Eco-Friendly Nanoemulsion Formulation of Mentha piperita Against Stored Product Pest Sitophilus oryzae
Received: 16-Oct-2018 / Accepted Date: 26-Nov-2018 / Published Date: 04-Dec-2018 DOI: 10.4172/2329-8863.1000404
Abstract
Mentha piperita concentrations based on LC50 were estimated against Rice weevil, Sitophilus oryzae the results indicated that an increase in the insecticidal effect of M. piperita essential oil against the insect species when formulated as a nano-emulsion the highest and fastest toxic effect were observed with M. piperita (4%) nanoemulsion via thin film residue method and treatment with wheat grains method, because of smaller particle size and increase biological activity due to increased surface area of emulsion droplets therefore more opportunity of the formulation to come in contact with the target insect. The nano-emulsion formulations of M. piperita essential oil containing surfactants were successfully created via the high-energy emulsification method. The formulation provided a nano particle-size, with the smallest size being 43.55 nm. The results concluded that M. piperita nanoemulsions may be used as an alternative for the control of other stored-product insect pests. They have the advantage of promising insecticidal activity and being eco-friendly and less toxic than synthetic pesticides.
Keywords: Rice weevil; Sitophilus oryzae; Mentha piperita L.; Nano formulation; EO nano-emulsion
Introduction
Stored-grain insects in developing countries cause huge losses of stored-grain products, amounting to 5-10% loss in temperate regions and 20-30% in the tropical regions [1]. In Egypt, the annual loss in wheat due to stored insects is estimated as equivalent to half a million tons of which 12% is caused by the rice weevil alone [2].
The rice weevil, Sitophilus oryzae (Coleoptera: Curculionidae) is a major primary pest, of stored grain-based products, including maize, rice and wheat, particularly in the tropic regions [3,4]. This pest can cause considerable damage to stored grains and completely destroy kernels. Moreover, the product quality is affected by presence of eggs and dead insects, and holes on the grains [5]. According to the global economic estimation, the related costs of pests to stored food stuffs could reach to about 500 million USD per year [6].
Massive applications of conventional pesticides to control these insects result in adverse effects on beneficial organisms, leaves their residues in the food leads to severe risks to human health and environment, it reduced the populations of natural enemies and developed the insect resistance to synthetic insecticides [7,8].
Recently scientists pointed to use naturally occurring insecticides. many of these compounds are secondary plant substances (allelochemicals) including alkaloids, quinones and essential oils [9]. These active substances extracted from plants are effective against wide range of insects and act as toxicants, as insect growth regulator (IGR), repellents or as phagodeterrent [10]. These properties make them suitable bioinsecticides for organic agriculture and could be an alternative to those chemical insecticides.
Owing to the fact, the most essential oils used as flavoring agents possessing insecticidal properties showed ovicidal, larvicidal, adulticidal against several insect species [11,12]. One of the most effective plants against stored pests is Mentha piperita L. or peppermint, a plant from the Lamiaceae family, Mentha piperita oil possesses diversified potential in the areas of food, cosmetics, medicines, and pest control [13]. The essential oils extracted from M. piperita have also been reported as a source of botanical insecticides [14].
However, the major inconvenience of the use of essential oils are their chemical instability in the presence of air, light, moisture and high temperature that can determine the rapid evaporation and degradation of some active components [15]. A method to overcome these problems is the incorporation of essential oils into a controlledrelease nano-formulation which prevents rapid evaporation and degradation, enhances stability and maintains the minimum effective dosage/application [16]. In addition, this nano-formulation compared with bulk formulation is expected to be more effective, showed less toxicity towards non-target organisms and increased persistence of the active ingredient [17,18].
This study aims to evaluate the efficiency of M. piperita (EO nanoemulsion) as possible as protecting agents of wheat grains against infestation by the rice weevil, Sitophilus oryzae .
Materials And Methods
Insect rearing
Cultures of the rice weevil, Sitophilus oryzae (L.), was maintained in (Stored Products Department, Plant Protection Research Institute, Agriculture Research Center, Dokki, Giza, Egypt.) over 5 years without exposure to insecticides and reared on sterilized whole wheat. Insect rearing and all experimental procedures were carried out at 26.1°C and 65.5% R.H. Adults used in studies was two weeks post-eclosion.
Materials
Tween 80, Acetone and Peppermint oil was purchased as pure oil from Department of Botany and microbiology, Faculty of Science, Alexandria University, Egypt.
Preparation of nano-emulsion
The oil-in-water nano-emulsion was formulated using M. piperita (peppermint) essential oil, non-ionic surfactant (tween 80) and deionized water, according to Ghotbi et al. [19] and Sugumar et al. [20]. The concentration of M. piperita (EO) (4%, v/v) was prepared. Initially, coarse emulsion was prepared by adding water to organic phase containing oil and surfactant in ratios 1:5 (v/v) using a magnetic stirrer, which was then subjected to ultrasonic emulsification using a 20 kHz Sonicator (BANDELIN Sonopuls ).
M. piperita nano-emulsion characterizations
Droplet size determination: The emulsion droplet size and size distribution was determined using particle size analyzer (Malvern-UK, 4700 model) Droplet size was analyzed using dynamic light scattering (DLS) technique [21]. Prior to all the experiments, the nano-emulsion oil formulations were diluted with water to get rid of the multiple scattering effects. The droplet size and the polydispersity index (PDI) of the formulated nano-emulsion oil were measured.
Morphology of M. piperita nano-emulsion: To visualize the shape and morphology of the formulated M. piperita nano-emulsion oil, transmission electron microscopy (TEM) at the EM Unit in the Faculty of Science, Alex. Univ. was carried out. One drop of emulsion was negatively stained with ethanol and was positioned on a copper grid. The TEM micrographs were acquired using a transmission electron microscope (JEOL JEM-1400Plus) with a tungsten source and operating at 80 kV.
Bioassay technique
Contact toxicity bioassay using thin film residue: The insecticidal activity of the tested essential oils against the adults of S. oryzae was determined by direct contact application [22,23]. A series of dilutions of M. piperita essential oils were prepared using acetone as a solvent. Aliquots of 1 ml of the dilutions were applied on the bottom of a glass Petri dish (9 cm diameter) to give deferent concentrations from the bulk of M. piperita and M. piperita nano-emulsion. After evaporation of the solvent for 2 min, 20 adults of tested insects were separately placed into each Petri dish. Control dishes with and without solvent were used. All treatments were replicated three times. Mortality percentages were recorded after 24, 48 and 72 h of treatment and LC50 values were calculated according to Finney [24]. The toxic index (TI) of the tested EO nano-emulsion or free EO was calculated by the following equation according to Yamamoto et al. [25] and Sun [26]:
The toxic index (%) (TI) was calculated (based on LC50 after 72 hrs.)
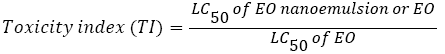
The compound has TI less than 1 (TI1) have high toxicity.
Toxicity increase (%)=(TI of EO-TI of EO nano-emulsion) × 100
Contact toxicity bioassay using treatment wheat grains with free M. piperita (EO) and M. piperita (EO) nano-emulsion: The essential oils were admixed with grains according to Qi and Burkholder [22]. Wheat grains were treated with both M. piperita (EO) and (EO nanoemulsion) at different concentration (0.8, 1.6, 3.3, and 13.3 ml/kg). The M. piperita (EO) and (EO nano-emulsion) were dissolved in acetone (2 ml) then mixed manually with grains (60 gm in 0.4-Litter) glass jars and were divided into three equal replicates. Notably, it has been previously elucidated that when the solvent evaporates, the nanoemulsion retains its properties [27,28]. After evaporation of acetone, the treated grains were infested by newly emerged adults (10 pairs). Mortality was recorded every week for two weeks. The number of progeny was recorded after six weeks of infestation.
Statistical analysis
Mortality rate was estimated and corrected according to formula [29] as follows:
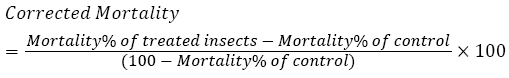
The toxicity data was analyzed using probit analysis to estimate the LC50 (Ldp line).
Results
M. piperita nano-emulsion characterizations
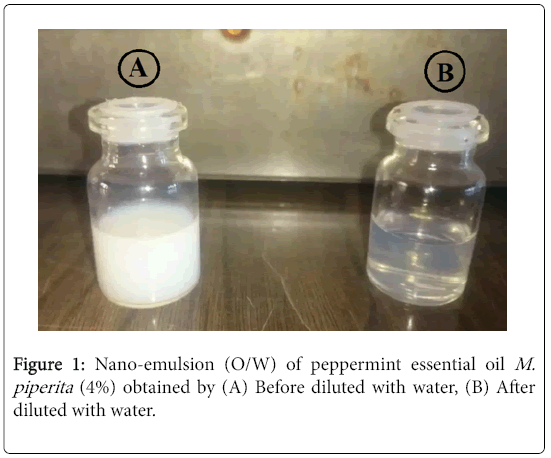
Nano-emulsion droplet size distribution and surface morphology: The nano-emulsion was obtained after sonicating coarse emulsion for 45 min Figure 1 the results showed that emulsion droplets were in the range of 43.55 nm.
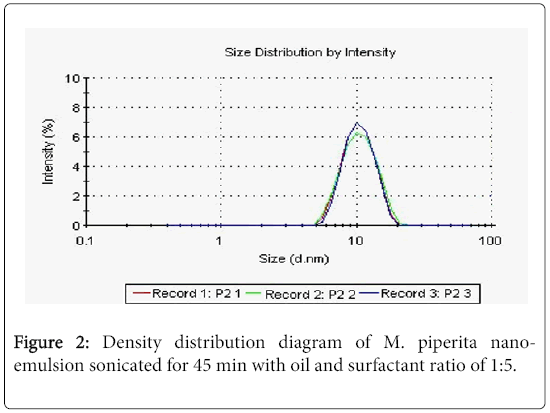
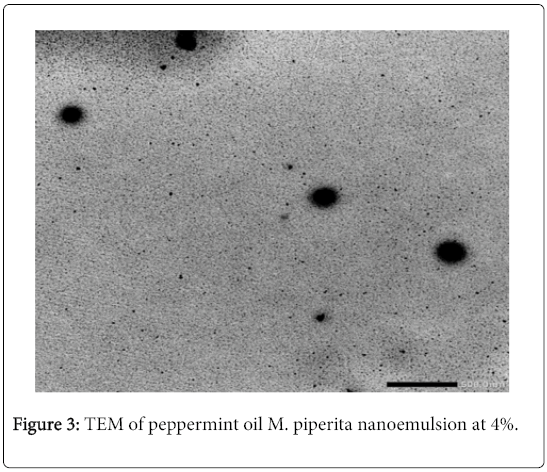
The average of droplets size recorded 43.55 nm with poly dispersity index (PDI) of 0.787, Figure 2 the morphology of peppermint oil M. piperita nano-emulsions was optically transparent or translucent appearance compared with micro-emulsion with the same formulation. The nano-emulsion was visualized using transmission electron microscopy (TEM). Figure 3 show the particles appeared round, spherical in shape, a good dispersion and narrow size distribution, when M. piperita (EO) used at 5% conc.
Contact toxicity bioassay using thin film residue
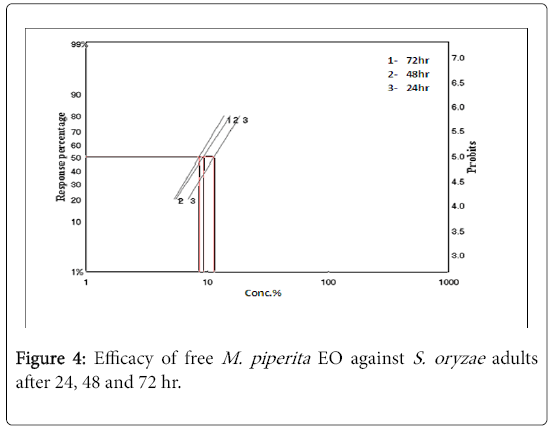
Insecticidal activity of M. piperita nano-emulsion and the free M. piperita EO were evaluated by Film residue contact toxicity against S. oryzae . Results showed that both of them have toxic effect against S. oryzae after, 24, 48 and 72 h of treatment. The lethal concentration (LC50). After 24 h of exposure, accounted 0.181 μl/cm2, after 48 h of exposure 0.147 μl/cm2 and after 72 h of exposure 0.136 μl/cm2 of free M. piperita EO against S. oryzae as show in Table 1 and the lethal concentration (LC50) after 24 h of exposure, accounted 0.127 μl/cm2, after 48 h of exposure 0.106 μl/cm2 and after 72 h of exposure 0.095 μl/cm2 of M. piperita nano-emulsion against S. oryzae , respectively as show in Table 2.
| Time | LC50 µl/cm2 | Confidence Limits | Slope | X2 | Toxicity Index (TI) | |
| Lower | Upper | |||||
| 24 hr | 0.181 | 0.159 | 0.199 | 3.861 ± 0.451 | 1.132 | |
| 48 hr | 0.147 | - | - | 3.696 ± 0.565 | 12.065 | |
| 72 hr | 0.136 | 0.109 | 0.155 | 4.063 ± 0.674 | 3.668 | 1 |
Table 1: Insecticidal effect of M. piperita on Sitophilus oryzae adults using thin film residue after 24, 48, and 72 hr post-exposure.
| Time | LC50 µl/cm2 | Confidence limits | Slope | X2 | Toxicity Index (TI) | Toxicity Increase (%) | |
| Lower | Upper | ||||||
| 24 hr | 0.127 | 0.097 | 0.145 | 5.102 ± 1.043 | 0.001 | ||
| 48 hr | 0.106 | 0.031 | 0.123 | 5.224 ± 1.737 | 0.576 | ||
| 72 hr | 0.095 | 0.023 | 0.123 | 5.576 ± 2.004 | 1.859 | 0.698 | 30.2 |
Table 2: Insecticidal effect of M. piperita EO Nano-emulsion on Sitophilus oryzae adults using thin film residue after 24, 48, and 72 h postexposure.
M. piperita Nano-emulsion caused high mortality and toxicity with less concentration compared to the bulk oil. The toxicity index (TI) accounted for S. oryzae 0.698 based on LC50 after 72 h of exposure as shown in Table 2. However, the mortality in S. oryzae was increased with increasing exposure time and concentration of M. piperita nanoemulsion or the bulk EO in a concentration-dependent manner. The mortality in S. oryzae as shown in Table 2 account 68.33, 91.67 and 100% after 24 hr and 86.67, 98.33 and 100% after 48 hr and 88.33, 100 and 100% after 72 hr of exposure to nano-emulsion at concentration 10, 15 and 30 μl (Figure 4). In contrast, the free M. piperita EO showed nearly the same effect with high concentrations ranged from 10-30 μg as shown in Table 2. These observations demonstrated that M. piperita nano-emulsion in lower concentrations compared with essential oil has a considerably greater effect on the adult of S. oryzae . The results concluded that, the toxicity effects were significantly more pronounced for the M. piperita nano-emulsion formulations compared with the bulk M. piperita oil (Figure 5).
Contact toxicity bioassay using treatment wheat grains with M. piperita (EO) and (EO nano-emulsion)
Mortality of S. oryzae via Contact toxicity using treatment with wheat grains method, were significantly affected by the exposure time, all main effects (formulations and concentrations) and associated interactions (Table 3).
| Essential oil | Conc. mL/kg | Mortality (%) after | Mean of emerged adults after | |||
|---|---|---|---|---|---|---|
| 1 week ± SD | 2 weeks ± SD | Mean | 6 weeks ± SD | Mean | ||
| M. piperita (EO nano-emulsion) 4% | 1.6 | 0.0 ± 0.0 | 44 ± 3.13 | 78.58 | 1.67 | 0.84 |
| 3.3 | 88 ± 0.57 | 98.33 ± 0.58 | 1.67 | |||
| 8.3 | 98.33 ± 0.58 | 100 ± 0.0 | 0.0 | |||
| 13.3 | 100 ± 0.0 | 100 ± 0.0 | 0.0 | |||
| M. piperita (EO) | 0.8 | 82.5 ± 2.52 | 95 ± 1.73 | 94.06 | 2.5 | 0.83 |
| 1.6 | 85 ± 1.73 | 100 ± 0.0 | 2.5 | |||
| 3.3 | 90 ± 2.64 | 100 ± 0.0 | 0.0 | |||
| 13.3 | 100 ± 0.0 | 100 ± 0.0 | 0.0 | |||
| Control | 0.0 | 0.0 | 0.0 | 115 | ||
Table 3: Effect of M. piperita (EO), (EO nano-emulsion) on mortality and emergence of S. oryzae adults.
After one week of exposure, the highest mortality was recorded for S. oryzae , exposed to 13.3 ml/kg of M. piperita nano-emulsion, where all exposed adults were dead. After two weeks of exposure, 8.3 mL/kg of the tested oil nano-emulsion formulations caused 100% mortality of exposed adults (Table 3).
Among the bulk of M. piperita (EO) the highest mortality recorded after one week for S. oryzae exposed to 13.3 ml/kg and after two weeks of exposure, 1.6 ml/kg. The results also showed significant differences in the adult emergence of S. oryzae in M. piperita (EO) and M. piperita nano-emulsion after 6 weeks from treatment (Table 3).
Discussion
Few reports are available on the insecticidal activity of M. piperita essential, with fast and high mortality, against stored-product pests. This study showed an increase in the insecticidal effect of M. piperita essential oil against the insect species when formulated as a nanoemulsion. In the present study, the results are in almost agreed with the results stated by earlier investigators. The differences in chemical components may be due to variations in environmental, climatic and geographical which effect on chemical composition of M. piperita . Formulating essential oils (EOs) into nano-emulsion which is transparent and can be used in food and beverage products, thereby, decreasing the amount of EOs required [30]. The TEM image of M. piperita confirmed the results that show the spherical shape and a good dispersion of droplets nano-emulsion agreed with Sugumar et al. [20] and Ostertag et al. [31] who reported that good nano-emulsion had droplets size between 20-200 nm. The tested M. piperita coarse emulsion was turbid and milky white in color due to droplet size in micrometer range. After sonication, the emulsion became optically transparent. This decrease in turbidity was due to minimized droplet diameter after sonication which results in relatively weak scattering making the emulsion system optically transparent agreed with McClements [32]; Pey et al. [33]; Abouelkassem et al. [34].
The bioassay of the nano-emulsion formulations of M. piperita demonstrated toxicity effects on S. oryzae. The insecticidal effects of the formulations varied with the insect species, concentration of the formulations, exposure time and the method of application. The results of contact toxicity in thin film residue method using glass Petri dish and contact toxicity using treatment with wheat grains method, showed that increasing mortality with increasing concentration and the exposure time for the tested insect [35-38]. The possible explanation for these results is the absorption of the toxic substance increases through insect’s body by increase time and concentration. The results indicated that the S. oryzae very sensitive when exposed to M. piperita (EO) and (EO nano-emulsion) [39-44]. Results agreed with the effectiveness of M. piperita EO with concentration 1.0% against S. oryzae evaluated by Magdy et al. [45], which lead to about 96.6% mortality after 24 hr and 100% mortality after 84 hr of exposure using thin film residue method.
The insecticidal activity of M. piperita EO is due to the presence of menthol, menthone, methyl acetate, mentho-furan and 1,8-cineole that found as major components. Moreover, the minor compounds can play an important role in EO toxicity. Previous studies showed that insecticidal and biological activity of M. piperita EO could be due to the present of major constituents such as menthol, menthone, and menthofuran [46-50]. It has been reported that M. piperita EO, have insecticidal activity against many insects such as S. oryzae [51-54].
Among the nano-emulsion formulations, the highest and fastest toxic effect were observed with M. piperita (4%) nano-emulsion against S. oryzae via thin film residue method and treatment with wheat grains method, because of smaller particle size and increase biological activity due to increased surface area of emulsion droplets therefore more opportunity of the formulation to come in contact with the target insect. Whereas, the lower mortality caused by M. piperita EO with the biggest particle size indicates that the smaller the particle size, the greater the probability of higher efficacy.
This finding is consistent with the studies of Anjali et al. [17]; Nenaah [55]; Pant et al. [56]; Sugumar et al. [20]; Abouelkassem et al. [34]; Nenaah et al. [28]; Oliveira et al. [57,58]; Choupanian et al. [59]; Mossa et al. [60]; Choupanian et al. [61]. The study showed that M. piperita essential oil based nano-emulsion formulations were able to increase the mean mortality rate of S. oryzae compared to the free M. piperita EO.
Conclusion
The nano-emulsion formulations of M. piperita essential oil containing surfactants were successfully created via the high-energy emulsification method. The formulation provided a nano particle-size, with the smallest size being 43.55 nm. The M. piperita with the smallest particle size was found to be most effective in controlling S. oryzae adults. Overall, the present study proved that M. piperita nanoemulsions are effective in controlling S. oryzae adults. These nanoemulsions may be used as an alternative for the control of other storedproduct insect pests. They have the advantage of promising insecticidal activity and being eco-friendly and less toxic than synthetic pesticides.
References
- Nakakita H (1998) Stored rice and stored product insects. Rice Inspection Technology Manual. ACE Corporation, Tokyo, Japan, pp: 49-65.
- Howe RW (1965) Losses caused by insects and mites in stored foods and feeding stuffs. In Nutrition Abstracts and Reviews 35: 285-303.
- González JOW, Gutiérrez MM, Ferrero AA, Band BF (2014) Essential oils nano-formulations for stored-product pest control-Characterization and biological properties. Chemosphere 100: 130-138.
- Lü JH, He YQ (2010) Fumigant toxicity of Ailanthus altissima Swingle, Atractylodes lancea (Thunb.) DC and Elsholtzia stauntonii Benth extracts on three major stored-grain insects. Industrial Crops and Products 32: 681-683.
- DomÃnguez Umpiérrez JE, Marrero Artabe L (2010) Catálogo de la entomofauna asociada a almacenes de alimentos en la provincia de Matanzas. Fitosanidad 14: 75-82.
- Sharma RP, Yadav RR (2001) Susceptibility Status of Helicoverpa armigera Hub. to some Synthetic Insecticides and Neem Seed Kernel Extract as Influenced by Host Plant. Pesticide Research Journal 13: 152-159.
- Saleem MA, Ahmad M, Ahmad M, Aslam M, Sayyed AH (2008) Resistance to selected organochlorin, organophosphate, carbamate and pyrethroid, in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. J Econ Entomol 101: 1667-1675.
- Appel AG, Gehret MJ, Tanley MJ (2001) Repellency and toxicity of mint oil to American and German cockroaches (Dictyoptera: Blattidae and Blattellidae). J Agric Urban Entomol 18: 149-156.
- Burfield T, Reekie SL (2005) Mosquitoes, malaria and essential oils. International Journal of Aromatherapy 15: 30-41.
- Souguir S, Chaieb I, Cheikh ZB, Laarif A (2013) Insecticidal activities of essential oils from some cultivated aromatic plants against Spodoptera littoralis (Boisd). J Plant Prot Res 53: 388-391.
- Adel MM, Atwa WA, Hassan ML, Salem NY, Farghaly DS, et al. (2014) Biological activity and field persistence of Pelargonium graveolens (Geraniales: Geraniaceae) loaded solid lipid nanoparticles (SLNs) on Phthorimaea operculella (Zeller)(PTM)(Lepidoptera: Gelechiidae). International Journal of Science and Research 4: 514-520.
- Shah PP, Mello PMD (2004) A review of medicinal uses and pharmacological effects of Mentha piperita.
- Aflatuni A (2005) The yield and essential oil content of mint (Mentha spp.) in Northern Ostrobothnia. PhD Thesis, University of Oulu.
- Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57: 405-424.
- Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnology Advances 29: 792-803.
- Anjali CH, Sharma Y, Mukherjee A, Chandrasekaran N (2012) Neem oil (Azadirachta indica) nanoemulsion-a potent larvicidal agent against Culex quinquefasciatus. Pest Manag Sci 68: 158-163.
- Devi N, Maji TK (2011) Study of complex coacervation of gelatin a with sodium carboxymethyl cellulose: microencapsulation of neem (Azadirachta indica A. Juss.) seed oil (NSO). International Journal of Polymeric Materials 60: 1091-1105.
- Ghotbi RS, Khatibzadeh M, Kordbacheh S (2014) Preparation of neem seed oil nano-emulsion. In Proceedings of the 5th International Conference on Nanotechnology: Fundamentals and Applications, Prague, Czech Republic, pp: 11-13.
- Sugumar S, Clarke SK, Nirmala MJ, Tyagi BK, Mukherjee A, et al. (2014) Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull Entomol Res 104: 393-402.
- Fernandes CP, Mascarenhas MP, Zibetti FM, Lima BG, Oliveira RP, et al. (2013) HLB value, an important parameter for the development of essential oil phytopharmaceuticals. Rev bras farmacogn 23: 108-114.
- Qi YT, Burkholder WE (1981) Protection of stored wheat from the granary weevil by vegetable oils. J Econ Entomol 74: 502-505.
- Broussalis AM, Ferraro GE, Martino VS, Pinzón R, Coussio JD, et al. (1999) Argentine plants as potential source of insecticidal compounds.J Ethnopharmacol 67: 219-223.
- Finney DJ (1971) Probit analysis. Cambridge University Press, London, pp: 318.
- Yamamoto I, Casida JE (1999) Nicotinoid insecticides and the nicotinic acetylcholine receptor.
- Sun YP (1950) Toxicity Index-an improved Method of comparing the relative 378 Toxicity of Insecticides. J Econ Entomol.
- Da Costa JT, Forim MR, Costa ES, De Souza JR, Mondego JM, et al. (2014) Effects of different formulations of neem oil-based products on control Zabrotes subfasciatus (Boheman, 1833) (Coleoptera: Bruchidae) on beans. J Stored Prod Res 56: 49-53.
- Nenaah GE, Ibrahim SI, Al-Assiuty BA (2015) Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.). J Stored Prod Res 61: 9-16.
- Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18: 265-267.
- Qian C, Decker EA, Xiao H, McClements DJ (2012) Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chemistry 132: 1221-1229.
- Ostertag F, Weiss J, McClements DJ (2012) Low-energy formation of edible nano-emulsions: factors influencing droplet size produced by emulsion phase inversion. J Colloid Interface Sci 388: 95-102. 388: 95-102.
- McClements DJ (2002) Colloidal basis of emulsion color. Curr Opin Colloid Interface Sci. 7: 451-455.
- Pey CM, Maestro A, Solé I, González C, Solans C, et al. (2006) Optimization of nano-emulsions prepared by low-energy emulsification methods at constant temperature using a factorial design study. Colloids Surf A Physicochem Eng Asp 288: 144-150.
- Sh A, Abdelrazeik AB, Rakha OM (2015) Nano-emulsion of jojoba oil, preparation, characterization and insecticidal activity against Sitophilus oryzae (Coleoptera: Curculionidae) on wheat.
- Scott IM, Jensen H, Scott JG, Isman MB, Arnason JT, et al. (2003) Botanical insecticides for controlling agricultural pests: piperamides and the Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Arch Insect Biochem Physiol 54: 212-225.
- Upadhyay RK, Jaiswal G (2007) Evaluation of biological activities of Piper nigrum oil against Tribolium castaneum. Bull Insectology 60: 57.
- Arabi M, Frankenberger JR, Engel BA, Arnold JG (2008) Representation of agricultural conservation practices with SWAT. Hydrological Processes: An International Journal 22: 3042-3055.
- Kraikrathok C, Ngamsaengi S, Bullangpoti V, Pluempanupat W, Koul O (2013) Bio efficacy of some piperaceae plant extracts against Plutella xylostella L.(Lepidoptera: Plutellidae). Communications in Agricultural and Applied Biological Sciences 78: 305-309.
- Abo-Arab RB, Helal RMY, Nadia A El-Aidy (1998) Bioresidul activity of certain oils and plant extracts on some stored grain insects in relation with quality of wheat grain. J Agric Sci Mansoura Univ 23: 5641-5653.
- Negahban M, Moharramipour S, Sefidkon F (2007) Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J Stored Prod Res 43: 123-128.
- Sahaf BZ, Moharramipour S, Meshkatalsadat MH (2007) Chemical constituents and fumigant toxicity of essential oil from Carum copticum against two stored product beetles. Insect Sci 14: 213-218.
- Sahaf BZ, Moharramipour S, Meshkatalsadat MH (2008) Fumigant toxicity of essential oil from Vitex pseudo-negundo against Tribolium castaneum (Herbst) and Sitophilus oryzae (L.). J Asia Pac Entomol 11: 175-179.
- Ogendo JO, Kostyukovsky M, Ravid U, Matasyoh JC, Deng AL, et al. (2008) Bioactivity of Ocimum gratissimum L. oil and two of its constituents against five insect pests attacking stored food products. J Stored Prod Res 44: 328-334.
- Saroukolai AT, Moharramipour S, Meshkatalsadat MH (2010) Insecticidal properties of Thymus persicus essential oil against Tribolium castaneum and Sitophilus oryzae. Journal of Pest Science 83: 3-8.
- El-Disouky M (2002) Efficacy of insecticide formulations and alternative methods against certain insects (Doctoral Dissertation, PhD Thesis in Chemistry of Pesticide, Fac Agric Alex Univ, Egypt).
- İşcan G, Ki̇ri̇mer N, Kürkcüoǧlu M, Başer HC, DEMIrci F (2002) Antimicrobial screening of Mentha piperita essential oils. J Agric Food Chem 50: 3943-3946.
- Behnam S, Farzaneh M, Ahmadzadeh M, Tehrani AS (2006) Composition and antifungal activity of essential oils of Mentha piperita and Lavendula angustifolia on post-harvest phytopathogens. Communications in Agricultural and Applied Biological Sciences 71: 1321-1326.
- Soković MD, Vukojević J, Marin PD, Brkić DD, Vajs V, et al. (2009) Chemical composition of essential oilsof thymus and mentha speciesand their antifungal activities. Molecules 14: 238-249.
- Hayes JR, Stavanja MS, Lawrence BM (2007) Biological and toxicological properties of mint oils and their major isolates: safety assessment. Mint: Genus Mentha. Taylor & Francis Group, Boca Raton, FL.
- Hussain AI, Anwar F, Nigam PS, Ashraf M, Gilani AH (2010) Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J Sci Food Agric 90: 1827-1836.
- Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A (2005) Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agric Food Chem 53: 1408-1416.
- Michaelraj S, Sharma RK (2006) Toxicity of essential oils against rice moth, Corcyra cephalonica Stainton in stored maize. Journal of Entomological Research 30: 251-254.
- Michaelraj S, Sharma K, Sharma RK (2007) Fumigation toxicity of some phyto essential oils against stored insect pests of maize. Pesticide Research Journal 19: 9-14.
- Varma J, Dubey NK (2001) Efficacy of essential oils of Caesulia axillaris and Mentha arvensis against some storage pests causing biodeterioration of food commodities. Int J Food Microbiol 68: 207-210.
- Nenaah GE (2014) Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind Crops Prod 53: 252-260.
- Pant M, Dubey S, Patanjali PK, Naik SN, Sharma S (2014) Insecticidal activity of eucalyptus oil nanoemulsion with karanja and jatropha aqueous filtrates. Int Biodeterior Biodegradation 91: 119-127.
- Oliveira AE, Duarte JL, Amado JR, Cruz RA, Rocha CF, et al. (2016) Development of a larvicidal nanoemulsion with Pterodon emarginatus Vogel Oil. PloS One 11: e0145835.
- Oliveira AP, Santana AS, Santana ED, Lima APS, Faro RR, et al. (2017) Nano-formulation prototype of the essential oil of Lippia sidoides and thymol to population management of Sitophilus zeamais (Coleoptera: Curculionidae). Ind Crops Prod, 107: 198-205.
- Choupanian M, Omar D, Basri M, Asib N (2017) Preparation and characterization of neem oil nanoemulsion formulations against Sitophilus oryzae and Tribolium castaneum adults. Journal of pesticide Science 42: 158-65.
- Mossa ATH, Abdelfattah NAH, Mohafrash SMM (2017) Nanoemulsion of Camphor (Eucalyptus globulus) Essential Oil, Formulation, Characterization and Insecticidal Activity against Wheat Weevil, Sitophilus granarius. Asian Journal of Crop Science 9: 50-62.
- Choupanian M, Dzolkhifli O (2018) Cytophilus oryzae (L., 1763) (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae) under the control of neem oil nano emulsions and physicochemical identification and formulation. Turkey Journal of Entomology 42: 127-139.
Citation: Massoud MA, Adel MM, Zaghloul OA, Mohamed MIE, Abdel-Rheim KH (2018) Eco-Friendly Nanoemulsion Formulation of Mentha piperita Against Stored Product Pest Sitophilus oryzae. Adv Crop Sci Tech 6: 404. DOI: 10.4172/2329-8863.1000404
Copyright: © 2018 Massoud MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7384
- [From(publication date): 0-2018 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 6247
- PDF downloads: 1137