Efficiency of Adipose-Derived versus Bone Marrow-Derived Stem Cells in Modulation of Histopathological Changes and CD31 Immunoexpression during Wound Healing in Rats
Received: 04-Apr-2018 / Accepted Date: 17-Apr-2018 / Published Date: 25-Apr-2018
Abstract
Objective: To evaluate therapeutic effect of intradermal injection of bone marrow-derived mesenchymal (BMMSCs) versus adipose-derived (ASCs) stem cells on surgical skin wound healing.
Methods: Forty albino rats were used and classified into four groups: I (Control), II (skin wound), III (skin wound with BM-MSCs treatment) and IV (skin wound with ASCs treatment). The rats were sacrificed on 7th and 14th days from the day of wound induction and skin specimens were processed to be examined by light microscope.
Results: Examination of skin sections of groups II, III and IV after 7 days revealed discontinuity of epidermis, granulation tissue formation and cellular infiltration which were more extensive in group II. After 14 days, these findings persisted in group II, partially subsided in group III and completely subsided in group IV. Collagen fibers were increased with change in their orientation as the healing progress. The CD31 immunoexpression in endothelial cells lining dermal blood vessels was increased in group IV after 7 days with significant reduction in the same group after 14 days when compared with group II and group III.
Conclusion: Wound healing was more advanced with ASCs treatment and that could suggest ASCs as a promising therapy for wound healing without complications and further investigations are recommended to enhance the efficacy of ASCs as a therapy for tissue repair.
Keywords: Adipose stem cells; CD31; Wound; Microscope
Introduction
The goals of skin wound treatment is the enhancement of wound closure with restoration of skin function as a barrier and for infection prevention and pain suppression and hence allow rapid return to a normal professional and social life [1]. Although several strategies were tried to treat wounds, no ideal therapy is available to date and poor responses and frequent reopening could be happened due to many systemic metabolic diseases and local conditions like low vascularization. Hence, new therapies are required for optimization of wound healing outcomes [2].
Stem cells have great potential for damaged tissues replacement through their unique ability for self-renewal and undergoing differentiation and so are promising for wound treatment [3]. Mesenchymal stem cells (MSCs) are multipotent non-hematopoietic cells could be derived from bone marrow, adipose tissue, skin, fetal liver and umbilical cord blood [4,5]. MSCs were tested in clinical trials and gave promising outcomes without any apparent toxicity in patients [6].
Bone marrow-derived MSCs (BM-MSCs) were detected in the bone marrow over the entire life span of mammals without exhibiting hemopoietic markers but only regulate hematopoietic cell development [7]. Moreover, it was reported that they can differentiate into neural cells, hepatocytes, lung, kidney, skin, and the gastrointestinal tract cells [8].
BM-MSCs are identical genetically to their hosts and so; do not lead to immune reaction. However, obtaining of them by bone marrow aspiration has the disadvantages of being an invasive process [9].
Adipose-derived stem cells (ASCs) are present in perivascular region of white adipose tissue of subcutaneous fat and have the same markers as other MSC isolated from bone marrow or umbilical cord. They also share many properties with MSCs such as multipotentiality and their ability to differentiate into adipocytes, chondroblasts, osteoblatses, myoblast, fibroblasts and endothelial cells [10]. However, they are more promising to be used in treatment applications than other stem cells because they could be easily isolated in large quantities and easy to be cultured [11]. The ability of ASCs for self-renewal and tissue regeneration has great influence on proper wound healing with invisible scars and thicker skin [12]. ASCs were reported to be suitable for the treatment of wounds due to their ability to differentiate into several cell types and secrete angiogenic and anti-apoptotic factors [13].
So, the aim of the present study was to reveal therapeutic effect of intradermal injection of BM-MSCs versus ASCs on the histopathological changes during surgical skin wound healing in adult male albino rats.
Materials and Methods
Stem cells
Bone marrow derived mesenchymal stem cells (BM-MSCs) and adipose derived stem cells (ASCs) were provided from Biochemistry Department, Kasr Al-Ainy Medical School.
Bone marrow was harvested by flushing the tibiae and femurs of a 6-weeks old male rats with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. Nucleated cells were isolated with a density gradient and re-suspended in complete culture medium supplemented with 1% penicillin- streptomycin. Cells were incubated at 37°C in 5% humidified C O2 for 12-14 days, until formation of large colonies (80~90% confluence). The culture was washed with phosphate buffered saline (PBS) and released with 0.25% trypsin in 1 mM EDTA (5 min at 37°C). After centrifugation, the cells were re-suspended with serum-supplemented medium and incubated in 50 cm2 culture flask (Falcon, USA). MSCs in culture were characterized by their adhesiveness and fusiform shape [14].
ASCs were derived from the adipose tissue around upper part of the intestine of 6 weeks old male rats and washed with saline solution, incubated in Dulbecco’s modified Eagle’s medium (DMEM). The cell suspension was centrifuged and the sediments of stromal vascular fraction (SVF) were collected. Isolated nucleated cells were resuspended in complete culture medium supplemented with 1% penicillin-streptomycin. Cells were incubated for 12-14 days as primary culture or upon formation of large colonies. When large colonies developed (80-90% confluence), cultures were washed twice with phosphate buffer saline (PBS) and cells were trypsinized with 0.25% trypsin in 1 mM (EDTA) for 5 minutes at 37°C. After centrifugation, cells were re-suspended with serum-supplemented medium and incubated in 50 cm2 culture flask (Falcon, USA). On day 14, the adherent colonies of cells were trypsinized, and counted [15].
Experimental animals
Forty adult healthy male albino rats (180-200 g body weight) were obtained from breading animal house, Faculty of Medicine, Zagazig University. The animals were maintained in accordance with the guidelines of stem cell research unit in the central laboratory, Zagazig University. Throughout the duration of the experiment, the rats were housed at room temperature with normal light/dark cycles and were allowed ad-libitum access to food and water. The experiment was carried out in compliance with the “Guide of the Care and Use of Laboratory Animals” [16]. Experimental protocols were approved by the ethical committee of the Faculty of Medicine, Zagazig University. One week after acclimatization, rats were randomly divided into four equal groups.
Group I (Control): The rats were equally subdivided into two subgroups (five rats each); subgroup Ia (negative control), the animals received no treatment and subgroup Ib, the animals were injected intradermally on the middle of the right dorsal side with 0.5 ml of phosphate-buffered saline (PBS), the vehicle of stem cells [1].
Group II (Skin wound): The rats were anesthetized using an intramuscular injection of ketamine and xylazine, at dose of 40 and 5 mg/kg body weight, respectively. The surgical area was shaved with an electric razor and disinfected using 70% ethanol. After a deep surgical plane of general anesthesia had been reached, a square of wound (1 cm2) were performed on the middle of the right dorsal side under standard sterile conditions. Both the epidermal and dermal layers were removed down to the subcutaneous connective tissue, creating a fullthickness wound [17].
Group III (Skin wound with BM-MSCs treatment): The rats were underwent surgical wound as group II then injected with BM-MSCs extracted from male albino rats once at a dose of 1×106 cells per rat suspended in 0.5 ml of PBS [18]. They were injected intradermally immediately after wound induction using Short Needle Insulin Syringe at cross shaped 4 injection sites in the margin of the surgical skin wound.
Group IV (Skin wound with ASCs treatment): The rats were underwent surgical wound as group II then injected with ASCs once at a dose of 1×106 cells per rat suspended in 0.5 ml of PBS [19]. They were injected intradermally immediately after wound induction using Short Needle Insulin Syringe at 4 cross shaped injection sites in the margin of the surgical skin wound.
The rats of the experimental groups (II, III and IV) received Penicillin (5 mg/kg intramuscular) and anti-inflammatory Meloxicam (0.2 mg/kg intramuscular) for three consecutive days after wound induction. Their wounds were covered with a non-adhering dressing allowing wound to heal and the bandages were replaced every other day [20]. The rats were housed individually to avoid infection or further damage to wound by the others. The state of wounds was observed to detect any oozing or infection was every day. The rats of experimental groups (II, III and IV) were sacrificed on 7th and 14th days of wound induction. The animals of control group were sacrificed at the same time with the corresponding experimental groups. Both control skin and skin wound specimens were taken with the same depth.
Histological and immunohistochemical study
Skin specimens (1 cm3) were fixed in in 10% formol saline for 48 hours and then processed to prepare paraffin blocks. Thick sections (5 μm) were prepared and stained by hematoxylin and eosin (H&E) and Mallory trichrome stains [21]. Immunohistochemical study was done for localization of CD31 in endothelial cells lining proliferating blood vessels. The deparaffinized sections on charged slides were used using avidin–biotin-complex (ABC) immunoperoxidase technique. The sections were incubated in hydrogen peroxide for 10 min to block the endogenous peroxidase then incubated with the primary anti-CD31 antibody at 1:5000 dilutions for 30 min at room temperature. The primary anti-body used was a mouse monoclonal antibody with cytoplasmic reaction specific for CD31 obtained from Lab Vision Corporation (Cat. #MS-1873-R7). Then the slides were washed with phosphate buffer then incubated with the secondary anti-mouse antibodies universal kits obtained from Zymed Corporation. Staining was completed by incubation with substrate chromogen DAB (3,3- diaminobenzidine) which resulted in brown-colored precipitate at the antigen sites and Mayer’s hematoxylin was used as a counter stain. Positive control was lung tissue. Negative control sections were prepared using PBS with-out using the primary antibody [22].
Morphometric study
The image analyzer computer system Leica Qwin at Pathology Department, Faculty of Dentistry, Cairo University was used to evaluate the following parameters in 10 consecutive fields from each rat in randomly chosen five animals of each group. Using anti-CD31 immunostained sections, the mean area percentage of positive immunoexpression for CD31 was measured at a magnification 400X. They were measured using the color detect menu and in relation to a standard measuring frame.
Using Mallory trichrom stained sections; the mean area percentage of collagen was measured. Three images per rat of each group were captured at 100X magnification. The ImageJ software was used to evaluate the percentage of blue staining (collagen) which was measured as the percentage of total pixels in each image using the “Threshold color”.
Statistical analysis
Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences) version 24.
Data was summarized using median and interquartile range. Comparisons between quantitative variables were done using the nonparametric Kruskal-Wallis and Mann-Whitney tests. P-values less than 0.05 were considered as statistically significant.
Results
Histological and immunohistochemical results
Examination of skin sections of subgroups Ia and Ib were similar and did not show any histological variations, therefore, results of subgroup Ia were represented as the control group (I).
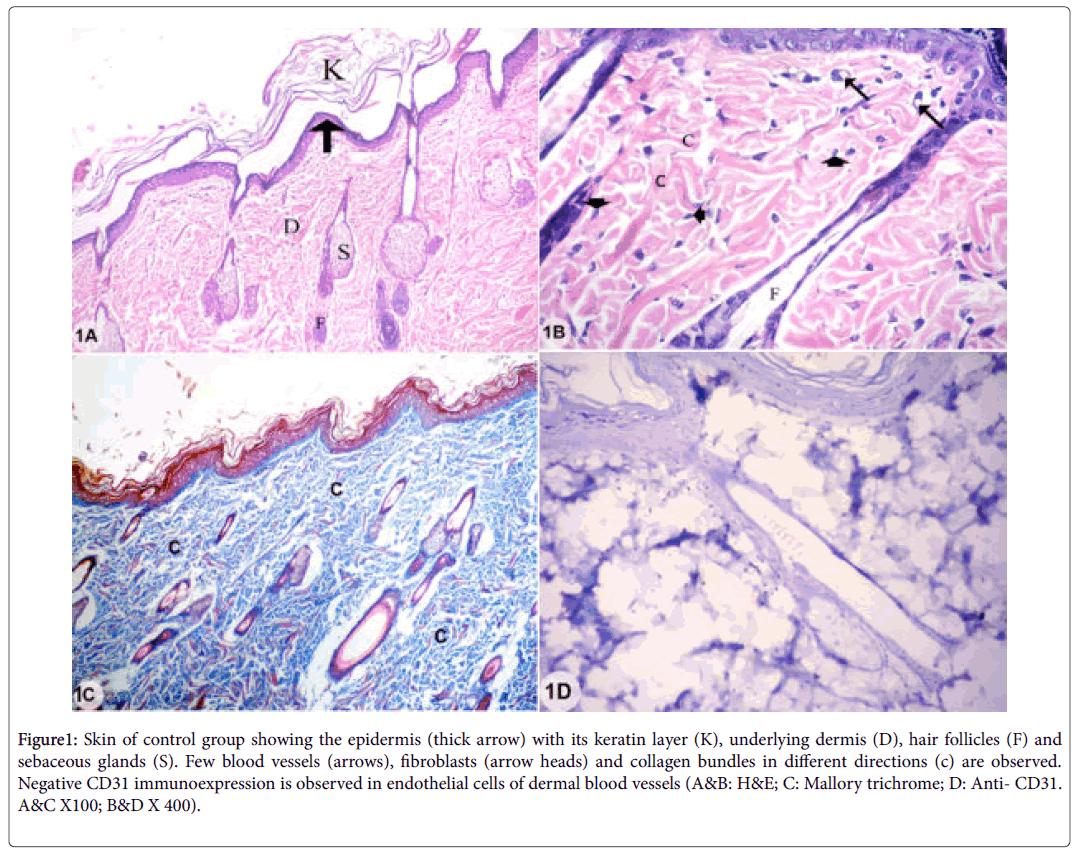
Examination of skin sections of control group showed the two layers of the skin; epidermis and dermis. The epidermis was formed of stratified squamous keratinized epithelium. The dermis consisted of connective tissue with collagen bundles in different directions, few blood vessels, fibroblasts, hair follicles and sebaceous glands. The endothelial cells lining dermal blood vessels showed negative CD31 immunoexpression (Figures 1A-1D).
Figure 1: Skin of control group showing the epidermis (thick arrow) with its keratin layer (K), underlying dermis (D), hair follicles (F) and sebaceous glands (S). Few blood vessels (arrows), fibroblasts (arrow heads) and collagen bundles in different directions (c) are observed. Negative CD31 immunoexpression is observed in endothelial cells of dermal blood vessels (A&B: H&E; C: Mallory trichrome; D: Anti- CD31. A&C X100; B&D X 400).
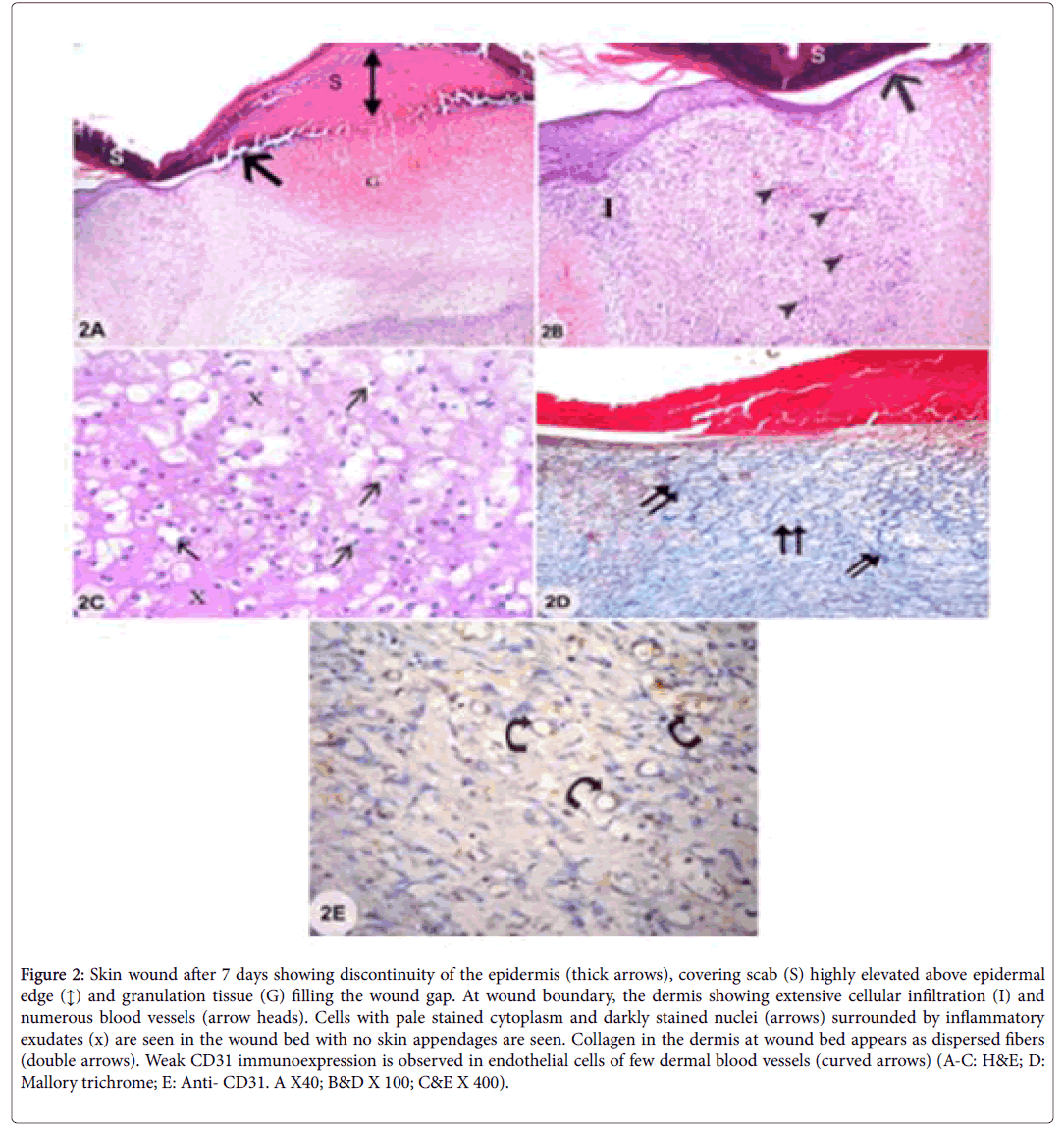
Examination of sections from skin wound after 7 days showed discontinuity of the epidermis at wound site which was covered by highly elevated skin scab above epidermal surface and the wound gap was filled with granulation tissue. The regenerating dermis at the wound bed contained cells with pale stained cytoplasm and small dark nuclei and surrounded by inflammatory exudates. The dermis at the boundaries of the wound showed the signs of inflammation in the form of extensive cellular infiltrations and numerous blood vessels. Hair follicles and sebaceous glands were absent. The dermis was filled with dispersed collagen fibers at the wound bed and showed weak CD31 immunoexpression of endothelial cells lining few of the blood vessels (Figures 2A-2E).
Figure2: Skin wound after 7 days showing discontinuity of the epidermis (thick arrows), covering scab (S) highly elevated above epidermal edge (↕) and granulation tissue (G) filling the wound gap. At wound boundary, the dermis showing extensive cellular infiltration (I) and numerous blood vessels (arrow heads). Cells with pale stained cytoplasm and darkly stained nuclei (arrows) surrounded by inflammatory exudates (x) are seen in the wound bed with no skin appendages are seen. Collagen in the dermis at wound bed appears as dispersed fibers (double arrows). Weak CD31 immunoexpression is observed in endothelial cells of few dermal blood vessels (curved arrows) (A-C: H&E; D: Mallory trichrome; E: Anti- CD31. A X40; B&D X 100; C&E X 400).
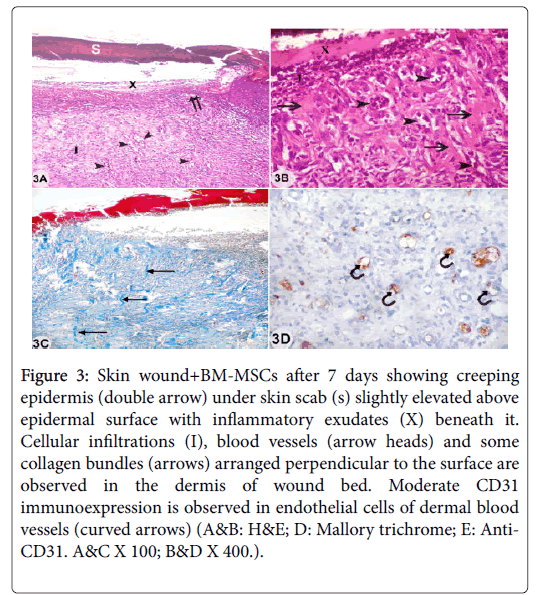
Examination of sections from skin wound with bone marrow derived mesenchymal stem cells treatment (wound+BM-MSCs) after 7 days showed discontinuity of epidermis with starting of epidermal creeping to cover wound gap. The covering skin scab was slightly elevated above epidermal surface with inflammatory exudates under it. Dense cellular infiltrations and blood vessels were detected in the regenerating dermis at wound bed with absence of hair follicles and sebaceous glands. Some collagen bundles arranged perpendicular to the surface and moderate CD31 immunoexpression of endothelial cells lining some dermal blood vessels were observed (Figures 3A-3E).
Figure 3: Skin wound+BM-MSCs after 7 days showing creeping epidermis (double arrow) under skin scab (s) slightly elevated above epidermal surface with inflammatory exudates (X) beneath it. Cellular infiltrations (I), blood vessels (arrow heads) and some collagen bundles (arrows) arranged perpendicular to the surface are observed in the dermis of wound bed. Moderate CD31 immunoexpression is observed in endothelial cells of dermal blood vessels (curved arrows) (A&B: H&E; D: Mallory trichrome; E: Anti- CD31. A&C X 100; B&D X 400.).
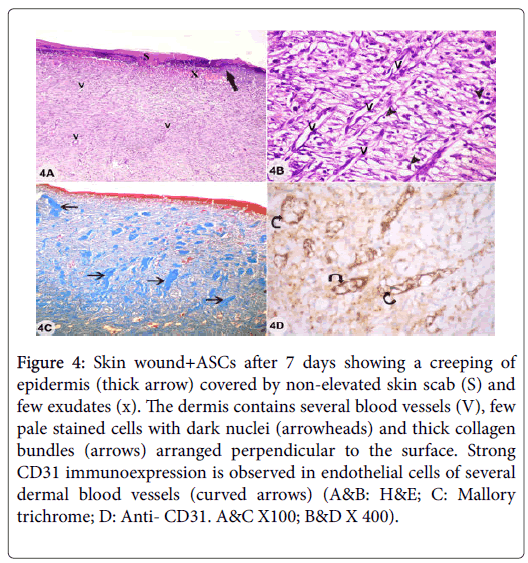
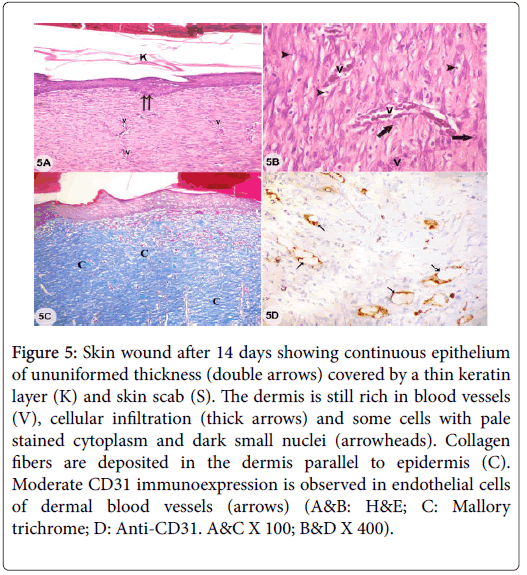
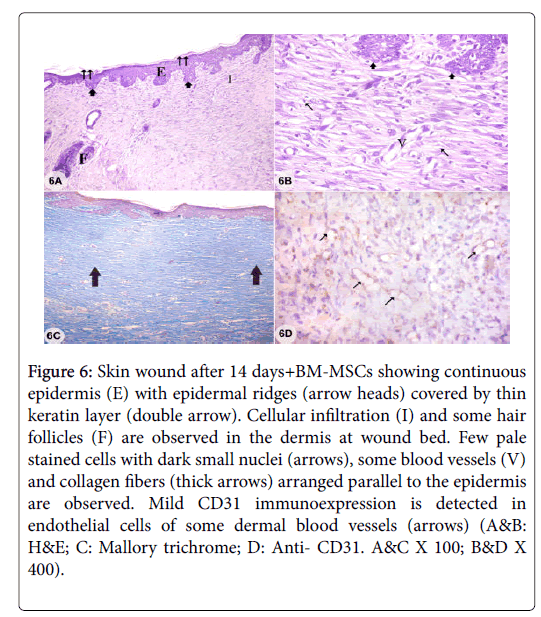
Examination of sections from skin wound with adipose derived stem cells treatment (wound+ASCs) after 7 days showed creeping of epidermis under skin scab which was non-elevated above epidermal surface with inflammatory exudates under it. Dense cellular infiltrations and several blood vessels were seen in the dermis with absence of hair follicles and sebaceous glands. Thick collagen bundles arranged perpendicular to the surface and strong CD31 immunoexpression in endothelial cells of several dermal blood vessels were observed (Figures 4A-4E). Examination of sections from skin wound after 14 days showed continuous epithelium of ununiformed thickness and covered by scab and a thin keratin layer. The dermis appeared with persistent numerous blood vessels and cellular infiltrations. Some cells had pale stained cytoplasm and dark shrunken nuclei with deposition of collagen fibers parallel to epidermal surface and the skin appendages were still absent. Moderate CD31 immunoexpression was observed in endothelial cells of some dermal blood vessels (Figures 5A-5D). Examination of sections from skin wound+BM-MSCs after 14 days showed continuous epidermis with epidermal ridges and thin keratin covering layer. The dermis showed cellular infiltration with few pale stained cells with dark small nuclei and some blood vessels were still detected. The hair follicles started to reappear and collagen fibers were arranged in dermis parallel to epidermis. Mild CD31 immunoexpression was observed in endothelial cells of some dermal blood vessels (Figures 6A-6D).
Figure 4: Skin wound+ASCs after 7 days showing a creeping of epidermis (thick arrow) covered by non-elevated skin scab (S) and few exudates (x). The dermis contains several blood vessels (V), few pale stained cells with dark nuclei (arrowheads) and thick collagen bundles (arrows) arranged perpendicular to the surface. Strong CD31 immunoexpression is observed in endothelial cells of several dermal blood vessels (curved arrows) (A&B: H&E; C: Mallory trichrome; D: Anti- CD31. A&C X100; B&D X 400).
Figure 5: Skin wound after 14 days showing continuous epithelium of ununiformed thickness (double arrows) covered by a thin keratin layer (K) and skin scab (S). The dermis is still rich in blood vessels (V), cellular infiltration (thick arrows) and some cells with pale stained cytoplasm and dark small nuclei (arrowheads). Collagen fibers are deposited in the dermis parallel to epidermis (C). Moderate CD31 immunoexpression is observed in endothelial cells of dermal blood vessels (arrows) (A&B: H&E; C: Mallory trichrome; D: Anti-CD31. A&C X 100; B&D X 400).
Figure 6: Skin wound after 14 days+BM-MSCs showing continuous epidermis (E) with epidermal ridges (arrow heads) covered by thin keratin layer (double arrow). Cellular infiltration (I) and some hair follicles (F) are observed in the dermis at wound bed. Few pale stained cells with dark small nuclei (arrows), some blood vessels (V) and collagen fibers (thick arrows) arranged parallel to the epidermis are observed. Mild CD31 immunoexpression is detected in endothelial cells of some dermal blood vessels (arrows) (A&B: H&E; C: Mallory trichrome; D: Anti- CD31. A&C X 100; B&D X 400).
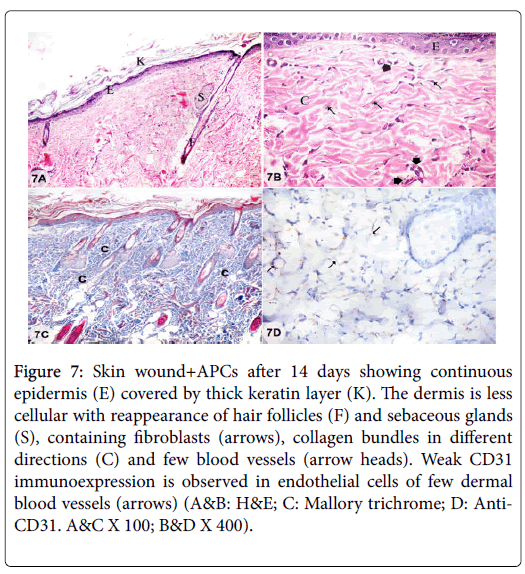
Examination of sections from skin wound+ASCs treatment after 14 days showed continuous epidermis covered by thick keratin layer. The dermis appeared less cellular as the cellular infiltration was greatly subsided with presence of few fibroblasts and few blood vessels. Collagen bundles appeared in different directions with reappearance of skin appendages. Weak CD31 immunoexpression was observed in endothelial cells of few dermal blood vessels (Figures 7A-7D).
Figure 7: Skin wound+APCs after 14 days showing continuous epidermis (E) covered by thick keratin layer (K). The dermis is less cellular with reappearance of hair follicles (F) and sebaceous glands (S), containing fibroblasts (arrows), collagen bundles in different directions (C) and few blood vessels (arrow heads). Weak CD31 immunoexpression is observed in endothelial cells of few dermal blood vessels (arrows) (A&B: H&E; C: Mallory trichrome; D: Anti- CD31. A&C X 100; B&D X 400).
Morphometrical and statistical results
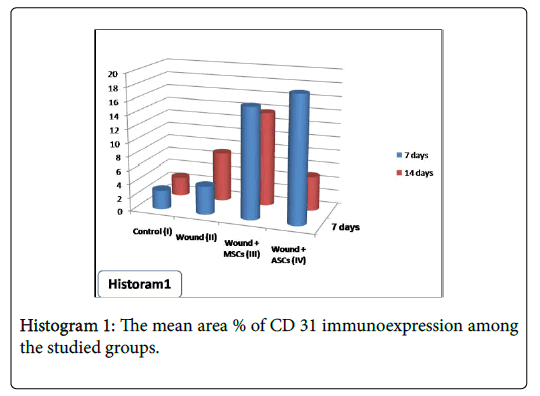
Statistical analysis of the of the mean area percentage of CD31 immunoexpression, as an indicator of proliferating new blood vessels, showed statistical differences among studied groups. After 7 days of wound induction, significant increase in CD31 immunoexpression was detected in group IV (wound+ASCs) when compared with other studied groups. Significant reduction in CD31 immunoexpression was observed in group II (wound) when compared with both group III (wound+BM-MSCs) and group IV (wound+ASCs). After 14 days, significant reduction in CD31 immunoexpression was observed in group IV (wound+ASCs) when compared with both group II (wound) and group III (wound+BM-MSCs) (Table 1 and Histogram 1).
| Groups | Control Group (I) Mean ± SD (n=10) |
Wound Group (II) Mean ± SD (n=10) |
Wound+BM-MSCs Group (III) Mean ± SD (n=10) |
Wound+ASCs Group (IV) Mean ± SD (n=10) |
|||
|---|---|---|---|---|---|---|---|
| After 7 d | After 14 d | After 7 d | After 14 d | After 7 d | After 14 d | ||
| Area % of CD31 | 2.80 ± 0.30 | 4.16 ± 0.30a | 7.13 ± 0.22 | 16.12 ± 0.15 | 13.79 ± 0.30a | 18.37 ± 0. 44a | 5.04 ± 1. 41* b |
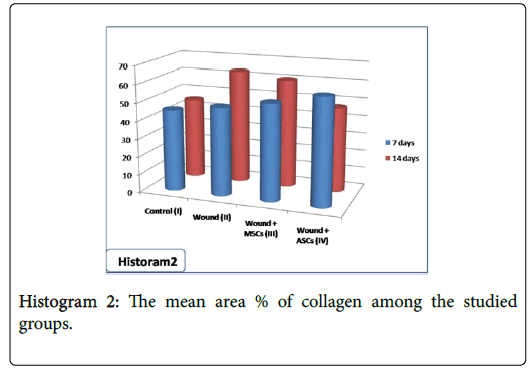
| Area % of collagen | 45.4 ±2.31 | 49.1 ±4.32 | 63.3 ±9.15a | 53.5 ±4.59 | 60.3 ±6.45a | 59.3 ±5.42a | 47.2 ±3.05b |
| aSignificant change (P<0.05) among studied groups. bSignificant change (P<0.05) when compared with group II&II). SD=Standard deviation; N=Number of animals; d=days. |
|||||||
Table 1: Statistical analysis of area % of CD31 immunoexpression and area % of collagen fibers among the studied groups.
After 7 days, statistical analysis of the mean area % of collagen showed significant increase in group IV (wound+ASCs) when compared with other studied groups. After 14 days, the mean area % of collagen showed significant increase in both group II (wound) and group III (wound+BM-MSCs) when compared other studied groups. However, significant reduction in collagen was observed in group IV (wound+ASCs) when compared with both group II (wound) and group III (wound+BM-MSCs) (Table 1 and Histogram 2).
Discussion
Skin wound is a health care problem as normal wound healing remains imperfect even under optimal conditions with failing to resume skin structure and function [23]. In the present work, the choice of MSCs treatment for wound healing was attributed to their reported ability of migration to sites of injury without immune reaction and ethically, MSCs use is not hindered, in contrast to the use of human embryonic stem cells [3,24]. The choice of ASCs, in the present study, was owing to their reported advantage of being easy isolated in large quantities, easy to be cultured and amplified and hence has more potential for treatment applications [11].
In the current work, after 7 days of wound induction, light microscopic examination of H&E stained wound sections of groups II, III and IV revealed discontinuity of epidermis, granulation tissue formation, skin scab and inflammatory infiltrate. In the same context, the inflammation phase was described to involve increased vascular permeability, migration of blood cells to wound site and plasma oozing into injured tissue [25]. This inflammatory process was reported to be important to start wound healing process and its suppression, as in cases of diabetes mellitus, caused abnormal healing [26].
However, in the current study, after 7 days, the granulation tissue and cellular infiltration were more extensive in group II than in both groups III and IV. That is in consistence with the suggested antiinflammatory effect of MSCs and particularly of ASCs [27]. That was explained by previous investigators who attributed this to their paracrine effect with secretion of trophic factors that lead to reprogramming of inflammatory macrophages from type 1 into an anti-inflammatory macrophages type II that modulating inflammation [3,28]. Moreover, enhanced expression of the anti-inflammatory protein, tumor necrosis factor, was observed in the lung tissue after MSCs trapping [29].
In the current study, apoptotic cells with pyknotic darkly stained nuclei were observed in granulation tissue at wound bed. Similarly, apoptosis of inflammatory cells was observed during first few days of wound healing and was suggested to be important to end the process of inflammation and initiate healing phase [30]. On the other hand, failure of apoptosis during healing was reported to lead to pathological repair with hypertrophic scar and keloid formation with hypervascularity and hypercellularity [31].
After 14 days of wound induction, some of the granulation tissue and the signs of inflammation persisted in group II and partially subsided in group III and completely subsided in group IV. Despite the importance of inflammatory reactions in wound healing process, the persistence of them could be considered as a bad sign in wound healing process as that may impair healing of the wound with formation of chronic unhealed wounds [32]. Hence, in the present work, the subsidence of cellular infiltration, after 14 days of wound induction in group IV could be considered as a good sign of wound healing.
In the present study, after 14 days, the wound of groups III and IV showed appearance of epidermal ridges and reappearance of some skin appendages that couldn’t be detected in the group II. Hence, these findings could be considered as a sign of proper healing of epidermis. In the same context, epidermal ridges were suggested to be taken as an indicator for full differentiation of epidermis as these ridges are essential to strength epidermal-dermal junction against shear stress and to prevent penetration by pathogens [33].
Examination of Mallory trichrome stained sections of skin wound after 7 days, showed collagen in the dermis of wound bed as dispersed fibers in group (II) and as bundles perpendicular to the surface in both III and IV groups which were thicker in group IV. These findings were in the same context with Kwon and his colleagues who stated that systemic and local injection of BM-MSCs caused significant increase in collagen production with improvement of wound healing in diabetic rats [24]. So, they suggested that collagen, as a major component of extracellular matrix, is crucial for tissue repair after injuries. In addition, collagen deposition defects were reported to be accompanied with delayed wound healing in diabetic patients [32]. These findings were explained by Xue and his colleagues who stated that MSCs could provide early signal for dermal fibroblast to start healing of skin wound and also have ability to differentiate into fibroblasts [34]. After 14 days, collagen bundles in group IV deposited in different direction more or less resembling that of control group. However, in II and III groups, the collagen failed to form bundles in different directions and appeared as fibers parallel to the surface. That could be attributed to the reported ability of ASCs to express extracellular matrix proteins including several types of collagen with formation of collagen type I network [35]. In the same context, the BM-MSCs treated wound showed thin collagen (type III) deposition with parallel orientation to skin surface and then after the third week, was replaced by thick collagen (type I) deposition in different directions with stress planes in a manner like normal skin [36]. In addition, during the late regenerative phase of wound healing, the fibroblasts were reported to undergo phenotypic alteration into myofibroblasts to begin wound contraction [37].
In the current work, skin wound after 7 days showed significant reduction in CD31 immunoexpression in endothelial cells lining dermal blood vessels in group II although the presence of several blood vessels within the granulation tissue and wound bed boundaries. However, in III and IV groups, there was early increase in CD31 immunoexpression denoting increased angiogenesis. That is in agreement with previous investigators who stated that BM-MSCs transplantation increases capillary angiogenesis which is critical for wound healing and skin grafting success as it restore vascular perfusion and deliver nutrients to the wound [38,39].
Hence, wound angiogenesis regulation could be used to improve healing particularly in cases of healing impairment [40]. The role of BM-MSCs in skin repair was attributed to their angiogenic effect through secretion of several arteriogenic cytokines such as vascular endothelial growth factor (VEGF), angiopoietin, apoptotic cells elimination and through enhancement of the endothelial cells proliferation and differentiation [18,41,42]. In the same context, MSCs were reported to differentiate into endothelial cells and release proangiogenic molecules attracting inflammatory and progenitor cells to provide microenvironment suitable for neoangiogenesis and subsequent wound healing. Hence, enhancement of angiogenic capacity of BM-MSCs was reported to provide simple and efficient therapy for the wounds with large defects [43]. In the present study, angiogenesis after 7 days was significantly high in wound with ASCs treatment when compared with wound with BM-MSCs treatment as denoted by significant increase in CD31 immunoexpression. Similarly, allo-ASCs transplantation was observed to induce neovascularization better than auto-BM-MSCs transplantation [44]. Also, ASCs was suggested to accelerate healing of skin ulcers as they differentiate into endothelial cells and through secretion of angiogenic factors [13,45,46]. In addition, ASCs were suggested as an inducer for angiogenesis in treatment of human limb ischemia [47]. This therapeutic effect was suggested to be attributed to microvesicles released from them [48]. However, after 14 days, the CD31 immunoexpression in the present work was significantly reduced in group IV when compared with that of groups II and III. That is in agreement with Basiouny and his colleagues who observed that vascular endothelial growth factor (VEGF) stimulated angiogenesis about 7 days after wounding and then showed reduction later on due to diminished inducers of VEGF such as hypoxia and accordingly, VEGF and neoangiogenesis decline again after wound healing [36].
Collectively, wound healing processes was more advanced, in the present work, with ASCs treatment than with BM-MSCs treatment. In same context, ASCs were reported to have ability to reduce wound healing time in rats within 7 days [49]. Similar results were observed after intradermal injection of allogenic BM-MSCs into linear wounds incision in rats [50]. Although both ASCs and BM-MSCs have improving effect on wound healing, ASCs have the advantage of being more available in adequate amount and have less invasive methods to be obtained [51]. In addition, in burn injury, where BM-MSCs were suppressed, ASCs were reported to be more benefit in wound healing especially when daily application is needed as ASCs were unaffected by burn injury or culturing procedures [11,52]. Also, ASCs injection was suggested to be a promising nonsurgical treatment for acute anal sphincter injury [53].
Conclusion
Wound healing processes was more advanced with ASCs treatment, more than with BM-MSCs treatment, as revealed by earlier restoration of skin appendages, normal collagen bundles pattern and earlier angiogenesis as indicated by increased CD31 immunoexpression which then decreased as wound healing progress. That could suggesting ASCs as a promising therapy for wound healing without keloids or scars besides their more adequacy, more availability and less invasive methods to be obtained. Further investigations are recommended to confirm and enhance the efficacy of ASCs to be used as a cell-based therapy for tissue repair.
Conflict of Interest
The authors have no conflicting financial interest.
Funding Source
No funding source
References
- Rodriguez J, Boucher F, Lequeux C, Josset-Lamaugarny A, Rouyer O, et al. (2015) Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Res Ther 6: 241.
- Leavitt T, Hu MS, Marshall CD, Barnes LA, Lorenz HP, et al. (2016) Scarless wound healing: Finding the right cells and signals. Cell Tissue Res 365: 483-493.
- Augello A, Kurth TB, De Bari C (2010) Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater 20:121-133.
- Silva JD, Lopes-Pacheco M, Paz AHR, Cruz FF, Melo EB, et al. (2018) Mesenchymal stem cells from bone marrow, adipose tissue, and lung tissue differentially mitigate lung and distal organ damage in experimental acute respiratory distress syndrome. Crit Care Med 46: e132-e140.
- Fu X, Li H (2009) Mesenchymal stem cells and skin wound repair and regeneration: Possibilities and questions. Cell Tissue Res 335: 317-321.
- Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, et al. (2005) Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 11: 389-398.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8: 315-317.
- Herzog EL, Chai L, Krause DS (2003) Plasticity of marrow-derived stem cells. Blood 102: 3483-3493.
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, et al. (2003) Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116: 1827-1835.
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, et al. (2003) Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 17: 101-119.
- Sterodimas A, De Faria J, Nicaretta B, Pitanguy I (2010) Tissue engineering with adipose-derived stem cells (ADSCs): Current and future applications. J Plast Reconstr Aesthet Surg 63: 1886-1892.
- Tabit CJ, Slack GC, Fan K, Wan DC, Bradley JP, et al. (2012) Fat grafting versus adipose-derived stem cell therapy: Distinguishing indications, techniques, and outcomes. Aesthetic Plast Surg 36: 704-713.
- Nie C, Yang D, Xu J, Si Z, Jin X, et al. (2011) Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 20: 205-216.
- Alhadlaq A, Mao JJ (2004) Mesenchymal stem cells: Isolation and therapeutics. Stem Cells Dev 13: 436-448.
- Takehara Y, Yabuuchi A, Ezoe K, Kuroda T, Yamadera R, et al. (2013) The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest 93: 181-193.
- Borena BM, Pawde A, Amarpal HP, Kinjavdekar P, Singh R, et al. (2010) Evaluation of autologous bone marrow-derived nucleated cells for healing of full-thickness skin wounds in rabbits. Int Wound J 7: 249-260.
- Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25: 2648-2659.
- Lim JS, Yoo G (2010) Effects of adipose-derived stromal cells and of their extract on wound healing in a mouse model. J Korean Med Sci 25: 746-751.
- Alsarra IA (2009) Chitosan topical gel formulation in the management of burn wounds. Int J Biol Macromol 45: 16-21.
- Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, London: Churchill Livingstone, New York.
- Kiernan JA (1999) Histological and histochemical methods: Theory and practice, Butterworth-Heinemann, Oxford.
- Hocking AM, Gibran NS (2010) Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 316: 2213-2219.
- Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, et al. (2008) Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 5: 453-463.
- Maslinska D, Gajewski M (1998) Some aspects of the inflammatory process. Folia Neuropathol 36: 199-204.
- Deveci M, Gilmont RR, Dunham WR, Mudge BP, Smith DJ, et al. (2005) Glutathione enhances fibroblasts collagen contraction and protects keratinocytes from apoptosis in hyperglycaemic culture. Br J Dermatol 152: 217-224.
- Zhang J, Huang X, Wang H, Liu X, Zhang T, et al. Â (2015) The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther 6: 234.
- Manferdini C, Maumus M, Gabusi E, Piacentini A, Filardo G, et al. (2013) Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum 65: 1271-1281.
- Sun YQ, Deng MX, He J, Zeng QX, Wen W, et al. (2012) Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cell 30: 2692-2699.
- Wu Y, Chen S (2014) Apoptotic cell: Linkage of inflammation and wound healing. Front Pharmacol 5: 1.
- Huang NF, Zac-Varghese S, Luke S (2003) Apoptosis in Skin Wound Healing. Wounds 15: 182-194.
- MartÃnez-SantamarÃa L, Conti CJ, Llames S, GarcÃa E, Retamosa L, et al. (2013) The regenerative potential of fibroblasts in a new diabetes-induced delayed humanised wound healing model. Exp Derm 22: 195-201.
- Jinnestal CL, Belfrage E, Back O, Schmidtchen A, Sonesson A, et al. (2014) Skin barrier impairment correlates with cutaneous Staphylococcus aureus colonization and sensitization to skin-associated microbial antigens in adult patients with atopic dermatitis. Int J Dermatol 53: 27-33.
- Xue SN, Lei J, Yang C, Lin DZ, Yan L, et al. (2012) The biological behaviors of rat dermal fibroblasts can be inhibited by high levels of MMP9. Exp Diabetes Res 2012: 494579.
- Tsuji W, Rubin JP, Marra KG (2014) Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells 26: 312-321.
- Basiouny HS, Salama NM, Maadawi ZM, Farag EA (2013) Effect of bone marrow derived mesenchymal stem cells on healing of induced full-thickness skin wounds in albino rat. Int J Stem Cells 6: 12-25.
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, et al. (2013) Distinct fibroblast lineages determine dermal architecture in skin development and repair Nature 504: 277-281.
- Sheng L, Yang M, Li H, Du Z, Yang Y, et al. (2011) Transplantation of adipose stromal cells promotes neovascularization of random skin flaps. Tohoku J Exp Med 224: 229-234.
- Hacker S, Mittermayr R, Nickl S, Haider T, Lebherz-Eichinger D, et al. (2016) Paracrine factors from irradiated peripheral blood mononuclear cells improve skin regeneration and angiogenesis in a porcine burn model. Sci Rep 6: 25168.
- Zhao S, Li L, Wang H, Zhang Y, Cheng X, et al. (2015) Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 53: 379-391
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, et al. (2004) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109: 1543-1549.
- Uchiyama A, Motegi SI, Sekiguchi A, Fujiwara C, Perera B, et al. (2017) Mesenchymal stem cells-derived MFG-E8 accelerates diabetic cutaneous wound healing. J Dermatol Sci 86: 187-197.
- Shou K, Niu Y, Zheng X, Ma Z, Jian C, et al. (2017) Enhancement of bone-marrow-derived mesenchymal stem cell angiogenic capacity by npwt for a combinatorial therapy to promote wound healing with large defect. Biomed Res Int 7920265
- Rigol M, Solanes N, Roura S, Roque M, Novensa L, et al. (2014) Allogeneic adipose stem cell therapy in acute myocardial infarction. Eur J Clin Invest 44: 83-92.
- Huang SP, Hsu CC, Chang SC, Wang CH, Deng SC, et al. (2012) Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann Plast Surg 69: 656-662.
- Kuo YR, Wang CT, Cheng JT, Kao GS, Chiang YC, et al. (2016) Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant 25: 71-81
- Lee HC, An SG, Lee HW, Park JS, Cha KS, et al. (2012) Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J 76: 1750-1760.
- Zhao L, Takerra J, Liu D (2017) Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther 8: 125.
- Ohashi CM, Caldeira FA, Feitosa-Junior DJ, Lopes VA, Witter DR, et al. (2016) Stem cells from adipose tissue improve the time of wound healing in rats. Acta Cir Bras 31: 821-825.
- Fang F, Huang RL, Zheng Y, Liu M, Huo R, et al. (2016) Bone marrow derived mesenchymal stem cells inhibit the proliferative and profibrotic phenotype of hypertrophic scar fibroblasts and keloid fibroblasts through paracrine signaling. J Dermatol Sci 83: 95-105.
- Zannettino AC, Paton S, Arthur A, Khor F, Itescu S (2008) Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol 214: 413-421.
- Prasai A, El Ayadi A, Mifflin RC, Wetzel MD, Andersen CR, et al. (2017) Characterization of adipose-derived stem cells following burn injury. Stem Cell Rev Rep 13: 781-792.
- Kuismanen K, Juntunen M, Narra Girish N, Tuominen H, Huhtala H, et al. (2018) Functional outcome of human adipose stem cell injections in rat anal sphincter acute injury model. Stem Cells Transl Med 7: 295-304.
Citation: Hashem HE , Mobasher MOI, Mohamed MZ, Alkhodary AAM (2018) Efficiency of Adipose-Derived versus Bone Marrow-Derived Stem Cells in Modulation of Histopathological Changes and CD31 Immunoexpression during Wound Healing in Rats. J Biochem Cell Biol 1: 106.
Copyright: © 2018 Hashem HE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 5445
- [From(publication date): 0-2018 - Dec 18, 2025]
- Breakdown by view type
- HTML page views: 4405
- PDF downloads: 1040