Effect of Hepatitis C Virus on Erythropoiesis among Sudanese Haemodialysis Patients at Ibn-sena Hospital and Alnao Teaching Hospital
Received: 24-Oct-2018 / Accepted Date: 17-Jan-2019 / Published Date: 25-Jan-2019 DOI: 10.4172/2332-0877.1000393
Abstract
Background: Anemia is an almost constant complication of advanced renal failure, which may worsen preexisting heart disease and as a consequence accelerate the progression of renal dysfunction. Patients with hepatitis C virus (HCV) infection are associated with higher hemoglobin and hematocrit values compared with those with no HCV infection.
Objective: To assess the effect of hepatitis C virus on erythropoiesis among Sudanese haemodialysis patients at Ibn-Sina Hospital and Al-Nao Teaching Hospital in 2017.
Material and methods: Prospective analytical case control study was conducted among 121 regularly dialyzed ESRD patients; 41 as cases (HCV- Positive) and 80 as controls (HCV-Negative). All patients were subjected to full medical history and examination to identify their age, original kidney disease, duration and route of haemodialysis, number of sessions per week, average duration of each session, dose of (ESAS) for patients on treatment and dose of parental iron for patients on treatment. Hb was examined in all patients.
Results: 121 ESRD patients under regular haemodialysis participated in this study, 41 as case group (HCVPositive) and 80 as control group (HCV-Negative). No significant difference in age and gender were encountered. The mean of Hb in case group was 11.6 ± 1.2 g/dl, while in the control group was 9.3 ± 1.8 g/dl. And the difference was statistically significant (P=0.000). The majority of the case group 24 (58.5%) did not require treatment with erythropoietin; whilst the majority of control group 49 (61.3%) were using erythropoietin as 8000 weekly. The difference was statistically significant (P=0.000). The majority of the case group 34 (82.9%) did not require iron treatment; moreover, the majority of control group 39 (48.8%) were using 200mg of iron weekly. Again, the difference was statistically significant (P=0.000)
Conclusion: This study concluded that haemodialysis patients with HCV infection tend to have higher haemoglobin, and lower erythropoietin and iron requirements than the patients with no HCV infection.
Keywords: HCV; Haemodialysis; Haemoglobin
Introduction
Approximately, 170 million people worldwide are chronically infected with the hepatitis C virus (HCV) [1]. The prevalence of HCV infection in patients undergoing dialysis is persistently greater than that in the general population [2] being endemic in haemodialysis (HD) units around the world, predominantly in Mediterranean and developing countries of the Middle and Far East [3].
Nosocomial transmission of HCV infection has been reported to be a considerable route in modern hospital dialysis units, particularly during the outbreaks of infection [4]. Anaemia is the most common haematological abnormality in chronic renal failure. In the past, blood transfusion was the essential method in the treatment of renal anaemia, whereas the transfusion requirement has recently lessened by the use of erythropoietin (EPO).
Iron deficiency is frequent in patients with renal failure and iron need is increased by erythropoietin therapy; therefore, iron replacement is very important in the treatment of renal anaemia. It is known that there is a debatable relationship between iron stores and HCV infection [5,6] as it is not clear whether HCV infection facilitates iron accumulation or increased iron storage predisposes to HCV infection. Further, the influence of HCV infection upon potential iron and erythropoietin therapy is controversial despite the frequent observation of high serum ferritin level and hepatosteatosis among patients with HCV infection that may depress the response to therapy [7]. However, erythropoietin requirements and levels in HCV-positive and negative patients were reported to be different in patients with end-stage renal disease (ESRD) [8,9]. Recently published data had reported higher haemoglobin and haematocrit levels in HCV-positive compared to HCV negative HD patient
The 2012 KDIGO guidelines suggest that among patients on regular haemodialysis the haemoglobin concentration should be monitored monthly [10]. Once therapy with ESAs is initiated, serial evaluation of iron indices is necessary for early detection of iron deficiency. The KIDIGO 2012 guidelines suggest evaluating iron status at least every three months during ESAs treatment, and more frequent on other circumstances [10].
Local health care systems may have failed to deliver adequate monitoring for both haemoglobin and iron indices due to limited resources, the unacceptably high cost to the patient and their families, therefore it is not uncommon that patient receive prophylactic ESAs with or without intravenous iron unaccompanied by adequate monitoring. ESAs are expensive, not available and it has major side effects. The most common side effects aside from elevated blood pressure and its related problems are headache, flu like symptoms and pure red cell aplasia.
Although iron is essential for erythropoiesis and parenteral iron has important role in management of anaemia in ESRD patients, but chronic HCV patients have evidence of iron overload [11]. The third National Health and Nutrition Examination Survey concluded that HCV infection is associated with higher serum levels of Ferritin and iron [12]. Also, iron overload has deleterious effect on the course of HCV infection; because iron overload enhances HCV replication [13]. Again, iron overload leads to hepatic fibrosis mediated by oxidant stress, leading to further deterioration in clinical course of HCV patients [14].
Materials and Methods
This was a prospective analytical case control study undertaken in the duration between July to December 2017 at Ibn-Senna hospital centre of renal disease and transplantation and Al-Nao teaching hospital centre of renal dialysis
The study included 121 end stage renal disease patients on regular haemodialysis for at least 3 months, 41 of them are HCV positive patients labelled as case group, and 80 age matched gender matched HCV negative patients labelled as control group. Patients labelled as HCV positive if they had positive anti-hepatitis C virus antibodies on two separate occasions
All patients with history of blood transfusion, massive blood loss in the last 6 months, active malignancy, poly cystic kidney disease, HBV positive patients and patients receiving anti-viral treatment were excluded from this study
All patients were consented, subjected to full medical history examination and routine lab investigation to identify their age, gender, original kidney disease, duration and route of HD, number of sessions per week and average duration of each session, Erythropoietin and iron requirements and haemoglobin concentration
The research was approved by the ethical committee of the Sudan medical specialization board (SMSB). The data was analysed by IBM SPSS statistics 23. The Chi-Square test was used as significant test for categorical variables and T-test for continuous variables, additionally P value of less than 0.05 was considered statistically significant.
Results
A total of 121 ESRD patients under regular haemodialysis participated in this study, 41 patients as study group (HCV-Positive) and 80 patients as control group (HCV-Negative). The majority of the study group 12 (29.3%) were among the age group between 40-49 years old. While in control group 25 (31.3%) were among age group between 50-59 years old, with no significant differences (P=0.089). In the gender distribution, most of the study group 29 (70.7%) and control group 51 (63.8%) were males, with insignificant difference (P=0.288) (Table 1).
| Group | |||
|---|---|---|---|
| Case (HCV- Positive) (N= 41) | Control (HCV- Negative) (N= 80) | P value | |
| Age (Years) | |||
| 18 – 29 | 6 (14.6%) | 8 (10%) | 0.089 |
| 30 – 39 | 9 (22%) | 12 (15%) | |
| 40 – 49 | 12 (29.3%) | 12 (15%) | |
| 50 – 59 | 9 (22%) | 25 (31.3%) | |
| = 60 | 5 (12.2%) | 23 (28.8%) | |
| Gender | |||
| Male | 29 (70.7%) | 51 (63.8%) | 0.288 |
| Female | 12 (29.3%) | 29 (36.3%) | |
Table 1: Age and gender distribution among study group (HCV- Positive) and control group (HCV-Negative).
Regarding duration of haemodialysis, most of the study group 31 (75.6%) has dialysis duration more than 5 years, while the control group 54 (67.5%) has dialysis duration between 1-5 years (P=0.000) (Table 2).
| Group | |||
|---|---|---|---|
| Case (HCV- Positive) (N= 41) | Control (HCV- Negative) (N= 80) | P value | |
| Duration of HD | |||
| 3–11 months | 1 (2.4%) | 12 (15%) | 0.000 |
| 1– 5 years | 9 (22%) | 54 (67.5%) | |
| >5 years | 31 (75.6%) | 14 (17.5%) | |
| Renal failure cause | |||
| Diabetes | 3 (7.3%) | 16 (20%) | 0.144 |
| Hypertension | 15 (36.6%) | 28 (35%) | |
| Obstructive uropathy | 7 (17.1%) | 6 (7.5%) | |
| PCKD | 0 (0%) | 0 (0%) | |
| Other | 16 (39%) | 30 (37.5%) | |
Table 2: Duration of haemodialysis and comorbidities among study group (HCV- Positive) and control group (HCV-Negative).
In the comorbidities, the majority of study group 15 (36.6%) and control group 28 (35%) has hypertension.
Concerning the dialysis characteristics; the vast majority of the study group 35 (85.4%) and control group 73 (91.3%) has dialysis rate of 2 sessions per week (P=0.304). Regarding the route of dialysis; the majority of study group 38 (92.7%) and control group patients 56 (70%) were using A-V Fistula (P= 0.016) (Table 3).
| Group | |||
|---|---|---|---|
| Case (HCV- Positive) (N= 41) | Control (HCV- Negative) (N= 80) | P value | |
| Dialysis session (Rate) | |||
| One session | 0 (0%) | 0 (0%) | 0.304 |
| Two sessions | 35 (85.4%) | 73 (91.3%) | |
| Three sessions | 6 (14.6%) | 7 (8.8%) | |
| Average of dialysis time | |||
| 2 – 3 hours | 25 (61%) | 12 (15%) | 0.000 |
| 3 – 4 hours | 16 (39%) | 68 (85%) | |
| Route of dialysis | |||
| A-V Fistula | 38 (92.7%) | 56 (70%) | 0.016 |
| Temporary Catheter | 0 (0%) | 4 (5%) | |
| Semi-Permanent Catheter | 3 (7.3%) | 20 (25%) | |
Table 3: Dialysis characteristics (rate, time and route) among study group (HCV- Positive) and control group (HCV-Negative).
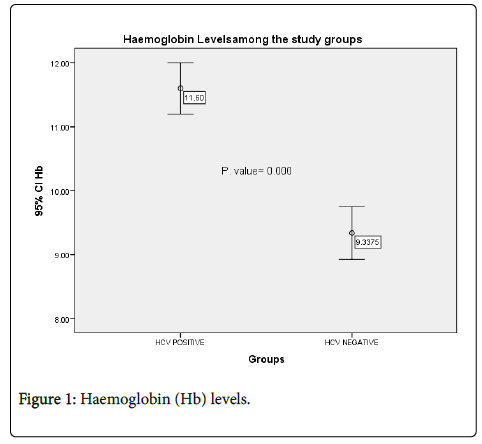
Regarding to the haemoglobin (Hb) levels; the mean of Hb in the study group was 11.6 ± 1.2 g/dl, while in the control group it was 9.3 ± 1.8 g/dl. And the difference was statistically significant (P=0.000) (Figure 1).
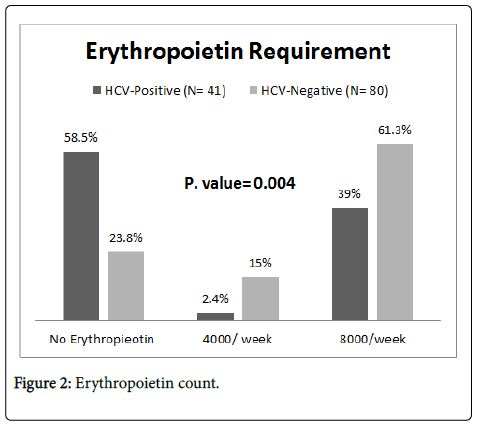
Concerning the erythropoietin requirements; the majority of the study group 24 (58.5%) did not require erythropoietin, while the majority of control group-49 (61.3%)-used erythropoietin in a dose of 8000 international units weekly. The difference was statistically significant (P= 0.004) (Figure 2).
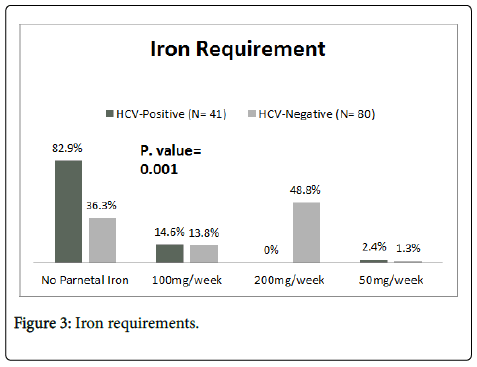
Regarding parental iron requirements; most of the study group 34 (82.9%) did not require iron; moreover, the majority of control group 39 (48.8%) used 200mg of iron dextran weekly. The difference was statistically significant (P= 0.001) (Figure 3).
Discussion
The long-term survival and good quality of patient’s life with CKD depend on many factors like haemoglobin, iron status, and bone marrow response to erythropoiesis stimulating agents (7). Patients with ESRD on maintenance haemodialysis are usually anaemic due to lack of EPO secretion from the kidney. In general, anaemia is normocytic normochromic.
Niu MT et al. followed up 499 patients in duration of eighteen months, they detected anti HCV in 10% of them, and they stated that anti HCV positivity was not related to blood transfusion, race or age [15]. Which indicates that Patients on regular haemodialysis are at higher risk of acquiring HCV infection, and consequently have a higher prevalence than general population. This is in concordance with our study, which showed that in spite of using dedicated machines, isolated areas for hepatitis C virus positive patients and strict enforcement with the universal precautions; all the patients in the study group were not infected with the virus before starting dialysis and develop the infection thereafter, making the haemodialysis an independent risk factor for acquiring the virus.
Several risk factors contribute to the high prevalence of HCV in dialysis centres. Male patients have been reported to have a higher prevalence of HCV infection than female patients in haemodialysis centres. Moreover, the male haemodialysis patients infected with the virus had a significantly higher concentration of serum HCV RNA than females; which corresponds with this study, as the majority of the study group were males (70.7%)
Natov et al. [2] concluded that the interval since the beginning of dialysis has been significantly longer among HCV positive patients. This finding is compatible with our study; compared with antihepatitis C virus negative control (17.5%), anti-hepatitis C virus positive patients (75.6%) had more than 5 years of HD duration since initiating renal replacement therapy.
Systemic hypertension appears on earlier age groups, it is generally more severe, and causes higher morbidity and mortality from cardiac stroke and end stage renal disease in patients from African origins. Data in this study was concordant with the literature as hypertension is the leading cause of end stage renal disease in both the study group (36.6%) and the control group (35%).
Interestingly, and similar to the previous studies this study demonstrated that haemodialysis patients with HCV infection tend to have higher mean haemoglobin than negative HCV group patients (11.6 ± 1.2 g/dl vs. 9.3 ± 1.8 g/dl; P=0.000).
Simon et al [4] found that patients receiving haemodialysis showed significant increase in haemoglobin level and reticulocyte count, and their blood transfusion requirement is reduced during an episode of viral or toxic liver cytolysis [16]. Also, AL Saran et al. studied eightythree patients, they found that ESRD patients on regular haemodialysis with hepatitis C virus infection have significant higher haemoglobin and haematocrit levels compared with hepatitis C virus negative patients [17].
In addition, Hefni et al. [18] found that red blood cells count, haemoglobin concentration, haematocrit values were significantly higher in haemodialysis patients with hepatitis C virus infection compared with non-infected patients.
Saifan et al. [19] concluded that patients with hepatitis C virus infection were found to have higher haemoglobin concentration and haematocrit levels.
However, Boubacar et al. [20] found that there is no correlation between severity of anaemia and hepatitis C virus infection. This disagreement might be due to variation in sample size, origins and other biological factors.
In spite of many studies and reports regarding improvement of the haemoglobin levels and reduced requirement for anaemia treatment among anti-hepatitis C virus positive patients, the mechanisms underlying this improvement are incompletely understood. It was suggested that the liver has potential to produce EPO apart from the kidneys. Thus, stimulation of hepatic EPO production has been considered as an explanation for lessened anaemia in HD patients with viral hepatitis. Sachin et al 2003 concluded from his study on 49 patients that higher Hb and HCT levels in HCV-positive compared to HCV-negative group were attributed most probably to increased production of EPO from HCV-infected patient’s liver [21].
The present study showed that patients with hepatitis C virus infection has lower erythropoietin requirements than those with no HCV (P<0.05), and this might be attributed to the higher levels of endogenous erythropoietin in infected HCV patients. These findings were agreed with the study of Altimeter et al who reported that antihepatitis C virus positive patients on haemodialysis had higher serum EPO and required less exogenous EPO [22].
Finally, the current study revealed that patients with hepatitis C virus infection have lower iron requirements than those with no HCV (P<0.05). This goes in the same line with other studies in literature [17–21].
Unfortunately, the endogenous erythropoietin level was not directly measured, which was the main limitation for the study. Additional clinical variables, such as parathyroid hormone, serum Calcium and Phosphorus and complete iron profile should be incorporated in future studies; because these variables may play a role in the responsiveness of the patient to ESAs.
Conclusion
This study concluded that haemodialysis patients with HCV infection tend to have higher haemoglobin, and lower erythropoietin and iron requirements than the patients with no HCV infection.
References
- Butt AA (2005) Hepatitis C virus infection: the new global epidemic. Expert Rev Anti Infect Ther 3: 241-249.
- Natov SN (2005) Hepatitis C virus in chronic dialysis patients. Minerva UrolNefrol 57: 175-197.
- Nguyen MH, Keeffe EB (2005) Prevalence and treatment of hepatitis C virus genotypes 4, 5, and 6. Clin Gastroenterol Hepatol 3: S97-S101.
- Savey A, Simon F, Izopet J, Lepoutre A, Fabry J, et al. (2005) Large nosocomial outbreak of hepatitis C virus infections at a hemodialysiscenter. Infect Control Hosp Epidemiol 26: 752-760.
- Silva IS, Perez RM, Oliveira PV, Cantagalo MI, Dantas E, et al. Iron overload in patients with chronic hepatitis C virus infection: clinical and histological study. J Gastroenterol Hepatol 2005;20: 243-248.
- Theurl I, Zoller H, Obrist P, Datz C, Bachmann F, et al. (2004) Iron regulates hepatitis C virus translation via stimulation of expression of translationinitiation factor 3. J Infect Dis 190: 819-825.
- Distante S, Bjoro K, Hellum KB, Myrvang B, Berg JP, et al. (2002) Raised serum ferritin predicts nonresponse to interferon and ribavirin treatment in patients with chronic hepatitis C infection. Liver 22: 187-189.
- Altintepe L, Kurtoglu E, Tonbul Z, Yeksan M, Yildiz A, et al. (2004) Lower erythropoietin and iron supplementation are required in hemodialysis patients with hepatitis C virus infection. Clin Nephrol 61: 347-351.
- Abdalla AH, Owda AK, Fedail H, Popovich WF, Mousa D, et al. (2000) Influence of hepatitis C virus infection upon parenteral iron and erythropoietin responsiveness in regular hemodialysis patients. Nephron 84:293-294.
- KDIGO clinical practice guidelines for anaemia in chronic kidney disease (2012) Kidney Int Suppl 2:288.
- Riggio O, Montagnese F, Fiore P, Folino S, Giambartolomei S, et al. (1997) Iron overload in patients with chronic viral hepatitis: how common is it? Am J Gastroenterol 92: 1298-1301.
- Shan Y, Lambrecht RW, Bonkovsky HL (2005) Association of hepatitis C virus infection with serum iron status: analysis of data from the third National Health and Nutrition Examination Survey. Clin Infect Dis (Internet) 40: 834-841.
- Kakizaki S, Takagi H, Horiguchi N, Toyoda M, Takayama H, et al. (2000) Iron enhances hepatitis C virus replication in cultured human hepatocytes. Liver 20: 125-128.
- Pietrangelo A, Gualdi R, Casalgrandi G, Montosi G, Ventura E (1995) Molecular and cellular aspects of iron-induced hepatic cirrhosis in rodents. J Clin Invest 95: 18241-1831.
- Niu MT, Coleman PJ, Alter MJ (1993) Multicenter study of hepatitis C virus infection in chronic hemodialysis patients and hemodialysiscenter staff members. Am J Kidney Dis 22: 568-573.
- Simon P, Meyrier A, Tanquerel T, Ang K (1980) Haemodialysed patients. Br Med J 892-894.
- Alsaran KA, Sabry AA, Alghareeb AH, Al Sadoon G (2009) Effect of hepatitis C virus on hemoglobin and hematocrit levels in saudihemodialysis patients. Ren Fail 31: 349-354.
- El Hefni A, Kamel Salem R, Ebian H (2015) Iron status and erythropoiesis in chronic hepatitis C patients on hemodialysis. Egypt J Haematol 40:80.
- Saifan C, El-Charabaty E, Kleiner M, El-Sayegh S (2013) Effect of hepatitis C virus infection on erythropoiesis in patients on hemodialysis. Int J NephrolRenovasc Dis 6: 121-124.
- Boubaker K, Mahfoudhi M, Battikh AG, Bounemra A (2015) Higher Endogenous Erythropoietin Levels in Hemodialysis Patients with Hepatitis C Virus Infection and Effect on Anemia. OJ Neph 5: 29-34.
- Sahin I, Arabaci F, Sahin HA, Ilhan M, Ustun Y, et al. (2003) Does hepatitis C virus infection increase hematocrit and hemoglobin levels in hemodialyzed patients. Clin Nephrol 60: 401-404.
- Altintepe L, Kurtoglu E, Tonbul Z, Yeksan M, Yildiz A, et al. (2004) Lower erythropoietin and iron supplementation are required in hemodialysis patients with hepatitis C virus infection. Clin Nephrol 61: 347-351.
Citation: Ibrahim EAA (2019) Effect of Hepatitis C Virus on Erythropoiesis among Sudanese Haemodialysis Patients at Ibn-sena Hospital and Alnao Teaching Hospital . J Infect Dis Ther 7: 393. DOI: 10.4172/2332-0877.1000393
Copyright: © 2019 Ibrahim EAA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3303
- [From(publication date): 0-2019 - Jun 28, 2025]
- Breakdown by view type
- HTML page views: 2471
- PDF downloads: 832