Doing the Food Allergy Diagnosis
Published Date: 09-Jan-2017 DOI: 10.4172/2161-119X.1000284
Abstract
Most people can eat a variety of foods without problems. However, in a small percentage of the population, certain foods can cause adverse reactions, from which the origin should be investigated: Perhaps food poisoning, a psychological aversion to a food, an intolerance to an ingredient in a meal or a true food allergy. Diagnosis of food allergy requires a combination of a detailed medical history, laboratory data and in many cases, an oral food challenge, which is confirmatory of either tolerance or an adverse food reaction (allergy or non-allergic). The cornerstone of food allergy management is the elimination of the allergen involved, however the allergen elimination process could predispose patients, especially children to inadequate diets and cause nutritional deficiencies thus eliminating diets should be done in the most specific way possible.
Keywords: Food allergy, Allergy; Patient; Children
253857Definition
Adverse reactions to food may be due to intolerance, toxicity or immunological mechanisms. Food allergy is defined as an adverse immune response that occurs reproducibly on exposure to a given food. This is different from food intolerance, which refers to a nonimmunological adverse reaction to food [1-4]. Food allergy is the result of an immune response, which may be mediated by IgE (type I) and non-IgE mediated (type II, III, IV or mixed hypersensitivity); and which occurs by sensitization to food allergens [5-8].
Food Allergy Epidemiology
Food allergy is common, in developed countries, the prevalence of food allergy has increased over the last two or three decades, with no universal theory to explain this phenomenon and no interventions appear to curtail a similar increase in developing countries. The prevalence of food allergy seems to vary greatly with age. Currently estimated to affect between 2 and 8% of the world's population. The prevalence of food allergy is highest in infants and toddlers; affecting 6% to 8% of children and can be both life-impacting and lifethreatening [1-3,8,9].
2.5% of infants suffer from milk allergy and up to 10% of 1-yearolds suffer from allergies to food, including cow’s milk, egg, nuts, soya, wheat and fish/shellfish [2,10].
Some children may “outgrow” their symptoms of allergy, but for others, the food allergies may be lifelong. Because reported cases increase each year, food allergy should be considered a public health problem. In fact, some authors have described it as "the second wave" of the allergic epidemic [8].
Cross-reactivity between different species of foods in allergic patients is also common. Dietary avoidance is currently the most essential component in the management of food allergy. However, it may not be beneficial for children during periods of active growth, as it can limit their intake of nutrients from different foods. Conflicting information regarding cross-reactivity often leads to an inconsistent understanding of food allergies among physicians and other health care givers which lead to disparate recommendations and confusion among patients and parents [1]. Because possible adverse outcomes such as anaphylaxis can be life-threatening, food allergy has always been a great concern for parents, caretakers, health care practitioners, the food industry and government regulators [1].
This review focuses on the various methodologies for the diagnosis of Food Allergy and discusses the pros and cons of the methodology used into clinical practice.
Diagnosis of Food Allergy
The diagnosis of food allergy is a challenge since mild cases are often ignored or misinterpreted because the symptoms are not specific or can be related to other diseases. In young children, the diagnosis is more complicated because parents should be observed to observe the symptoms [11,12]. Otherwise food allergy only when the child unexpectedly reacts on exposure, with potentially severe consequences. On the other hand, the child may be unnecessarily restricted from consuming foods considered ‘high-risk [2].
When to suspect
The clinical presentation of food allergy includes a wide spectrum of signs and symptoms ranging from cutaneous manifestations (urticaria, angioedema, atopic dermatitis), gastrointestinal (vomiting, colic, abdominal pain, diarrhea or constipation), respiratory (rhinorrhea, wheezing, dyspnea) or even cardiovascular collapse. They are classified into immediate or late reactions. The former refer to allergic reactions occurring within the first two hours after ingestion of the food and the latter refer to reactions occurring after two hours of intake. However, this differentiation is based only on the onset of symptoms and does not necessarily describe a difference in mechanism of injury [4,13-16].
The presence of eczema in infancy is an important risk factor for the development of IgE-mediated food allergy. Hill and colleagues showed that increasing severity of eczema during infancy and earlier age of onset are both risk factors for development of allergy. Mailhol and colleagues demonstrated that children who are less than 2 years old and who have early-onset or severe eczema are at higher risk of food allergy and may be candidates for food allergy evaluation [2].
The Japanese Society of Allergy and Clinical Immunology classifies food allergy into 4 representative clinical patterns: which are concentrated in Table 1.
| Clinical presentation | Onset´s age | Frequently associated foods | Acquisicition of tolerance | Possibility of anaphylactic shock | Probable immunological mechanism |
|---|---|---|---|---|---|
| 1. Neonatal and infant gastrointestinal allergy | Neonatal or nursing | Cow´s milk | Frequent | +/- | Non IgE |
| 2. Atopic dermatitits associated to food allergy | Infants | Egg, cow´s milk, wheat, soy, etc. | Frequent | + | IgE |
| 3. Food allergy immediate onset (urticaria, anaphylaxis) | Any age | Infants and young children: egg, cow's milk, wheat, buckwheat, fish, peanut Schoolchildren and adults: shrimp, fish, wheat, fruit, buckwheat, peanut | Frequent: egg, cow´s milk, wheat, soy. Less frequent to other foods. | ++ | IgE |
| 4. specific forms | |||||
| Food-induced exercise-dependent anaphylaxis | School children-adults | Wheat, shrimp, squids, etc | Less probably | +++ | IgE |
| Oral allergy syndrome (OAS) | Any age | Fruits, vegetables, etc | Less probably | +/- | IgE |
Table 1: Clinical patterns of food allergy.
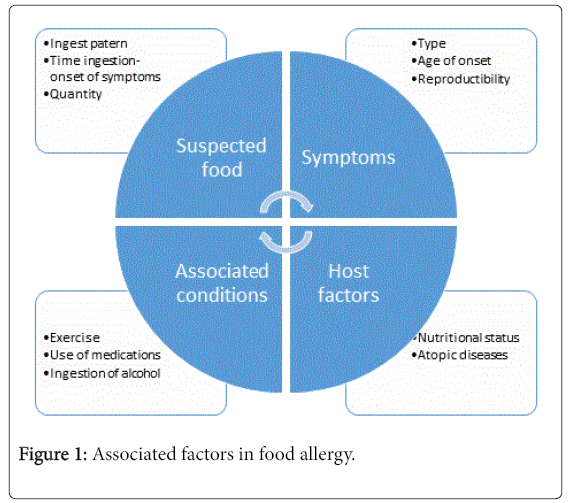
The clinical presentation of a food-induced allergic reaction depends on a number of variables (Figure 1). The information collected may include whether the participant had a reaction due to food ingestion, whether this reaction always occurred after eating the same food, what type of food was ingested, the amount (dose) ingested, the age of onset of symptoms, the type and severity of reaction and their reproducibility, the last moment symptoms occurred, details of symptoms that occurred, other associated conditions (exercise, medication intake, intake alcoholic beverages, etc.), whether the food was ingested alone or in combination with other foods that could delay its absorption and, importantly, host factors associated with the disease such as nutritional status or the presence of other atopic diseases and the treatment regimen. The use of feeding reminders (gathering information on the patient's eating habits for at least the past two weeks) is very useful for this purpose. The way in which the questions are asked is crucial to obtain the appropriate information. Questions can be misleading, depending on the choice of words or how they were phrased. For infants or young children who cannot express or communicate well enough, the parents or guardians would have to recall the allergic incident and help them answer the questionnaire. On the other hand, those with higher education or an aware of their food allergy might be more willing to participate. However, in systematic reviews, the positive predictive value of a careful medical history barely reaches 50% [1,8,10,17-19].
Several studies have now highlighted not only the gastrointestinal symptoms commonly seen in children with non IgE mediated food allergy but also the high prevalence of feeding difficulties, such as vomiting, constipation and abdominal discomfort and the extraintestinal manifestations, should be considered alarm data to investigate food allergy [2].
Patients with non-IgE-mediated AA have, predominantly, gastrointestinal symptoms, although it is not exclusive. The pathophysiology of non-IgE-mediated food allergy remains poorly described; however, there have been some advances in recognizing the overlap between IgE-mediated and non-IgE-mediated allergy; in particular, the more recent National Institute of Allergy and Infectious Diseases (NIAID) guidelines now include a mixed IgE-mediated and non-IgE-mediated allergy and eosinophilic esophagitis (EoE) and atopic eczema fall within this category [2].
Over the last decade, various non-IgE-mediated allergic conditions —in particular, EoE and food protein-induced enterocolitis syndrome (FPIES)—have received significant attention. Eosinophilic gastrointestinal disorders are classified according to the site of the inflammation and the depth and severity of the inflammation, which influences the presenting symptoms. The spectrum of pathologies (often with overlapping symptoms) includes EoE (the most common of these conditions), eosinophilic gastroenteritis and eosinophilic gastroen-terocolitis [2,20-22].
Recent guidelines on the diagnosis of EoE recommend that at least two to four biopsies be taken from both the proximal and distal esophagus to maximize diagnostic sensitivity. In symptomatic children with positive histological findings for EoE, a trial of proton pump inhibitors is recommended for 8 weeks in order to diagnose responsive esophageal eosinophilia. Studies have shown that up to 40% of children with esophageal eosinophilia have responded histologically to proton pump inhibitor therapy [2].
FPIES is another non-IgE-mediated disorder that occurs in young infants and the prevalence has been estimated at 0.36% by a retrospective study in the UK. The most commonly reported FPIES food is cow’s milk; however, it is also frequently reported with grains (that is, rice) and fish. FPIES presents with profuse vomiting or diarrhea or both; additional symptoms may include pallor, lethargy and neutrophilia, usually occurring within 2 to 4 hours after ingestion of the food. This condition is frequently misdiagnosed; there may be long delays in reaching the correct diagnosis and infants often have to undergo multiple unnecessary investigations. Fortunately, the prognosis is good and most children outgrow FPIES by 3 years of age [2].
Non-IgE-mediated food allergy is also associated with extraintestinal manifestations such as joint pain, fatigue, night sweats and headaches, although much more work is needed to understand the underlying pathophysiological mechanisms. In addition, these children often suffer from frequent, prolonged respiratory infections and may have associated minor immune deficiencies [2].
Foods Involved
Almost any food is capable of causing a set of allergic symptoms. Once a patient is diagnosed with a food allergy, it becomes important to identify the allergen(s) that cause the disorder and determine if it is mediated by IgE or not [3]. In young children, food allergy is usually acquired via the gastrointestinal tract and directed toward egg and milk mostly (type 1 or primary food allergy). Adolescent and adult patients, however, mainly acquire food allergy via primary sensitization to inhalant allergens on the basis of cross-reactivity between proteins in inhalant sources and in food. This type of food allergy is frequently mediated by sensitization to broadly represented allergens, or so-called panallergens (type 2) [3,23-25]. In this group of reactions, the presence of IgE antibodies cannot be demonstrated by conventional methods. The onset of symptoms is slower than in type I reactions, surfacing between hours to weeks after the intake of the allergen, requiring sometimes repeated exposure to the allergen to unchain symptoms [26].
Diagnostic tests for IgE-mediated food allergy
Sensitization is defined as the presence of a specific IgE response that occurs upon exposure of the immune system to an allergen. Both skin tests and in vitro tests use mast cell reactivity as a form of reading to detect the presence of IgE antibodies specific for an antigen. Although skin tests also provide information on the potency of specific IgE antibodies to trigger a biological effect (eg induction of the release of mast cell mediators), both tests assess sensitization. Clinical allergy is defined as the appearance of symptoms after food intake and this cannot be predicted based on sensitization as there may be sensitization with or without clinical allergy [8,10,27].
The demonstration of specific IgE antibodies supports the diagnosis of IgE-mediated food allergy, but with the exception of challenge tests with the offending food, no in vivo or in vitro test is able to provide a reliable prediction of the clinical reactivity of a patient [15] and the presence of specific IgE-like antibodies with or without clinical allergy may occur.
Determination of specific IgE
Skin prick tests
They are the first line to determine the presence of IgE antibodies specific for a food allergen [28]. The skin prick test (SPT), which utilizes commercial extracts, is usually performed using a skin prick test device on the back or the volar surface of the forearm. There is a wide variety of skin testing devices available worldwide. Devices that facilitate simultaneous application of multiple tests are also available. Standardized allergen extracts are used when available commercially, but most extracts are not standardized. Skin responses are measured in 15 or 20 min and compared with positive or negative controls consisting of 10 mg/ml of histamine or saline, respectively. Based on the wheal diameter, a measurement greater that is 3 or 5 mm greater than the wheal for the negative control is considered a positive skin reaction. In expert hands, the skin tests are performed easily, are safe. The choice of evidence should be based on a careful and detailed clinical history. They can be performed in patients of any age even though the skin reactivity is lower in small children and possibly in older adults. In both cases, it is possible to find patients in whom no specific IgE antibodies are detected in the blood but with positive skin tests. The use of good quality food extracts is highly recommended when available, however when there is a discrepancy between a suggestive medical history and negative skin tests (possibly because in commercial extracts there is a decrease in the concentration of minor or unstable allergens that may be relevant to the sensitization) it is recommended to perform prick to prick skin tests in which the skin is punctured with a lancet with which the fresh food has previously been punctured. Since the concentration of labile extracts is drastically reduced while stable allergens remain present in commercial extracts of plant-derived foods, it has been suggested that this observation be used as a measure to perform a differential diagnosis among patients sensitive to stable allergens (for example LTP, storage proteins, etc.) or labile (for example homologues of Bet v1, profilins). The main disadvantage of prick to prick tests is their low specificity resulting in a high percentage of false positive results, the inability to standardize the source of allergens and their high dependence on the availability of the fresh food in question. The reduced specificity is, among other reasons, an expression of cross-reactivity between pollen and other related foods and the only way to control the clinical relevance of positive skin tests is by performing controlled oral challenge tests [1].
Intradermal tests with food antigens should be avoided because false positives or anaphylactic reactions may occur [29].
In children with AD and food allergy to egg, milk, peanut and fish, skin tests have excellent sensitivity and negative predictive value (generally> 90%) but poor specificity and positive predictive value (50-85%) so that A negative skin test represents a good method to rule out an IgE-mediated food allergy while a positive result does not confirm the diagnosis [29,30].
Before taking skin tests the use of medications such as antihistamines or steroids should be discontinued because they influence the results. A positive skin test indicates the presence of specific IgE antibodies, more than 95% of patients with negative skin tests will not have symptoms of immediate food allergy. However, this result alone does not support the diagnosis of IgE-mediated food allergy especially in children whose skin is less reactive [29,33]. Studies are needed to define the size of the wheal that determines positive predictive value for different foods, ages and populations. The limitation of skin testing to foods is that the results may not correlate with a clinically significant allergy. SPT is also prone to examiner variability in the measurement and interpretation of the size of the reaction and to device variability [1,30-32,34].
Patch testing
A patch test in which a food antigen is applied to the skin is useful to predict non-immediate allergic reactions by IgE in the diagnosis of atopic dermatitis. They are difficult to interpret, when they do not have specialized training since they can present non-specific reactions. Some authors have reported that patch tests have poor reliability and do not increase the speed of the diagnostic process. However, there is no consensus [16,35,36].
In vitro tests: Allergen-specific IgE antibody determination
One of the major problems with using in vitro testing to study food allergies is that, while the methodology is the same in principle, there are several test standards for this procedure. These include the ImmunoCAP, the Hycor system and the Immunlite System from Siemens.
In vitro tests to measure specific IgE were developed beginning in 1974 with the well-known radioallergosorbent or RAST test. This was a proprietary test done developed by Pharmacia and although many allergists and lay people recognize and use the term RAST or CAPRAST, this test is no longer used because of the development of newer and better assays [1].
The presence of IgE-type antibody titers specific to a particular food, as a result of either direct sensitization or cross-reactivity, suggests sensitization to that particular antigen, but not necessarily to induce symptoms. The absence of clinical allergy in the presence of specific IgE antibodies can be caused by various variables including the absence of cofactors, extremely low levels of specific IgE, low affinity or inactive IgE, or a high threshold. However, for some antigens (egg, cow's milk and peanut) a probability curve can be made to indicate the correlation between specific IgE antibodies and the positive rates of immediate reactions in food challenge tests. In vitro tests may be more sensitive in infants and may be the method of choice in the case of extensive dermatitis or dermographism, or if the use of antihistamines cannot be discontinued [2,3,16].
Skin test results and serum specific IgE determinations are often used interchangeably in clinical practice, but there is little evidence of how these two diagnostic methods correlate in young children, so allergy testing should be done in Children with significant symptoms and not as a screening tool [3,37].
The different reference laboratories utilize different specific methodologies. When comparing studies, it is important to realize that the results may differ based on the test that is used. The level of specific IgE represents a likelihood of being allergic to that particular allergen but has no predictive value regarding the severity of a potential reaction. Even when the same methods are used to measure the serumspecific IgE, the positive predictive values of reacting to a particular food in a population is quite different [1].
Elimination diets
Elimination diets with and food challenges are required to establish the precise diagnosis of allergy, mediated or not by IgE [2-4].
A diagnostic elimination diet is to avoid suspected food based on a good medical history for no more than four weeks in the case of IgEmediated allergy and up to 6 weeks if IgE-mediated allergy is suspected, sufficient time to demonstrate An improvement in symptoms. Food should be carefully monitored and evaluated to avoid unnecessary food restrictions. If the elimination diet does not give clear results, it should be reassessed if co-factors could be involved. When a well-done elimination diet does not improve symptoms, the diagnosis of food allergy is unlikely. The phase of elimination must be followed by a progressive reintroduction of the eliminated food. When there is no risk of a serious reaction, it can be done at home. If symptoms are reported, they should be confirmed by an oral challenge at the hospital [4].
Food challenge
Three types of challenges may be performed in a food allergy study: open, single-blind, placebo-controlled, or double blind placebo controlled food challenge (DBPCFC). The open challenge is an unblinded feeding with a food in its natural form. It is used if the concern for participant bias is low and clinical symptoms are not expected to occur. It is usually performed in the office setting with a 1– 2-h observation period and it is aceptable for use in challenging young children in most cases. Because it is not blinded to the investigators, physicians and participants, the interpretation of the challenge can be subjective. However, it is still useful in eliminating potential food culprits when the clinical record or laboratory testing indicates that the food is unlikely to be causative.
Another approach is blinding or masking the challenge food with a vehicle by placing the food in an opaque titanium-dioxide-coated gelatin capsule or by mixing in another food to reduce bias. The placebo is usually a food that is similar to the challenge food in terms of taste, consistency, texture, appearance, smell and sensation within the mouth. In the single blind placebo controlled food challenge (SBPCFC), the patient is unaware of the challenge content, but the physician is. As long as the investigators remain careful not to inadvertently reveal to the patient what he or she will be receiving and be consistent when they hand out the food, this approach is fairly reliable. The last method is the double-blind, placebo-controlled food challenge, which is the gold standard for diagnosing. They are indicated to 1) confirm the diagnosis, 2) monitor the food allergy, or 3) to assess the occurrence of tolerance. There are guidelines that describe the procedure for the food challenge in detail in order to avoid severe reactions with doses calculated based on logarithmic increments and time intervals [13,38]. For many foods, such as milk, egg, peanut or walnuts, ranges vary from 3 mg to 3 g of protein. A food challenge is discontinued at the time of clinical reactions or when the last dose has been consumed even though no symptoms have appeared. To optimize safety, vital signs should be monitored closely and medical personnel should be trained to treat any possible allergic reaction, including anaphylaxis [1,3,39,40].
They are generally used in research studies, in chronic diseases such as atopic dermatitis, for patients who apparently have allergic reactions to multiple foods and when the patient's subjective perception could act as a distractor in the assessment of symptoms [4,35,41].
Patients from different geographic areas may not only be exposed to the same species of a particular food, but the method of preparation may be different which will affect the results of evaluation. Protein extracts used for analysis may vary from region to region which may affect the results of objective skin or serum testing and the cut-off of each food has to be determinate [1,3].
Determination of sensitization to a suspected food allergen includes the evaluation of cross-reactive cosensitizers and allergens from other foods or aeroallergens. To avoid the identification of food allergens that have no clinical relevance, only food allergens or aeroallergens that are related to clinical presentation, age, eating habits and geographical location of the patient should be investigated [3,4,13,35,42].
Other tests
Molecular diagnosis or component diagnosis
The diagnosis of food and other allergies has transitioned from the identification of allergen sources without knowledge of the molecules that cause the symptoms, to the precise identification of allergyinducing molecules. These processes are called “component-resolved allergy diagnosis” and “molecular allergy diagnosis”. Specific IgE antibodies are measured against individual allergenic food molecules with the potential to improve the specificity of serum tests and the specificity of the selected foods. This can be done through singleallergen or microarray measurement formats, which test a variety of simultaneously purified allergens [3,4,42].
Cross-reactivity increases the number of allergenic sources against which a subject displays allergic reactions. More cross-reacting allergens are detected with skin tests and/or in vitro tests than using the history of clinical symptoms to corresponding foods. Reports on specific food allergies linked with specific aero-allergens are inconsistent. This apparent inconsistency is not surprising, as today the majority of allergic patients are sensitized toward pollen or other inhalant allergens from more than one plant species and therefore, there is a plethora of possible cross-reactions. Moreover, geographic differences and different nutritional habits may also play an important role in this context, For example, the so-called Bet v1 family. Apart from allergens of the Bet v1 family, pollen-related food allergies can also be bases on profiling, proteins of the Bet v6 family or nsLTPs (in Southern European Countries) [43].
Basophil activation tests (BAT)
They have been used in the diagnosis of allergy to cow's milk, egg and peanut, as well as in the diagnosis of pollen-food syndromes. It has shown a high specificity and negative predictive value that the skin tests and the determination of specific IgE, without losing positivity and positive predictive value. However BAT requires a specialized laboratory and evidence of clinical studies is lacking [2,39].
Another area of promising research is the determination of IgE antibodies against synthetic linear peptides superimposed on food allergens. Studies have already been done for milk, peanut, egg and shrimp but more studies are needed [2-4].
Non-IgE mediated food allergy
The diagnosis of non-IgE-mediated food allergy is generally based on elimination diets, followed by re-introduction of the suspected foods, with the addition of intestinal biopsies, when needed. Recently, the lymphocyte stimulation test (LST) for k-casein was proposed as an alternative diagnostic test for intestinal cow’s milk allergy (ICMA) [2].
Unconventional tests
The determination of IgG and IgG4, bioresonance, kinesiology, iridology, hair analysis, cytotoxicity tests are not validated tests and therefore are not recommended for the diagnosis of food allergy [4,15].
Treatment
Treatment of the acute process
In the past, the cornerstone was avoidance of the allergen [4,16,35] and the emergency treatment of accidental exposures. In these cases, identifying patients at risk for anaphylaxis is important. These include: 1) previous anaphylaxis, 2) cofactors such as NSAIDs, exercise, infections, 3) mastocytosis. There is no evidence that antihistamines are effective in the treatment of severe symptoms and their "prophylactic" administration may mask the early symptoms of anaphylaxis and lead to delayed treatment [4,40].
Long-term treatment
One of the main objectives of clinical allergy care in a patient with AA should be to preserve quality of life by avoiding unnecessary dietary restrictions. To this end, patients should be educated to recognize the initial symptoms of an allergic reaction, learn to read labels and recognize high-risk food sources (particularly occult allergens). Patients at risk of severe reactions should learn to use selfadministered epinephrine and should have a written emergency plan [15].
To ensure that all nutrients present in the food or foods removed from the diet are covered by alternate sources, a plan should be established, preferably prepared by a nutritionist specializing in the subject. The risk factors for malnutrition in children with AA include delayed diagnosis, early onset of disease, multiple food allergy, active disease, persistent (subclinical) intestinal inflammation, elimination of many foods in the diet, The diet of foods with high nutritional value (milk, egg), poor adherence to dietary management (reluctance to expand the diet), extreme dietary self-limitation, association with atopic diseases (asthma, atopic dermatitis) or chronic diseases [4,16,44].
Oral immunotherapy may be useful in the development of tolerance, but is still in the experimental phase [45].
Omalizumab is a monoclonal antibody, licensed in the treatment of asthma. Its usefulness in food allergy is being investigated where it is suggested that it could accelerate the acquisition of tolerance. In non- IgE-mediated forms, anti-IL-5 antibodies (mepolizumab and reslizumab) have been used, but studies are still lacking [4,9].
When to send to a specialist
The reference should be considered when: 1) the child has a growth arrest associated with gastrointestinal symptoms, 2) there is no response to a single food elimination diet, 3) systemic reactions have occurred, 4) IgE-mediated food allergy associated with asthma, 4) Food allergy associated with severe atopic dermatitis, 5) multiple food allergy suspicion, 6) persistent suspicion of food allergy by parents (especially in young children or with unexplained symptoms) despite an unlikely clinical history, 7) history (1) accurate diagnosis (a challenge test is required), (2) providing instruction in diets, including elimination diets and dietary alternatives, especially when requested at day care, Kindergarten, school, etc. 3) provide instructions on the use of self-administered adrenaline for anaphylaxis [15,16,40].
Conclusion
In summary, the diagnosis of food allergy is based on strong clinical suspicion. A detailed medical history should be elaborated, where, if a particular food is suspected, the pattern of food intake should be established, as well as the presence of associated factors. If there have been no life-threatening reactions, it is recommended to perform skin tests as the first diagnostic study, since it is useful in cases of allergy mediated by IgE as in mixed forms, if these are positive, then a elimination diet Directed to the identified food and later confirmed with an oral challenge. If the skin tests are negative and no particular food has been identified, then an oligo-allergenic elimination diet should be performed. If life-threatening reactions have been previously reported, skin tests should be performed and if they are positive, specific serum IgE determination may be performed to determine whether oral challenge can be performed. In the case of negative cutaneous tests, a specialist-supervised food challenge will be performed.
The cornerstone of food allergy management is the elimination of the allergen involved, however the allergen elimination process could predispose patients, especially children to inadequate diets and cause nutritional deficiencies [33], thus eliminating diets Should be done in the most specific way possible. In the case of patients with cow's milk protein allergy (APLV), nutritional deficiencies with growth arrest, calcium and vitamin D deficiencies have been documented, which may also impact the absorption of micronutrients [33] and due to The similarities between food allergy and some symptoms of malnutrition, it becomes imperative to understand and evaluate the interaction between food allergy and nutrition in order to properly protect and identify food sources for particular subpopulations in economically disadvantaged countries and communities [46]. This is complicated but justifies the need for a detailed diagnosis in order to avoid the diets of elimination of multiple foods imposed by a bad diagnosis [30,47].
Knowing the clinical manifestations as well as the allergens involved and the triggers is crucial to establish strategies for proper diagnosis, prevention and treatment of food allergy. Educational programs for general practitioners, pediatricians and health personnel are required to improve awareness of this condition [48].
References
- Shu S, Chang C, Leung PSC (2014) Common methodologies in the evaluation of food allergy: Pitfalls and prospects of food allergy prevalence studies. Clin Rev Allergy Immunol 46: 198–210.
- Anagnostou K, Meyer R, Fox A, Shah N (2015) The rapidly changing world of food allergy in children. F1000Prime Rep 7: 35.
- Valenta R, Hochwallner H, Linhart B, Pahr S (2015) Food allergies: The basics. Gastroenterology 148: 1120-1131.
- Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, et al. (2014) EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 69: 1008-1025.
- Puc M (2003) Characterisation of pollen allergens. Ann Agric Environ Med 10: 143-149.
- Kurowski K, Boxer RW (2008) Food allergies: Detection and management. Am Fam Physician 77: 1678-1686.
- Cianferoni A, Spergel JM (2009) Food allergy: Review, classification and diagnosis. Allergol Int, 58: 457–466.
- Prescott S, Allen KJ (2011) Food allergy: Riding the second wave of the allergy epidemic. Pediatr Allergy Immunol 22: 155-160.
- Sicherer SH, Sampson HA (2014) Food allergy: Epidemiology, pathogenesis, diagnosis and treatment. J Allergy Clin Immunol 133: 291–307.
- Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JK, et al. (2013) A global survey of changing patterns of food allergy burden in children. World Allergy Organ J 6: 21.
- Chapman JA, Bernstein IL, Lee RE, Oppenheimer J, Editors A, et al. (2006) Food allergy: A practice parameter. Annals of Allergy, Asthma & Immunology 96: 1–68.
- Urisu A, Ebisawa M, Mukoyama T, Morikawa A, Kondo N (2011) Japanese guideline for food allergy. Allergology International 60: 221–236.
- Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, et al. (2012) ICON: food allergy. J Allergy Clin Immunol 129: 906-920.
- Longo G, Berti I, Burks AW, Krauss B, Barbi E (2013) IgE-mediated food allergy in children. Lancet 382: 1656-1664.
- Sherwood E, Boyd A (2012) Food allergy in children and young people. NICE Clinical Guideline.
- Urisu A, Ebisawa M, Mukoyama T, Morikawa A, Kondo N, et al. (2014) Japanese guideline for food allergy. 2014. Allergology International 60: 399–419.
- Pawankar R, Canonica GW, Holgate ST, Lockey RF (2011) White Book on Allergy. Milwaukee, Wisconsin: World allergy Organization.
- Demoly P, Hellings P, Muraro A, Papadopoulos NG, van Ree R (2014) Global atlas of global atlas of allergy. In: Akdis CA, Agache I (eds.) Global atlas of allergy. European Academy of allergy and clinical Immunology.
- eSicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, et al. (2014) The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol 133: 492-499.
- Dellon ES (2011) Approach to diagnosis of eosinophilic esophagitis. Gastroenterol Hepatol 7: 742–744.
- Epstein J, Warner J (2014) Recent advances in the phatophysiology and management of eosinophilic oesophagitis. Clinical and Experimental Allergy 44: 802–812.
- Morita H, Nomura I, Matruda A, Saito H, Matsumoto K (2013) Gastrointestinal food allergy in infants. Allergology International?: Official Journal of the Japanese Society of Allergology 62: 297–307.
- Ballmer-Weber BK, Fernandez-Rivas M, Beyer K, Defernez M, Sperrin M, et al. (2015) How much is too much? Threshold dose distributions for 5 food allergens. J Allergy Clin Immunol 135: 964-971.
- Bonds RS, Midoro-Horiuti T, Goldblum R (2008) A structural basis for food allergy: The role of cross-reactivity. Curr Opin Allergy Clin Immunol 8: 82-86.
- Hofmann A, Burks AW (2008) Pollen food syndrome: update on the allergens. Curr Allergy Asthma Rep 8: 413-417.
- Ronchetti R, Kaczmarski M, Haluszka J, Jesenak M, Villa MP (2007) Diagnostic algorithm of food allergy at the child age Food allergies, cross - reactions and agroalimentary biotechnologies. Advances in Medical Sciences 52.
- Sampson HA, Aceves S, Bock SA, James J, Jones S, et al. (2014) Food allergy: A practice parameter update-2014. J Allergy Clin Immunol 134: 1016-1025.
- de Silva D, Geromi M, Halken S, Host A, Panesar SS, et al. (2014) Primary prevention of food allergy in children and adults: systematic review. Allergy 69: 581-589.
- Wang J, Sampson HA (2009) Food allergy: recent advances in pathophysiology and treatment. Allergy Asthma Immunol Res 1: 19-29.
- Tan TH, Ellis JA, Saffery R, Allen KJ (2012) The role of genetics and environment in the rise of childhood food allergy. Clin Exp Allergy 42: 20-29.
- Wang J (2010) Management of the patient with multiple food allergies. Curr Allergy Asthma Rep 10: 271-277.
- Hong X, Wang X (2012) Early life precursors, epigenetics and the development of food allergy. Semin Immunopathol 34: 655-669.
- Martino DJ, Prescott SL (2010) Silent mysteries: Epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy 65: 7–15.
- Ibáñez MD, Garde JM (2009) Allergy in patients under fourteen years of age in Alergológica 2005. J Investig Allergol Clin Immunol 19 Suppl 2: 61-68.
- Asero R, Ballmer-Weber BK, Beyer K, Conti A, Dubakiene R, et al. (2007) IgE-mediated food allergy diagnosis: Current status and new perspectives. Molecular Nutrition & Food Research 51: 135–147.
- Turjanmaa K, Darsow U, Niggemann B, Rancé F, Vanto T, et al. (2006) EAACI/GA2LEN position paper: Present status of the atopy patch test. Allergy 61: 1377–1384.
- Schoos AM, Chawes BL, Følsgaard NV, Samandari N, Bønnelykke K, et al. (2015) Disagreement between skin prick test and specific IgE in young children. Allergy 70: 41-48.
- Sampson HA, Wijk RGV, Bindslev-Jensen C, Sicherer S, Teuber SS, et al. (2012) Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol 130: 1260–1274.
- Muraro A, Halken S, Arshad SH, Beyer K, Dubois AEJ, et al. (2014) EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy Eur J Allergy Clin Immunol 69: 590-601.
- Simons FE, Ardusso LR, Bilò MB, Cardona V, Ebisawa M, et al. (2014) International consensus on (ICON) anaphylaxis. World Allergy Organ J 7: 9.
- Burks AW, Jones SM, Boyce JA, Sicherer SH, Wood RA, et al. (2011) NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics 128: 955-965.
- Werfel T, Asero R, Ballmer-Weber BK, Beyer K, Enrique E, et al. (2015) Position paper of the EAACI: Food allergy due to immunological cross-reactions with common inhalant allergens. Allergy 70: 1079-1090.
- Lorenz AR, Scheurer S, Vieths S (2015) Food allergens: Molecular and Immunological Aspects, Allergen Databases and Cross-Reactivity. In: Ebisawa M, Ballmer-Weber BK, Vieths S, Wood RA (eds.) Food Allergy: Molecular Basis and Clinical Practice pp: 18–29.
- Giovannini M, D’Auria E, Caffarelli C, Verduci E, Barberi S, et al. (2014) Nutritional management and follow up of infants and children with food allergy: Italian Society of Pediatric Nutrition/Italian Society of Pediatric Allergy and Immunology Task Force Position Statement. Ital J Pediatr 40: 1.
- Tham EH, Rajakulendran M, Shek LP (2014) Prevention of food allergy in the real life. Asian Pac J Allergy Immunol 32: 16-24.
- Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, et al. (2014) Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy 69: 992-1007.
- Schaub B, Liu J, Höppler S, Schleich I, Huehn J, et al. (2009) Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 123: 74-782.
- Medina-Hernández A, Huerta-Hernández RE, Góngora-Meléndez MA, DomÃnguez-Silva MG, Mendoza-Hernández DA, et al. (2015) Clinical-epidemiological profile of patients with suspicion of alimentary allergy in Mexico. Mexipreval Study. Rev Alerg Mex 62: 28-40.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5460
- [From(publication date): 0-2017 - Aug 31, 2025]
- Breakdown by view type
- HTML page views: 4514
- PDF downloads: 946