Research Article Open Access
Dual Process of Bio-Phytoremediation of Arsenic from Contaminated Industrial Samples: An Alternative to Traditional Methods
| Jayashree Baviskar W, Vrushali Lawar V and Sharad Khandelwal R* | |
| Department of Microbiology, Institute of Life Sciences, HPT Arts and RYK Science College, Affiliated to Savitribai Phule Pune University, Nasik, India | |
| Corresponding Author : | Sharad Khandelwal R Department of Microbiology, Institute of Life Sciences HPT Arts and RYK Science College Affiliated to Savitribai Phule Pune University, Nashik, India Tel: 09689410568 E-mail: baviskarjayashree@gmail.com |
| Received February 11, 2016; Accepted March 14, 2016; Published March 21, 2016 | |
| Citation: Jayashree Baviskar W, Vrushali Lawar V, Sharad Khandelwal R (2016) Dual Process of Bio-Phytoremediation of Arsenic from Contaminated Industrial Samples: An Alternative to Traditional Methods. J Bioremed Biodeg 7:342. doi:10.4172/2155-6199.1000342 | |
| Copyright: © 2016 Jayashree Baviskar W, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Arsenic (As) is highly toxic, metalloid present in environment due to natural and anthropogenic sources. Arsenic III is highly toxic than Arsenic V. Arsenic removal by biological means is an upcoming and eco-friendly technique, where microorganisms have the ability to detoxify heavy metals from the environment. In the present work, Arsenic was detected from industrial effluents, and observed to be significantly higher than WHO limits where Dye industrial effluent showed highest Arsenic concentration of 249 ppm. Arsenic bioremediating bacteria were isolated from these effluents and identified as Klebsiella pneumonae Isolate-II, Lysinibacillus fusiformis Isolate I and III and Bacillus subtilis Isolate-IV. The cultures were subjected to bioremediation and highest removal was found to be 80% by isolate III.
The effluents were also subjected to biosorption and phytoremediation. Highest biosorption rate was found to be 47.2% by isolate II within 48 hours of incubation. Consortium of Klebsiella pneumonae+Pseudomonas putida showed 92.8% degradation and Lysinibacillus fusiformis+Bacillus subtilis showed 94% degradation within 4 days. This work will help us in careful handling of issues like Arsenic pollution, over exploitation of natural resources and sustainable development.
| Keywords |
| Arsenic; Rhodamine B; P. putida; Bioremediation; Nanoparticles |
| Introduction |
| Due to anthropogenic sources large amount of toxic compounds have been released in biosphere. Many industries release harmful pollutants such as As, mercury and lead in effluents and contaminate the soil. Arsenic is the most abundant toxic substance in environment. It is ranked first on the U.S. Environmental protection agency superfund list. Arsenic III and Arsenic V are the two inorganic forms [1]. It resembles phosphate and is a competitive inhibitor of many phosphate utilizing enzymes. Arsenic has shown many deleterious effects on kidneys, nervous system, which may lead to mental disorders. Chronic exposure may lead to permanent damage to organelles [2,3]. |
| Arsenic can be removed by several means from the contaminated sites using chemical precipitation, dialysis, ion exchange, reverse osmosis, and solvent extraction. These methods are costly and have low efficiency and lead to change in soil properties and leads to contamination at another site [3]. |
| Due to potent application of biological processes much attention is paid for using biotechnological approaches such as Bioremediation and phytoremediation [4]. The enzymes involved in the reduction reaction are mainly the oxidoreductases. These reactions produce less harmful contaminants and are extremely efficient in heavy metal removal, with cost effective and ecofriendly approach. |
| Materials and Methods |
| Microbial cultures and chemicals used |
| Water hyacinth (Eichhornia crassipes) was collected from Godapark, Nasik. Pseudomonas putida (MTCC -102), Bacillus subtilis (NCIM 3610), Phenorochaete chrysosporium (MTCC-787). Sodium arsenite (As III), Sodium arsenate (As V), Hydrochloric acid, Potassium iodate, Rhodamine B and Arsenic detection kit were procured from Hi Media, Mumbai. |
| Sample collection |
| Arsenic containing industrial effluents were collected from different industries viz. Metal washing industry, SIP injecto industry, Asian paints industry, CPC dye industry, Reliance dye industry, Paper mill industry, Rangrez dye industry, Marco trading chrome plating industry from Nasik, Ankleshwar and Surat. Pesticides contaminated soil was collected from grape farm of Makhmalabad, Nasik. Coal ash was collected from Eklahara thermal power plant, Nasik. Samples were collected in sterile containers and were stored at 4°c. pH of effluents was recorded for experimental purpose. |
| Detection of arsenic in samples |
| Spectrophotometric method: A stock solution of 1 mg/l was prepared by dissolving 173.33 mg of sodium Arsenite in 100 ml distilled water; working standard was prepared by appropriate dilution of stock solution. 0.05% solution was prepared of Rhodamine B dye by dissolving 2.5 mg in volumetric flask of 100 ml distilled water stored in ambered color bottle [5,6]. |
| To an aliquot of working standard solution containing 1-100 μg of arsenic in 25 ml calibrated flask, 2 ml potassium iodate 2% and 0.4 M 1 ml HCL was added and mixture was shaken gently. This was followed by addition of 2 ml of 0.05% Rhodamine B dye solution. The solution was kept for 15 min. and made up to the mark with distilled water. Absorbance was measured at 553 nm against a reagent blank, prepared in same way as above [6,7]. |
| Isolation and Identification of Arsenic oxidizing microorganisms from effluent: Out of collected industrial effluents, 04 different types of samples were chosen for bioremediation studies such as dye effluent, Coal Ash, Pesticide sample and chrome plating effluent. Enrichment of culture was done by adding 1% of effluent to nutrient broth containing Arsenite 50, 100, 150, 200, and 250 ppm, and was incubated at 28°C (Table 1). Microbial Culture from enriched flasks was plated on nutrient agar plates with increasing Arsenic concentrations. Isolates with confluent growth were identified from Bac-Test Laboratory, Nasik [8]. |
| Biomass determination |
| Isolates along with Pseudomonas putida in the effluents and in the MSM medium containing standard arsenic of 250 ppm were grown [8,9]. The growth of the microorganisms was determined after every 24 hrs by taking absorbance at 545 nm. |
| Bioremediation studies |
| Bioremediation of Arsenic was done using different isolates, standard strain of Pseudomonas putida, in Erlenmeyer flask containing sterile 50 ml double strength MSM media (Nacl=5 gm/L, mgCl2=0.5 gm/L, KH2PO4=0.45 gm/L, KH2PO4=0.9 gm/L, NH4Cl=0.3 gm/L, KCl=0.3 gm/L, Glucose=1 gm/L, Yeast extract=1 gm/L) and sterile 50 ml effluents. Beside this 250 ppm/ ml of standard Arsenic (III) was also considered for bioremediation studies using all the isolates [10]. Oxidation was measured after every 48 hrs spectrophotometrically [7]. Bioremediation of Arsenic was also performed using consortium Klebsiella pneumonia+P. putida and Lysinibacillus fusiformis+Bacillus subtilis. |
| Biosorption studies |
| Bacterial cells from actively growing culture were harvested and dry mass of 0.1 gm/10 ml of MSM was used for biosorption studies. Biomass was suspended in a flask containing Arsenic concentration of 250 ppm/ml. The binding capacity of the cells was calculated from the initial and final concentrations [11]. |
| Arsenic removal by Biosorption and Phytoremediation |
| 1% of water hyacinth extract were added to MSM medium containing 250 ppm/ml of Arsenic, 1% of biomass was inoculated and Arsenic concentration was determined spectrophotometrically after every 24 hrs [11]. |
| Biosorption kinetics studies |
| The metal biosorption (q) and bioremoval efficiency (R) using bacterial biomass and plant extract was calculated using following formulae. |
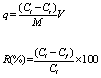 |
| Where: q=Metal adsorption (mg/g); M=dry biomass; V=total volume of sample treated; R=bioremoval efficiency %; Ci=initial concentration of metal in aqueous solution (ppm/L) and Cf=final concentration of metal in aqueous solution (ppm/L). |
| Toxicity testing on plants |
| Toxicity of Arsenic (III) and As (V) was observed on fenugreek plants with various dilutions of standard arsenic and different parameters root length, shoot length, size of leaves of fenugreek plant were determined. |
| Detoxification studies of effluents |
| Culture of Bacillus subtilis was inoculated in sterile Nutrient broth and incubated at room temperature for 24 hrs. to get cell density upto 106` cells/ ml. Detoxification of treated and untreated effluent against culture of Bacillus subtilis was studied on Mueller Hinton medium using Kirby Bauer’s well diffusion method. Distilled water was considered as control [12]. |
| Results and Discussion |
| Sample analysis |
| Determination of pH and different parameters of industrial effluents, pH of all effluents was lower than neutrality i.e., 6.0, Pesticide effluent was found to be neutral around 7.1 (Figure 1). |
| Isolation and identification of Arsenic oxidizing organisms |
| Arsenic oxidizing bacteria have been identified from various arsenic rich environments [13]. Microorganisms were isolated on plates containing increasing Arsenic concentrations of 50, 100, 150, 200, and 250 ppm and the results as per Bac-Test Laboratory (Nasik, India) were as follows: |
| We could isolate four effective microorganisms from various sources viz. Dye effluent, Chrome plating effluent, Coal ash sample, and pesticide contaminated soil, at a maximum range of arsenic concentration of 250 ppm tolerable by these organisms. |
| Arsenic determination using spectrophotometer |
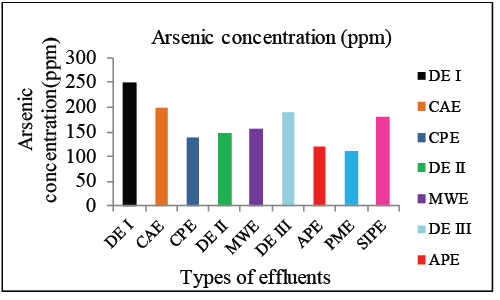
| Arsenic concentrations in different industrial effluents were determined using UV-Vis Spectrophotometer. Highest concentration was found to be 249 ppm in Dye effluent I (Graph 1). This value of As concentration in the effluent was found to be in the moderate range as compared to the industrial effluents. |
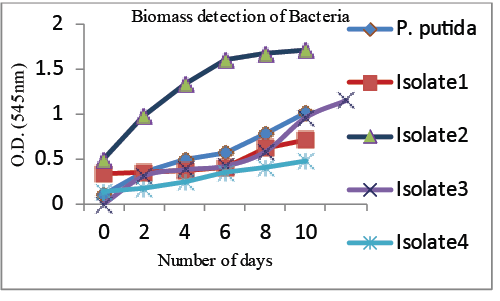
| Biomass determination |
| The highest biomass was obtained using Isolate II with 250 ppm Arsenic concentration (Graph 2). The isolate was found to grow luxuriantly in As containing effluent considered for the study. Other isolates also showed moderate growth. |
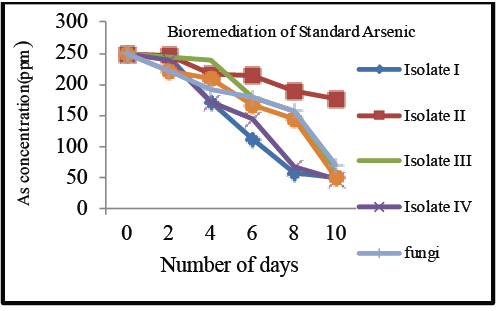
| Bioremediation of arsenic |
| Studies have been also carried out on tolerance of arsenic and its bioremoval using Acidithiobacillus ferrooxidans [14]. Plants and microorganisms are able to bioremediate metal ions [7]. Methodology for As removal from drinking water was developed, consisting of arsenate transport through an anion exchange membrane followed by coagulation [15]. The process was not cost effective therefore we decided to work on known concentration of Arsenic salt. |
| The salts were sterilized and inoculated with the respective isolates, (Isolate I- Dye isolate, Isolate II- Chrome isolate: Isolate III- Coal ash isolate: Isolate IV- Pesticide isolate) For pure 250 ppm of Arsenic, highest removal i.e., 81% was obtained by Isolate IV followed by isolate I i.e., 80%, Isolate III of 77%, followed by Isolate II i.e., 30% and Pseudomonas putida 76%. The samples were analyzed at the intervals of two days upto 10 days respectively (Graph 3a). |
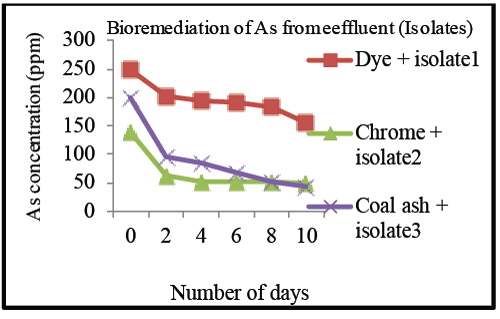
| Arsenic, removal from effluents was obtained by Isolate III i.e., 75% followed by isolate I (37.35%) for concentration of 249 ppm and isolate II (60%) for concentration of 120 ppm in 10 days at room temperature (Graph 3b). |
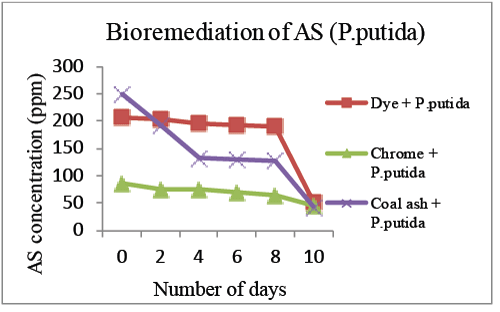
| Bioremediation of effluent was also carried out using Standard strain of Pseudomonas putida which showed 83.1%, 75.3% and 48% removal of Arsenic from Coal ash effluent, Dye effluent and Chrome Plating effluent respectively (Graph 4). |
| Bioremediation of arsenic by consortium |
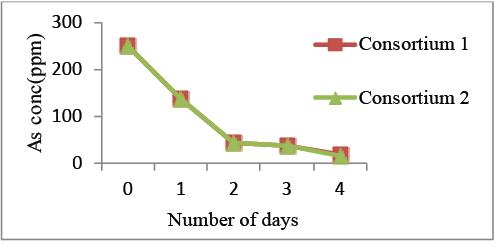
| Using the concentration of 250 ppm, Arsenic removal was obtained upto 92.8% by consortium of Klebsiella pneumonia and P. putida and around 94% by consortium of Lysinibacillus fusiformis and Bacillus subtilis (Graph 5). |
| Biosorption |
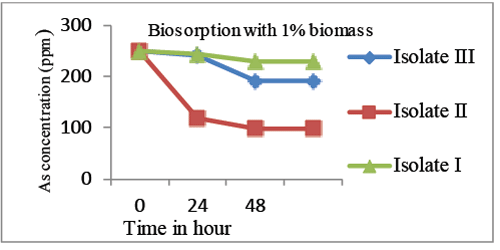
| Biosorption of As is studied using fungal biomass, and also fresh water micro algae [16]. In our study dry bacterial biomass of the isolates was used. Where 1% dry biomass showed significant Arsenic biosorption of 58 mg/g, 151 mg/g and 22 mg/g by isolate III, isolate II and isolate I with 8.8%, 60.4% and 23.2% bioremoval efficiency respectively (Graphs 6 and 7). |
| Biosorption and phytoremediation |
| Combination of 1% biomass and 1% plant extract showed 41 mg/g, 59 mg/g, 51.5 mg/g and 53 mg/g using Arsenic biosorption from isolate III from isolate II from isolate I from isolate IV and 32.8%, 47.2%, 41.2% and 42.4% bioremoval efficiency respectively within 48 hrs. Phytoremediation of As is tried using Chinese baker’s fern (Pteris vittata) Indian mustard (Brassica juncea) [16]. In our study we used water hyacinth extract for bioremoval of As. |
| Detoxification of the effluents |
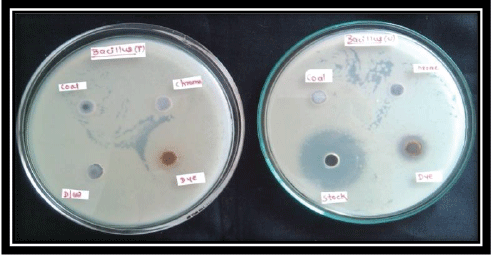
| Toxicity testing of the effluents was done to check the toxic effects of treated and untreated effluents on the standard strain of Bacillus subtilis. The nutrient butts containing 25 ml of Muller Hinton medium inoculated with 0.1 ml of Bacillus subtilis suspension were poured into Petri plate, four wells were bored and each well was inoculated with the untreated effluents along with the stock of standard arsenic and on the other hand treated effluents were inoculated in the other plate along with distilled water and the plates were incubated at 28°C for 24 hrs. |
| After 24 hrs the plates were observed for the growth in which the untreated Effluent showed zone of inhibition on both the plates containing culture of Bacillus subtilis (Figure 2). On plate containing Bacillus subtilis, zone of inhibition was observed after 24 hrs of incubation around the Dye effluent as well as stock of standard arsenic, while no zone of inhibition was observed on plates containing the treated effluent for the Bacillus culture indicating that the effluent was detoxified. |
| Toxicity Testing |
| Toxicity testing on plants |
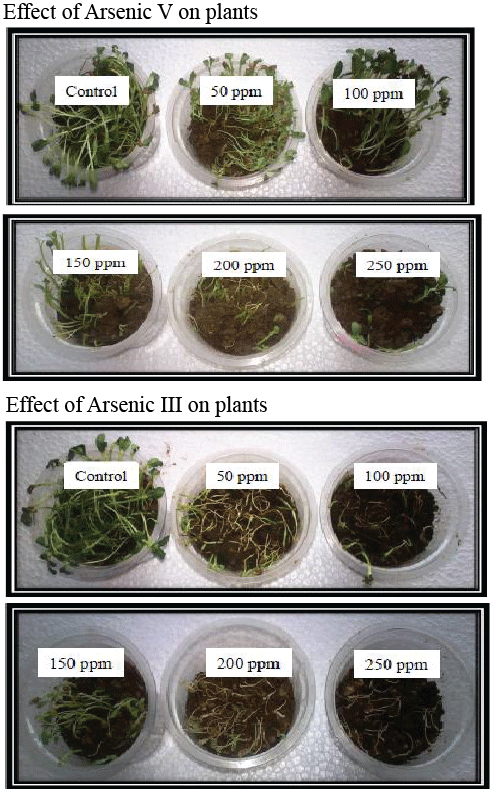
| With increase in concentration of As III, root length, shoot length and leaf length of plant decreased as compared with control, with stunted plant growth at high concentrations. With increasing concentration of As V, root length, leaf length and shoot length of plant decreased as compared with control, with altered transpiration of plant for high concentration (Table 2). As III was found to be more toxic than As V (Figure 3). |
| Conclusion |
| Bioremediation and Biosorption studies were carried out using microorganisms isolated from different industrial effluents viz: Lysinibacillus fusiformis from dye effluent and Coal Ash effluent, Bacillus subtilis from Pesticide sample and Klebsiella pneumoniae from chrome plating effluents. Arsenic levels in these effluents were above permissible limits. |
| Bioremediation resulted into an 80% removal of Arsenic using Klebsiella pneumonia. Biosorption studies turned out to be more efficient and less time consuming. The study reveals that isolated organisms used for the removal of Arsenic can be used as biosorption agent which would result in efficient removal of pollutants from the environment. Dual process of Biosorption along with Phytoremediation was performed where maximum Arsenic removal upto 40% was obtained within 2 days. We also consortia’s of Lysinibacillus fusiformis+Bacillus subtilis and Klebsiella pneumonia+Pseudomonas putida which showed almost 94% and 92% Arsenic removal within 3 days respectively. |
| Trivalent As was found to be more toxic than pentavalent As from the toxicity testing performed on plants. |
| This technique will be useful on large scale applications of industrial effluents. It will be advantageous for the design and fabrication of an eco-friendly and economically favorable treatment technology for the bioremediation of Arsenic from the environment. |
| Acknowledgements |
| Authors are thankful the UCG-Delhi for financial assistance and to the Department of Microbiology of HPT Arts and RYK Science College, Nasik for providing research facility and the staff of microbiology Department helping throughout the work. |
| References |
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Graphs at a glance
 |
 |
 |
 |
| Graph 1 | Graph 2 | Graph 3a | Graph 3b |
 |
 |
 |
|
| Graph 4 | Graph 5 | Graph 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 11510
- [From(publication date):
May-2016 - Aug 15, 2025] - Breakdown by view type
- HTML page views : 10437
- PDF downloads : 1073
