Research Article Open Access
Detection of Chikungunya virus (CHIKV) in urine of infected mice: a Potential Non-invasive Diagnostic Tool for CHIKV
| Jones PH1 and Okeoma CM1,2* | |
| 1Department of Microbiology, Carver College of Medicine, University of Iowa, Iowa City, IA, USA | |
| 2Interdisciplinary Graduate program in Molecular and Cellular Biology (MCB), University of Iowa, Iowa City, IA, USA | |
| Corresponding Author : | Chioma M Okeoma Department of Microbiology Carver College of Medicine University of Iowa, Iowa City, USA Tel: 319-335-7906 E-mail: chioma_okeoma@uiowa.edu |
| Received: June 03, 2015 Accepted: August 01, 2015 Published: August 08, 2015 | |
| Citation: Jones PH and Okeoma CM (2015) Detection of Chikungunya virus (CHIKV) in urine of infected mice: a Potential Non-invasive Diagnostic Tool for CHIKV. J Infect Dis Ther 3:226. doi:10.4172/2332-0877.1000226 | |
| Copyright: © 2015 Jones et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Background: The reemergence of Chikungunya virus (CHIKV) infection and the spread of the virus throughout the world call for a fast, reliable, and improved laboratory diagnostic procedures. The aim of this study was to determine if CHIKV genome is present in other body fluids besides plasma as a tool for developing non-invasive diagnostic method.
Methods: This study utilized a sensitive and less invasive assay that combines the specificity of immunocomplexing and the sensitivity of real-time PCR to detect CHIKV in urine and plasma of infected mice.
Results: CHIKV RNA was detected by immuno-capture-quantitative reverse transcriptase PCR (IC-qPCR) in urine and plasma of infected mice. Viral RNA was detected in urine up to day 30, long after viral clearance in plasma. Furthermore, infectious CHIKV was detected in urine by cell-based immunofluorescence and 50% tissue culture infective dose (TCID50) assays, suggesting the presence of infectious virus in urine.
Conclusion: We demonstrate a potential non-invasive diagnostic approach that could be adapted to screen forCHIKV infection in viremic and aviremic patients using urine.
With the rapid and continued spread of CHIKV outside and within the US, it is imperative to understand the pathogenesis of CHIKV and to develop a reliable non-invasive method of detection and/or diagnosis of CHIKV. Infection with CHIKV results in a spectrum of clinical symptoms, such as fever, encephalitis, neuropathy, and myelopathy [1-3]. Urine retention and paraparesis have also been associated with CHIKV infection [3]. Compared to healthy controls, CHIKV-infected patients were shown to have high levels of proline, hydroxyproline, and mucopolysaccharides in their urine [4].
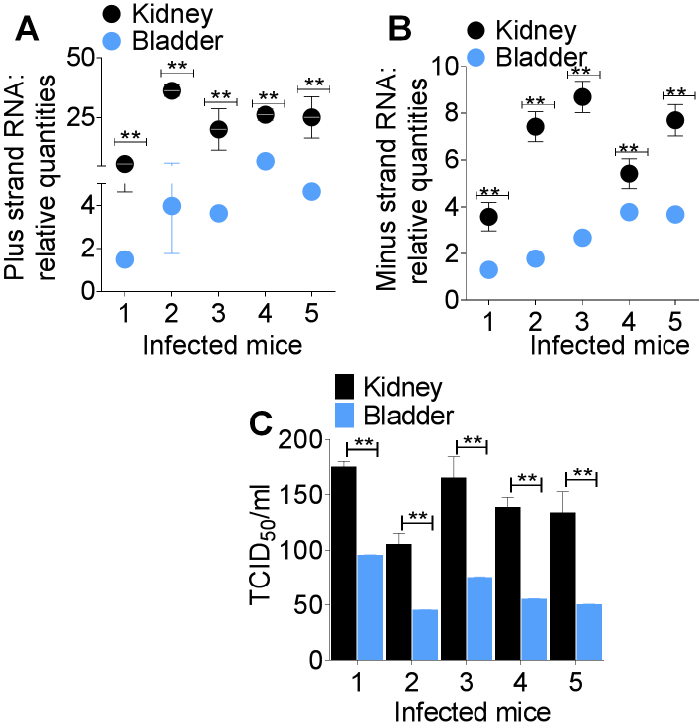
Most but not all sites of CHIKV replication and CHIKV-associated disease have been identified [5-10]. Infection with CHIKV is presumed to cause encephalitis, myelopathy and neuropathy and patients infected with CHIKV present with urinary symptoms [1-3]. However, it is unknown whether the urinary tract organs and tissues such as kidney and bladder are infected with CHIKV. This current research was conducted to assess whether CHIKV infects urinary tract organs and tissues and if CHIKV could be detected in the urine of infected hosts.
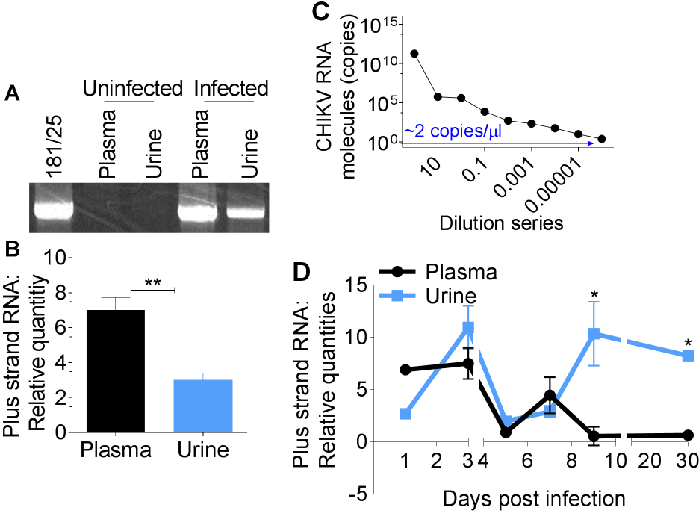
Immuno-capture of CHIKV particles shed into urine samples of infected mice was performed with anti-CHIKV polyclonal antibody followed by PCR amplification of CHIKV E1 sequences. PCR amplicons were separated by agarose gel electrophoresis and visualized with ethidium bromide as previously described [15,16].
Results show that CHIKV genome is present in both plasma and urine of infected mice (Figure 2A). Comparative analysis of CHIKV RNA in plasma and urine by quantitative PCR reveals a significant difference between plasma and urine CHIKV RNA content (Figure 2B).
However, plasma samples were positive for CHIKV on days 1 to 7. By day 9, plasma viral RNA has diminished below day 1 level, while urine viral RNA peaked (Figure 2D), suggesting that viral shedding into urine may be occurring.
Although declining, viral load in urine remained high up to day 30 post infection. These results reveal that each sample type could be of use in detecting CHIKV. However, urine sample has more value as CHIKV could be detected in urine long after the virus is cleared from the blood. Therefore, detection and quantitation of urine-associated viral load by IC-qPCR may provide a reliable and non-invasive method of CHIKV detection.
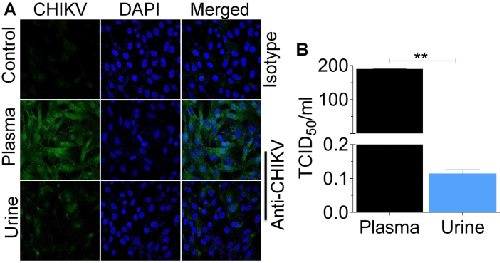
Alternatively, serial dilutions of infectious fluids were inoculated onto replicate Vero cells using a previously published protocol [6]. Cells were examined for cytopathicity to quantify virus titer using endpoint dilution assay [6], and results were expressed as TCID50. About 2 × 102 CHIKV particles/ml was detected in Vero cells inoculated with plasma compared to 1 × 10-1 particles/ml present in urine-inoculated Vero cells (Figure 3B). These results indicate that while comparable levels of CHIKV genome may be present in both urine and plasma, less infectious particles reside in urine.
In this study, we reveal that CHIKV infects the bladder and kidney of the host and that CHIKV RNA is present in urine of infected mice. Interestingly, infectious CHIKV was also detected in the bladder and kidney, as well as in urine samples. However, the titre of urine-associated infectious virus was significantly lower compared to plasma-associated virus from the same mice. It is not surprising that the titre of infectious CHIKV in urine is extremely low since other mosquito borne viruses such as West Nile Virus [17] and Dengue virus [18] have detectable viral genome in the absence of infectious virus in the urine of infected patients.
The occurrence of CHIKV minus strand RNA and infectious virus in urine samples is indicative of low but active viral replication in the urinary tract. Replication of CHIKV in the kidney and bladder could be the pathological mechanism for the presence of CHIKV RNA in urine. Presence of CHIKV RNA in urine beyond 7 days meant that CHIKV diagnosis could still be made even during the aviremic stage of infection.
References
- Chandak NH, Kashyap RS, Kabra D, Karandikar P, Saha SS, et al. (2009) Neurological complications of Chikungunya virus infection. Neurol India 57: 177-180.
- Rampal, Sharda M, Meena H (2007) Neurological complications in Chikungunya fever. J Assoc Physicians India 55: 765-769.
- Baishya R, Jain V, Ganpule A, Muthu V, Sabnis RB, et al. (2010) Urological manifestations of Chikungunya fever: A single centre experience. Urol Ann 2: 110-113.
- Lokireddy S, Vemula S, Vadde R (2008) Connective tissue metabolism in chikungunya patients. Virol J 5: 31.
- Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE Jr, et al. (1986) Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine 4: 157-162.
- Mahauad-Fernandez WD, Jones PH, Okeoma CM (2014) Critical role for bone marrow stromal antigen 2 in acute Chikungunya virus infection. J Gen Virol 95: 2450-2461.
- Jones PH, Maric M, Madison MN, Maury W, Roller RJ, et al. (2013) BST-2/tetherin-mediated restriction of chikungunya (CHIKV) VLP budding is counteracted by CHIKV non-structural protein 1 (nsP1). Virology 438: 37-49.
- Jones PH, Mehta HV, Maric M, Roller RJ, Okeoma CM (2012) Bone marrow stromal cell antigen 2 (BST-2) restricts mouse mammary tumor virus (MMTV) replication in vivo. Retrovirology 9: 10.
- Jones PH, Okeoma CM (2013) Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated BST-2/tetherin regulation. Cell Signal 25: 2752-2761.
- Mehta HV, Jones PH, Weiss JP, Okeoma CM (2012) IFN-α and lipopolysaccharide upregulate APOBEC3 mRNA through different signaling pathways. J Immunol 189: 4088-4103.
- Jones PH, Mahauad-Fernandez WD, Madison MN, Okeoma CM (2013) BST-2/tetherin is overexpressed in mammary gland and tumor tissues in MMTV-induced mammary cancer. Virology 444: 124-139.
- Madison MN, Roller RJ2, Okeoma CM3,4 (2014) Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 11: 102.
- Mahauad-Fernandez WD, DeMali KA, Olivier AK, Okeoma CM (2014) Bone marrow stromal antigen 2 expressed in cancer cells promotes mammary tumor growth and metastasis. Breast cancer res 16:493.
- Pongsiri P, Praianantathavorn K, Theamboonlers A, Payungporn S, Poovorawan Y (2012) Multiplex real-time RT-PCR for detecting chikungunya virus and dengue virus. Asian Pac J Trop Med 5: 342-346.
- Okeoma CM, Stowell KM, Williamson NB, Pomroy WE (2005) Neosporacaninum: quantification of DNA in the blood of naturally infected aborted and pregnant cows using real-time PCR. ExpParasitol 110: 48-55.
- Okeoma CM, Williamson NB, Pomroy WE, Stowell KM, Gillespie L (2004) The use of PCR to detect Neosporacaninum DNA in the blood of naturally infected cows. Vet Parasitol 122: 307-315.
- Tonry JH, Brown CB, Cropp CB, Co JK, Bennett SN, et al. (2005) West Nile virus detection in urine. Emerg Infect Dis 11: 1294-1296.
- Hirayama T, Mizuno Y, Takeshita N, Kotaki A, Tajima S, et al. (2012) Detection of dengue virus genome in urine by real-time reverse transcriptase PCR: a laboratory diagnostic method useful after disappearance of the genome in serum. J ClinMicrobiol. 50:2047-2052.
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 16077
- [From(publication date):
August-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11243
- PDF downloads : 4834
