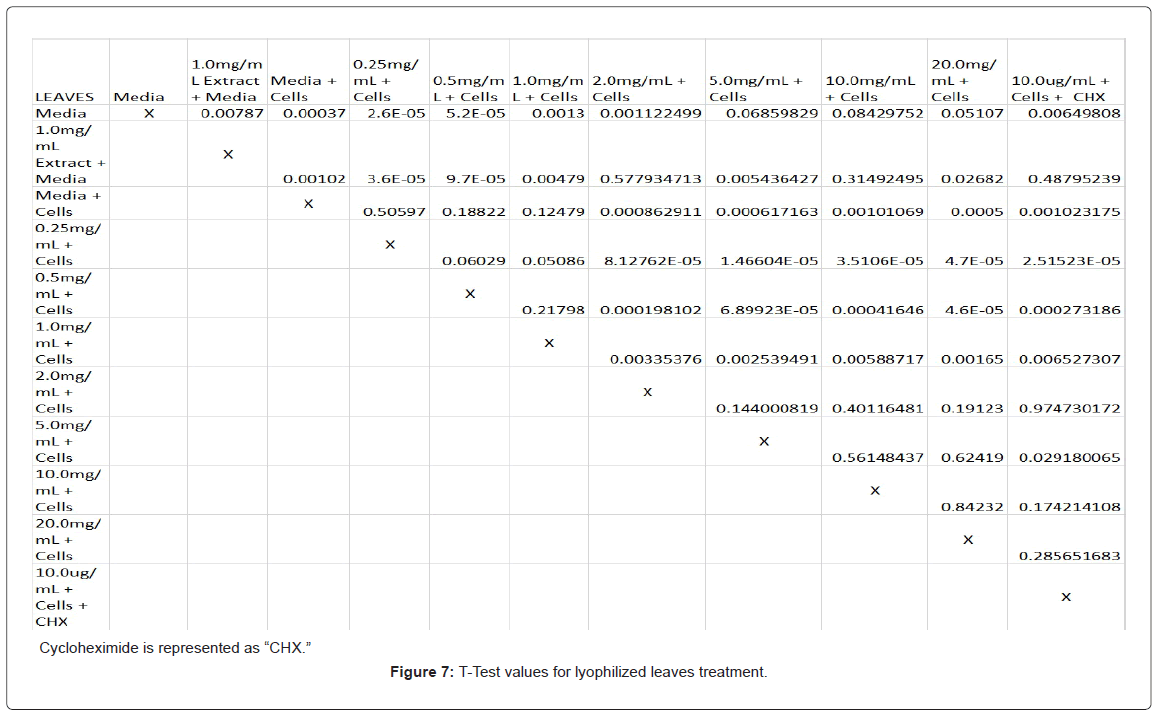
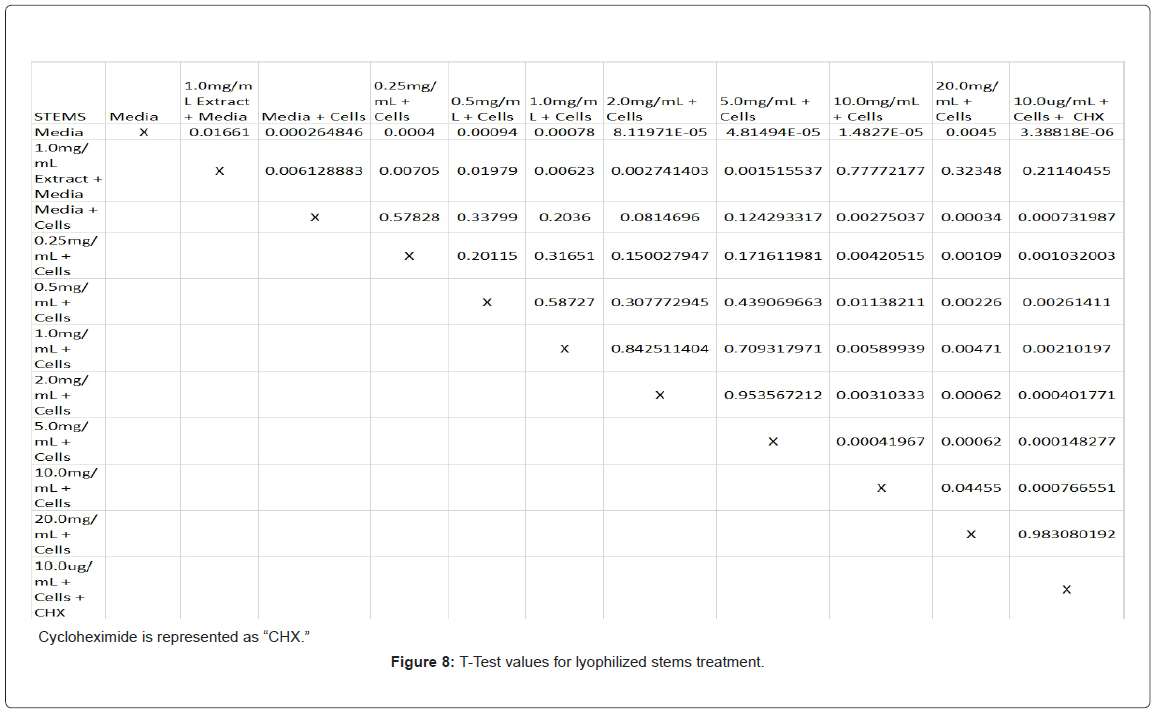
Absorbency readings for both the non-lyophilized and lyophilized extractions were subjected to a statistical T-test to evaluate the efficiency of the extractions at their respective concentrations. Statistical trends were analyzed in relation to the values found from the media and cell control and the 0.25 mg/mL treatment for each extraction. Likewise, the alpha value for this test was set to 0.05, where a p-value equal to or less than the alpha value was considered statistically significant and a p-value greater than the alpha value, not statistically significant.
Results
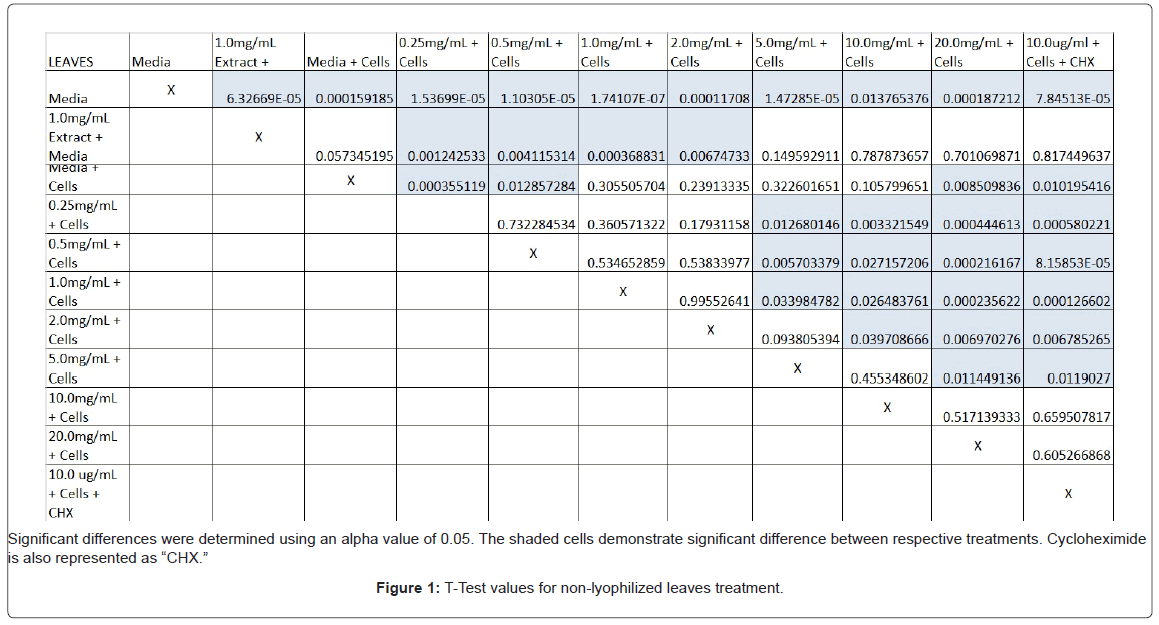
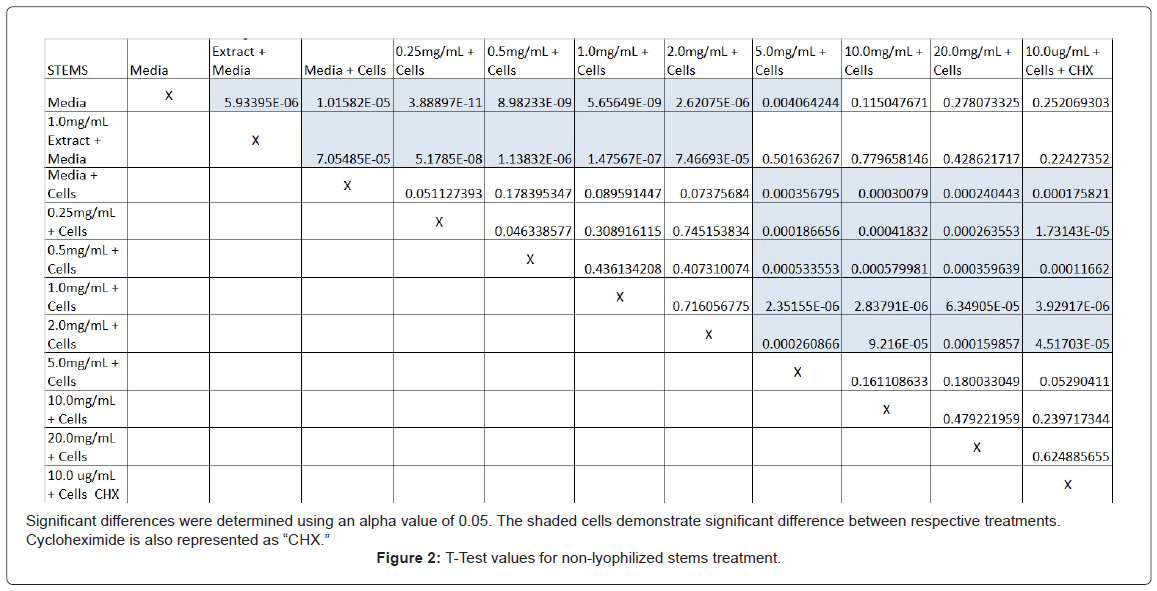
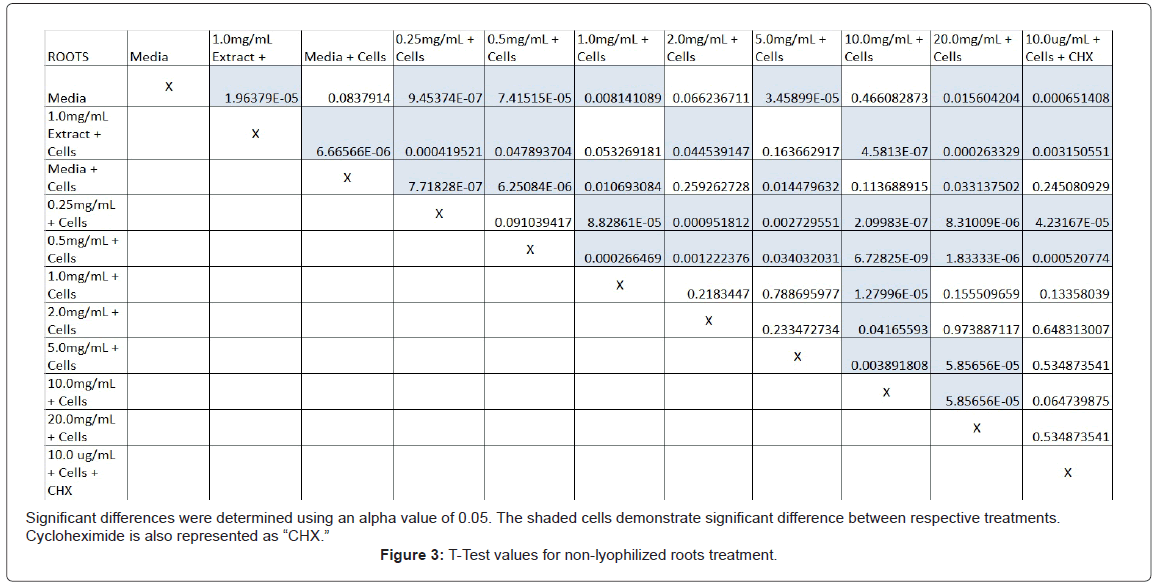
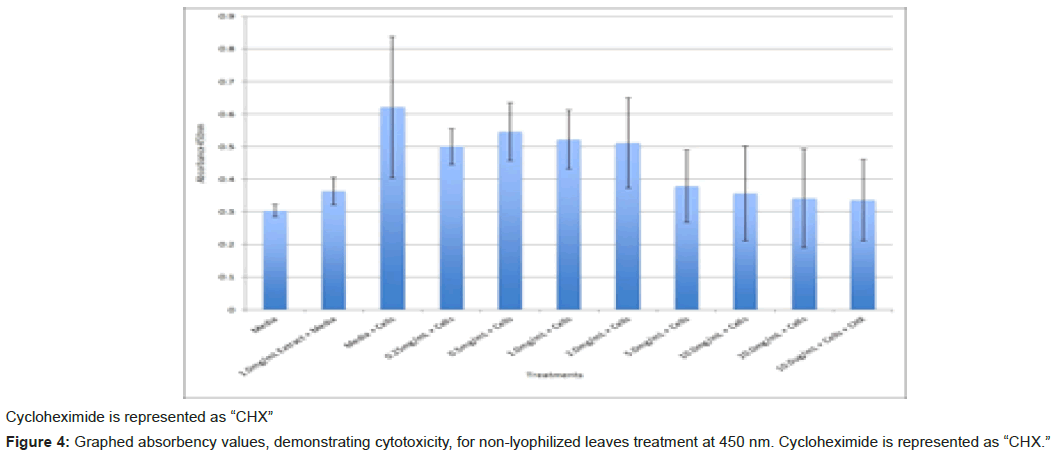
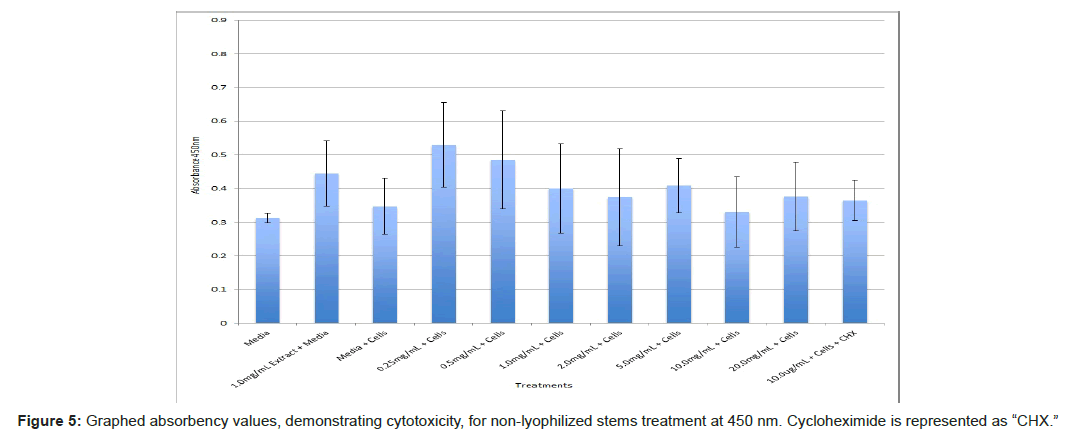
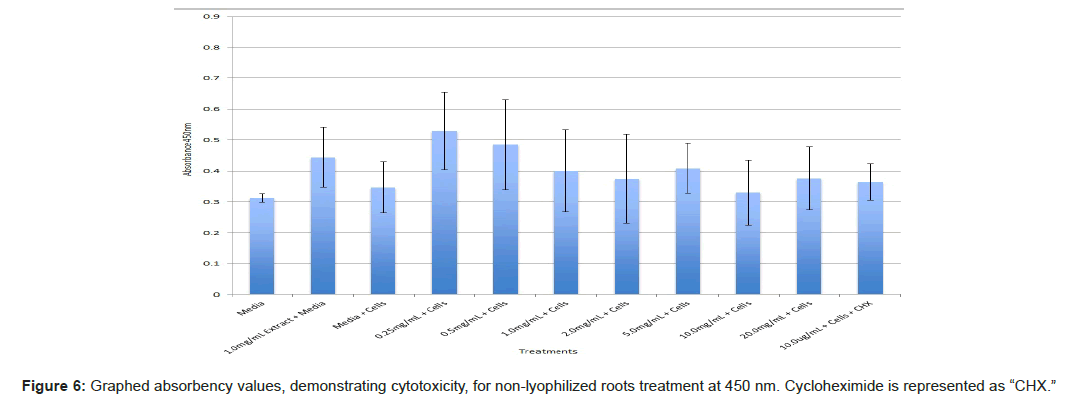
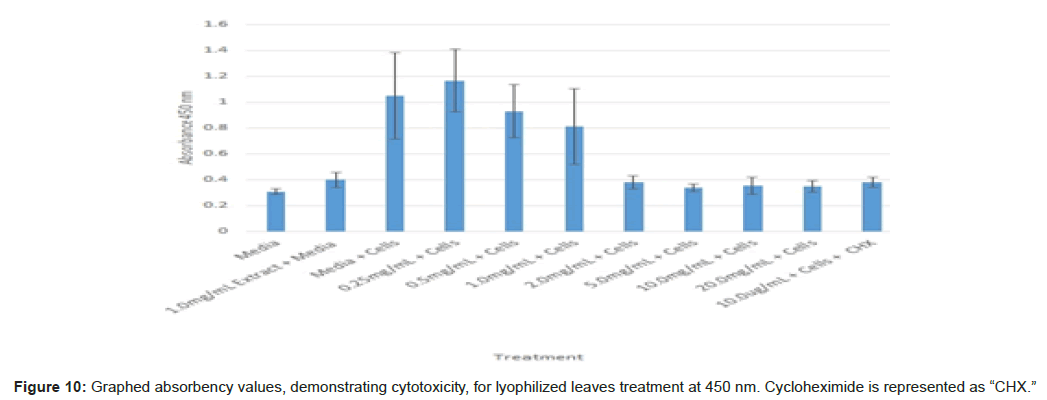
The study found that the non-lyophilized leaves treatment was statistically significant at 5.0 mg/mL through 20.0 mg/mL when considering the absorbency value for the 0.25 mg/mL treatment. Only the 0.25 mg/mL, 0.5 mg/mL, and 20.0 mg/mL were statistically significant compared to the media and cell control absorbency value (Figure 1). The p-values for the non-lyophilized stem treatments were considered statistically significant at concentrations 0.5 mg/mL and 5.0 mg/mL through 20.0 mg/mL in comparison to the 0.25 mg/mL value. Only the p-values from 5.0 mg/mL to 20.0 mg/mL were statistically significant to the media and cell absorbency value (Figure 2). As for the non-lyophilized root treatments, p-values from 1.0 mg/mL through 20.0 mg/mL were statistically significant in relation to the 0.25 mg/mL p-value, and p-values found at 0.25 mg/mL, 0.5 mg/mL, 1.0 mg/mL, 5.0 mg/mL, and 20.0 mg/mL concentrations were found to be significant in relation to the media and cell control (Figure 3). For all three nonlyophilized treatments, the absorbency values, collected at 450 nm, never exceeded above 0.7 for all the experimental concentrations and the controls (Figures 4-7).
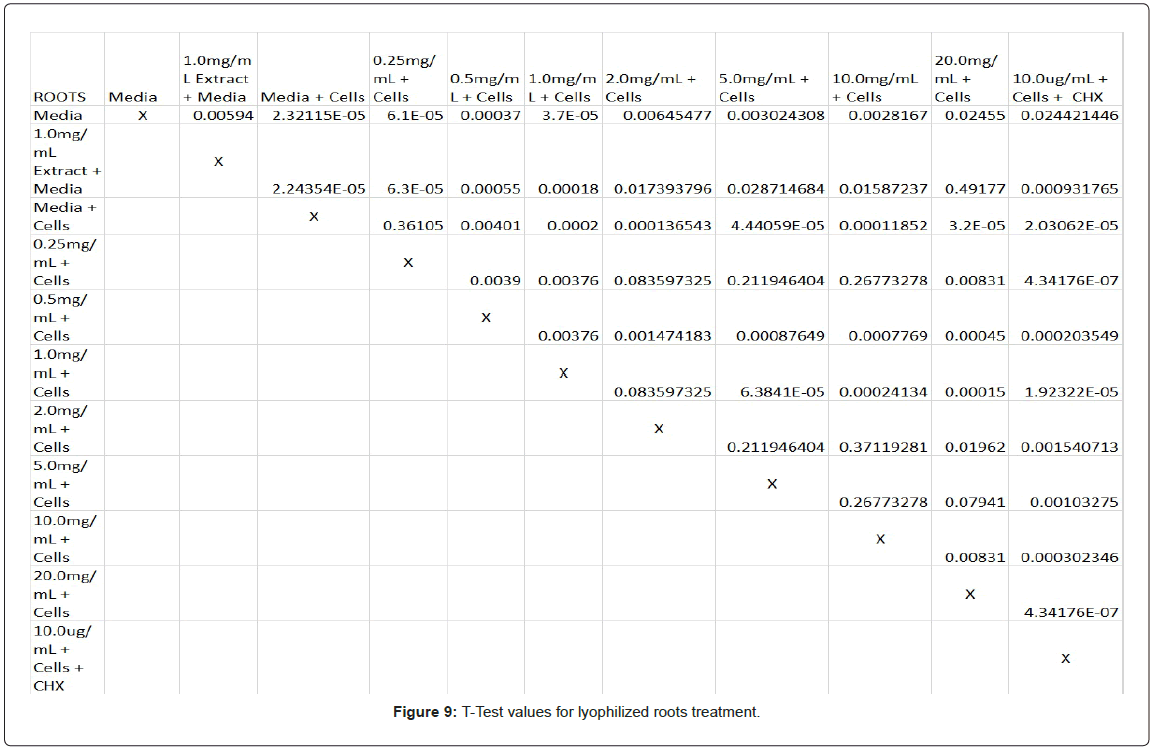
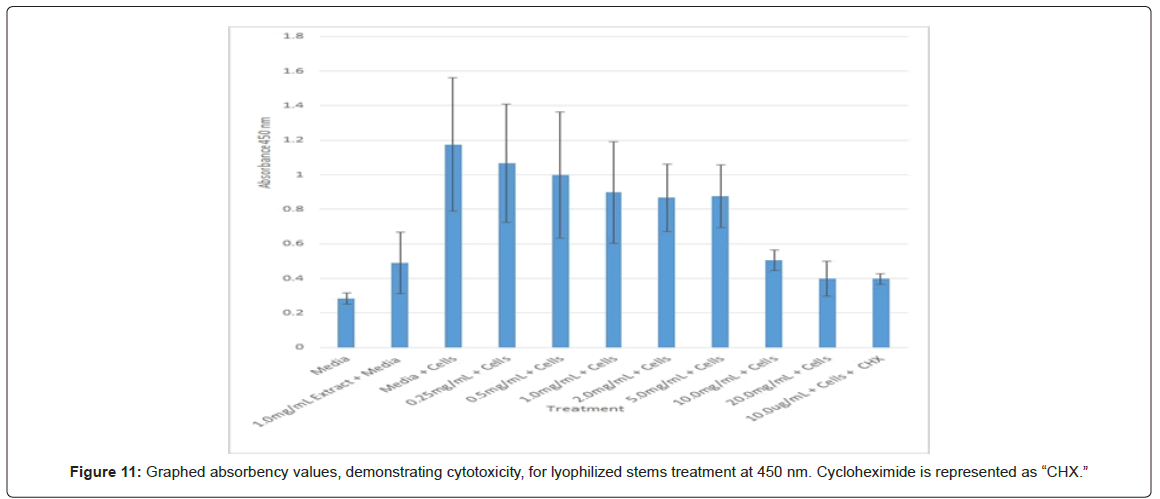
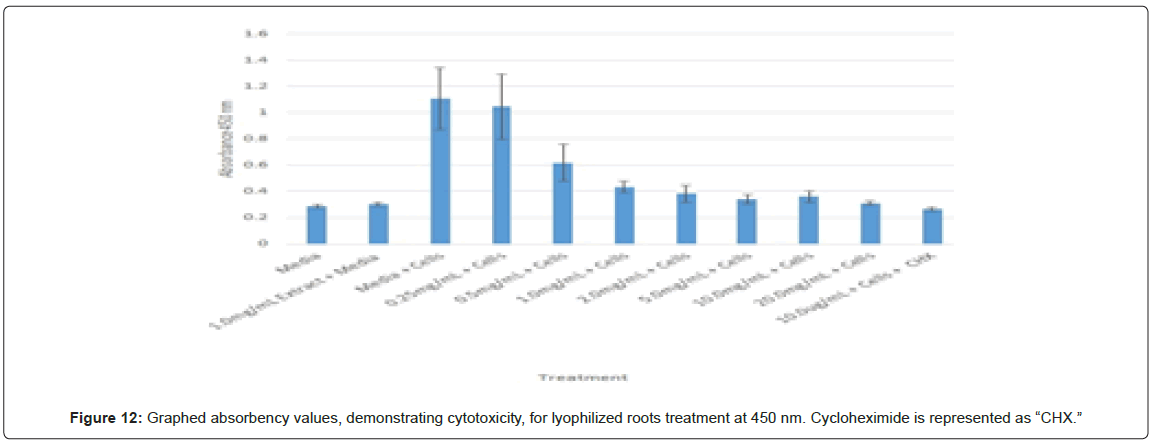
Statistical analysis was conducted for the lyophilized extractions as well. The study found that the p-values for the leaves treatment from 2.0 mg/mL to 20.0 mg/mL were statistically significant compared to the 0.25 mg/mL treatment and the media and cells control (Figure 8). In contrast, only the 10.0 mg/mL and 20.0 mg/mL p-values for the lyophilized stem treatments were statistically significant when juxtaposed to 0.25 mg/mL and the media and cells control (Figure 9). In comparison to the 0.25 mg/mL p-value, 0.5 mg/mL, 1.0 mg/mL, and 20.0 mg/mL were found to be statistically significant in the lyophilized root extractions, as well as p-values from 0.5 mg/mL through 20.0 mg/ mL, when assessed with the media and cells control (Figure 10). Many of the absorbency readings taken at 450 nm read approximately at 0.4, but a few readings, particularly those found from the lyophilized stems, read between 0.8 and 1.2 (Figures 9-12).
Discussion
The non-lyophilized extractions exhibited lower absorbency readings at 450 nm than did the lyophilized extractions. A possible explanation for this occurrence could be that there were larger traces of methanol in the non-lyophilized administered extractions than that of the lyophilized. This also could account for the “plateau-like” appearance of the non-lyophilized leaves treatment, as seen from concentrations 0.25 mg/mL to 2.0 mg/mL (Figure 4). Various concentrations of methanol traces could also explain the undulation in the non-lyophilized root extraction concentrations (Figure 11). Otherwise, there is a general trend amongst the data (both non-lyophilized and lyophilized) where a decrease in absorbency values occur. This may suggest a steady increase in cell cytotoxicity with increasing extraction concentrations. This trend, however, is not as clearly visible in the non-lyophilized treatments as it is in the lyophilized. In fact, at some concentrations the extractions aided cell growth, as exhibited by the 0.25 mg/mL concentration in the non-lyophilized and lyophilized leaves and the non-lyophilized roots when compared to the media and cell control absorbencies. Only the non-lyophilized stems and lyophilized roots and stems demonstrated no additional cell growth at the 0.25 mg/mL concentration (Figures 2,11,12). Overall, due to the additional traces of methanol that may account for the lower non-lyophilized absorbency values, the lyophilized extractions may be a truer indication of Amorpha fruticosa’s efficiency in regards to its cytotoxicity on PC-12 cells. When solely analyzing the lyophilized extraction treatments, the study suggests that the roots were the most effective, and the stems, the least effective against PC-12 cells. This finding is supported both through statistical T-test values and visible representation in graphical form (Figure 12).
The findings of this study conclude that the lyophilized root extractions from Amorpha fruticosa were the most effective when measuring its cytotoxicity on PC-12 adrenal neural cells and may aid advancements in finding alternative means of treating pheochromocytomas. However, it is recommended for future study that root extractions be further examined for antioxidant properties to explore a potential correlation between polyphenol and flavonoid content and the root’s cytotoxicity against carcinogenic cell activity. Likewise, this study suggests using primary cultured adrenal medulla cells from Rattus norvegicus to test plant extractions on a noncarcinogenic cell line. It should be noted that healthy adrenergic rat cells are difficult to culture and obtain commercially, but successful culturing would provide a solid testing model for prospective experimentation.
References
- Non-native invasive freshwater plants (2012-2014). Department of Ecology State of Washington.
- Lady Bird Johnson Wildflower Center. Amorpha fruticosa (2012). Plant Database.
- Ivanescu B, Lungu C, Spac A, Tuchilus C (2014) Essential oils from Amorpha fruticosa L. fruits – chemical characterization and antimicrobial activity. Analele Stiintifice Ale Universitatii Alexandru Ioan Cuza Din Iasi. Sectiunea II A, Biologie Vegetala 60: 33-39.
- Xueling Qu, Diao Yunpeng, Zhang Zhen, Wang Shouyu, Jia Yujie (2013) “Evaluation of anti-bacterial and wound healing activity of the fruits of Amorpha fruticosa L. Afr J Tradit Complement Altern Med 10: 458-468.
- HealthWatch EÂ Zh, Â Zheleva-Dimitrova D (2013) Antioxidant and acetylcholinesterase inhibition properties of Amorpha fruticosa L. and Phytolacca americana L. Pharmacognosy Magazine 9: 109-113.
- Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NV, Dahia PL, et al. (2004) Malignant pheochromocytoma: current status and initiatives for future progress. Society for Endocri11: 23-436.
- Chrisoulidou A, Kaltsas G, Ilias I Grossman (2007) The diagnosis and management of malignant phaeochromoctyoma and paraganglioma. Society for Endocri p: 569-585.
- Adler JT, Meyer-Rochow G, Chen H, Benn DE, Robinson BG, et al. (2008) Pheochromocytoma: current approaches and future directions. The Oncologist 13: 779-793.
- Bravo EL, Tagle R (2003) Pheochromocytoma: State-of-the-art and future prospects. Endocrine Rev 24: 539-553.
- Darr R, Lenders JWM, Hofbauer LC, Naumann B, Bornstein SR, et al. (2012) Pheochromocytoma update on disease management. Sage j 3: 39.
- American Type Culture Collection (2016) ATCC animal cell culture guide: Tips and techniques for continuous cell lines. Manassas (VA): ATCC p: 39.
- Promega (2012) Technical bulletin: CellTiter 96 ® AQueous One Solutioin Cell Proliferation Assay. Madison (Wisconsin): Promega.
Citation: Daugherty MK, Abramovitch D, Ivankovic DS, Weinbrenner DR (2016) Cytotoxic Effects of Amorpha Fruticosa Leaf, Stem, and Root Extractions on PC-12 Adrenal Neural Cells From Male Rattus Norvegicus. Adv Cancer Prev 2: 120. DOI: 10.4172/2472-0429.1000120
Copyright: © 2016 Daugherty MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4124
- [From(publication date): 0-2017 - Jul 27, 2025]
- Breakdown by view type
- HTML page views: 3205
- PDF downloads: 919