Research Article Open Access
Efficiency of In Vitro Regeneration is Dependent on the Genotype and Size of Explant in Tef [Eragrostis tef (Zucc.) Trotter]
| Zerihun Tadele*, Sonia Plaza-Wüthrich and Regula Blösch | |
| Institute of Plant Sciences, University of Bern, Altenbergrain 21, 3013 Bern, Switzerland | |
| Corresponding Author : | Zerihun Tadele Institute of Plant Sciences University of Bern, Altenbergrain 21 3013 Bern, Switzerland Tel: +41 31 631 4956 Fax: +41 31 631 4942 E-mail: zerihun.tadele@ips.unibe.ch |
| Received July 01, 2015; Accepted July 29, 2015; Published August 04, 2015 | |
| Citation: Tadele Z, Wüthrich SP, Blösch R (2015) Efficiency of In Vitro Regeneration is Dependent on the Genotype and Size of Explant in Tef [Eragrostis tef (Zucc.) Trotter]. Adv Crop Sci Tech 3:179. doi:10.4172/2329-8863.1000179 | |
| Copyright: © 2015 Tadele Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Tef [Eragrostis tef (Zucc.) Trotter] is the major cereal crop in the Horn of Africa particularly in Ethiopia where it is staple food for about 50 million people. Its resilience to extreme environmental conditions and high in nutrition makes tef the preferred crop among both farmers and consumers. The efficiency of in vitro regeneration plays significant role in the improvement of crops. We investigated the efficiency of regeneration in 18 tef genotypes (15 landraces and three improved varieties) using three sizes of immature embryos (small, intermediate and large) as an explant. In vitro regeneration was significantly affected by the genotype and the size of the immature embryo used as a donor. Intermediate-size immature embryos which were 101-350 µm long led to the highest percentage of regeneration. Interestingly, the three improved varieties presented very low regeneration efficiencies whereas the landrace Manyi resulted in consistently superior percentage of in vitro regeneration from all three sizes of explants. The findings of this work provide useful insight into the tef germplasm amenable for the regeneration technique which has direct application in techniques such as transformation. It also signifies the importance of using tef landraces instead of improved varieties for in vitro regeneration.
| Keywords |
| Eragrostis tef; Immature embryo; Plantlet; Regeneration; Embryogenesis; tef |
| Introduction |
| Tef [Eragrostis tef (Zucc.)Trotter] is a cereal crop extensively cultivated in the Horn of Africa where it is annually cultivated on about 3 million hectares of land in Ethiopia alone [1]. This extensive cultivation of the crop is related to some traits beneficial for farmers and consumers including, i) its tolerance to extreme environmental biotic and abiotic conditions, ii) its gluten-free seeds, hence considered as a healthy food, and iii) high palatability of its straw by livestock. Despite all these useful traits, tef is considered as an orphan crop due to the little scientific research done on the crop. As a result, the crop remains largely unimproved which is associated with poor productivity lodging or displacement of the plant from its upright position due to wind and rain is the major cause for inferior yield in tef [2]. Tef has a very tall and weak stem which falls on the ground due to wind and rain. The majority of research on tef improvement has been done at the Ethiopian Institute of Agricultural Research where conventional techniques of selection and hybridization are widely implemented to release 35 improved varieties which are suited to diverse agro-ecological regions [3]. The widely cultivated and popular variety called Quncho was developed by the intra-specific crossing between two improved cultivars [4]. The recently published tef genome [5] will accelerate the breeding program if integrated with improvement methods such as tissue culture and genetic transformation. |
| Tissue culture or also commonly known as in vitro regeneration plays a key role in crop improvement. In addition to its significant contribution in genetic transformation of plants, tissue culture is also usefulin developing large-scale clonal propagation of genotypes of interest and producing and propagating disease-free plants [6]. The somaclonal variations induced in the tissue culture are also source of variability in plant breeding [7,8]. The percentage of initial explants converted to plantlets or whole plants referred to the culture efficiency of regeneration. This efficiency is mainly affected by the genotypes and explants. The presence of a strong genetic effect was reported for Arabidopsis [9], wheat [10-12] and rice [13]. Considerable differences in regeneration ability were observed among four Arabidopsis ecotypes, namely Columbia, Landsbergerecta, Cape Verde Island and Wassilewskija based on the source of explant and composition of the culture medium [9]. In wheat, embryogenic capacity or number of somatic embryos formed from cultured immature embryos was mainly altered by the genotype whereby the best performing cultivars scored 1.4-1.8 plants/ explant [10]. |
| The source and size of explant affects the efficiency of regeneration. The study in malting barley called Morex showed that smaller embryos (0.5-1.5 mm) had higher regeneration efficiency than larger embryos (1.6- 3.0 mm) [14]. Similarly, in Sudan grass (Sorghum sudanense Piper)smaller immature embryos (0.7-1.5 mm) were better than larger ones (1.6-2.5 mm) in the speed and frequency of callus and shoot formation [15]. |
| Diverse in vitro regeneration techniques were studied for tef. The explants used for these investigations were seedlings, roots, and leaves [16,17], seeds [18], immature spikelets or panicles [19,20], and immature embryos [21] in which the latter resulted in substantially high percentage of regeneration. However, since earlier study using immature embryo was made on only two tef genotypes, it did not represent the existing tef germplasm with huge variations. Hence, the present study was made to investigate the efficiencies of in vitro regeneration in 18 tef genotypes with diverse morphological and agronomic properties [22]. |
| Materials and Methods |
| Plant material |
| Fifteen selected landraces and three improved varieties of tef were used. The 15 landraces were obtained from the National Plant Germplasm System (NPGS) of the United States Department of Agriculture [23]. They are: Ada (NPGS accession number: 524433), Addisie (524434), Alba (524435), Balami (524436), Beten (524437), Dabbi (524438), Enatite (524439), GeaLamie (524440), Gommadie (524441), Karadebi (524442), Manyi (524443), Red dabi (524457), Rosea (524444), TulluNasy (524445) and Variegata (524446) while the three improved varieties were Dukem (DZ-01-974), Magna (DZ-01- 196), and Tsedey (DZ-Cr-37). Donor plants were grown for three weeks under long-day conditions (16 h light, 8 h darkat 21+/- 1°C) before plantlets were transferred to short day conditions (8 h light, 16 h dark at 20+/-2°C). The soil used consisted of 5/11 parts of topsoil, 4/11parts of turf and 2/11parts of quartz sand. Plants were fertilized once a week with Hauert Plantaktiv 16-6-26 N-P-K (Hauert HBG Dünger Schweiz, Grossaffoltern, Switzerland). |
| Embryo isolation and in vitro regeneration |
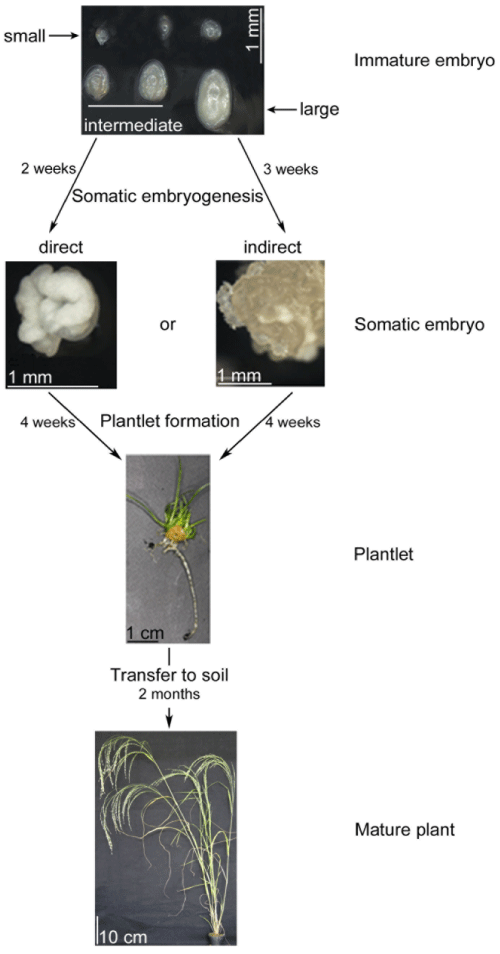
| The procedure for isolating immature embryos from panicles was based on earlier work [21]. Panicles were surface sterilized for 10 minutes with 1% HCl followed by three washings with sterile water. Immature embryos were separated from the sterilized caryopses by squeezing them out through an incision made at its base. Three different sizes of embryos were selected: small and globular (50-100 μm), intermediate (101-350 μm) and large embryos (351-750 μm) (Figure 1). Another distinction between the small and intermediate embryos was the loss of the globular shape in the intermediate ones. Large embryos were extracted from solid endosperm. Thirty immature embryos were plated on 3.5 cm diameter petri dishes containing K99 medium [24] placing the scutellum facing up and incubating in the dark at 25°C ± 2°C. The K99 media contains 90 g/l maltose, 1 g/l glutamine and 2 mg/l of 2,4-D. After two to three weeks in the dark, somatic embryos were transferred to K4NB medium [25] containing 36 g/l maltose, 0.15 g/l glutamine and 0.22 mg/l BAP. The pH of the medium was adjusted to 5.8 where 0.4% phytagel was used as a gelling agent. Plantlets were regenerated under 14-hour photoperiodic conditions for 4 weeks with a sub-culture to fresh medium after 2 weeks. The growth conditions consist of a relative humidity of 50% all day-long and a temperature of 21 ± 2°C during the dark and 25 ± 2°C during the light. The photon luminosity was set to 70 μmol/m2 s during the light period. Plantlets with well-developed root systems were transferred to soil and grown under the same conditions than for the donor plants (see above). After three weeks of hardening in long-day conditions, plants were transferred to short-day room for the production of seeds. Five replicates each containing 30 immature embryos were tested for each tef cultivar and size of the explant (Figure 1). |
| The efficiencies in somatic embryogenesis, regeneration and culture were enumerated as follows: |
| Embryogenesis efficiency, direct (%)=number of embryos/number of explants × 100 |
| Embryogenesis efficiency, indirect (%)=number of callus/number of explants × 100 |
| Regeneration efficiency, direct (%)=number of plantlets/number of embryos × 100 |
| Regeneration efficiency, indirect (%)=number of plantlets/number of callus × 100 |
| Culture efficiency=number of plantlets/number of explants × 100 [26]. |
| Determination of morphological, phenotypic and yield related traits |
| Morphological traits including numbers of tillers, panicles and internodes, and lengths of culm, panicle and the second culm internode (starting from the base of the plant) were determined at the flowering time. The length of second culm internode was earlier reported to determine the lodging tolerance [27]. Days to heading or flowering and days to maturity and the grain filling period were also determined. Days to heading was defined as the number of days from sowing until the first flower appeared and days to maturity was the number of days for all the grains of a panicle to mature. The time between flower emergence and grain maturity was defined as grain filling period. At harvesting time, shoot biomass was separated into culms and panicles and dried for 24 hours at 60°C in order to determine the dry weight of all culms and panicles. Harvest index was calculated as the ratio of the grain yield to the shoot biomass. |
| Statistical analysis |
| Statistical analysis was performed using SPSS Statistical 17.0 (IBM, Chicago, IL). Non-parametric tests were chosen as it is appropriate for the number of replicates used and the non-homogeneity of the variance in order to compare differences between the treatments (p ≤ 0.05). For K independent samples, Kruskal-Wallis tests were used, whereas for two independent samples, Mann-Whitney U tests were employed. For correlation analysis, the Pearson correlation test was used on the mean values of selected traits among efficiencies of embryogenesis, regeneration, and culture. |
| Results |
| In vitro regeneration |
| Steps and the timeline from excising the three sizes of immature embryos to the two paths of somatic embryogenesis, plantlet formation and to finally grown on soil to full maturity are shown in Figure 1. The efficiency of somatic embryogenesis was determined for the three sizes of immature embryos used as an explant as well as for the two types of embryos formed. While the three groups of immature embryos were small (50-100 μm), intermediate (101-350 μm) and large (351- 750 μm), the two types of embryos were those formed directly from the immature embryos and indirectly through callus. Somatic embryos made from immature embryos can be either directly without passing an intermediate callus forming step or indirectly through callus. While direct embryogenesis took two weeks once the embryos were placed on the appropriate media, the indirect embryogenesis took an additional week. The whole procedure starting from embryo isolation to fully mature plants on soil takes 12-13 weeks or about 3 months. |
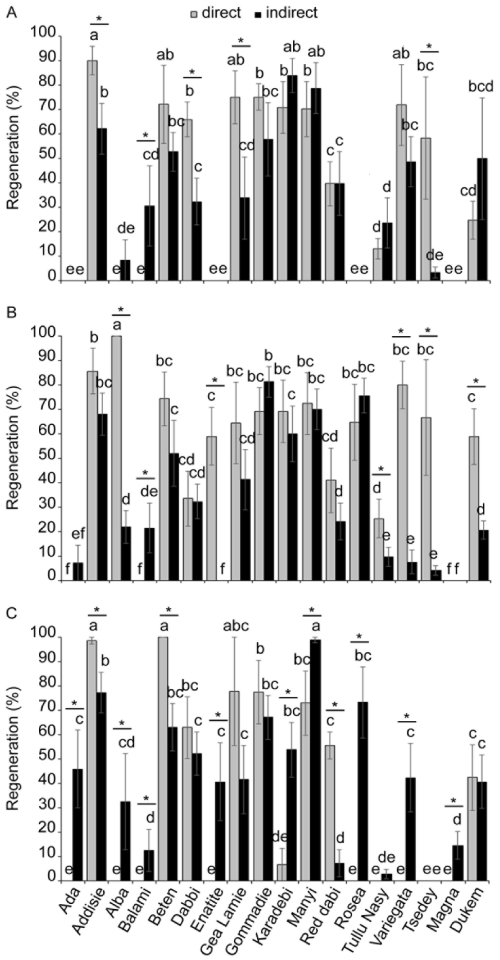
| A high diversity was found in the somatic embryogenesis depending on the size of the explant and the type of the tef ecotype. While intermediate-size immature embryos resulted in high percentage of somatic embryogenesis, only low proportion of small explants formed somatic embryos (Figure 2). Regarding small immature embryos, the efficiency of somatic embryos form edvaried from less than 10% in Ada, Enatite, Rosea, and the two improved varieties (Magna and Dukem) to more than 70% in Manyi (Figure 2A). Surprisingly, small-size explants from the landrace Rosea did not produce any somatic embryos. The proportions of embryos formed through direct and indirect somatic embryogenesis were similar except in Gommadie where significantly higher percentage was formed through direct somatic embryogenesis. In the case of intermediate immature embryos, the percentage of somatic embryogenes is ranged from less than 20% for Ada to around 90% for Gommadie and Manyi (Figure 2B). Unlike small-size embryos which formed the direct or indirect somatic embryogenesis in a similar proportion, in the intermediate-size embryos, the indirect embryogenesis was dominant over the direct one. This favor to the indirect embryogenesis was significant in six landraces, namely Alba, Enatite, GeaLamie, Karadebi, Rosea andVariegataas well as all the three improved varieties. For large immature embryos used as explants, the percentage of somatic embryogenesis varied from less than 20% in Balami, Rosea and Magna to more than 65% in Addisie, Gommadie and Many (Figure 2C). Similar to the intermediate-size embryos, the proportion of indirect embryogenesis was significantly higher than the direct ones for large-size explants except in Gea Lamie land race where the difference between direct and indirect embryogenesis was negligible. |
| The efficiency of regeneration which refers to the proportion of somatic embryos that result in plantlet formation were quantified for the three types of explants (small, intermediate and large) and the two forms of somatic embryos (direct and indirect) (Figure 3). Substantial variability in regeneration capacity was observed among the ecotypes, sources of explants and forms of embryos. Using small explants, four genotypes, namely Ada, Dabi, Rosea and Magna, did not form any plantlet while Alba and Balami did not regenerate from indirectly formed embryos (Figure 3A). However, over 70% of regeneration was obtained for Karadebi and Manyi. Regeneration efficiencies were variable between direct and indirect embryogenesis in each genotype although significantly higher values were obtained for directly formed embryos. The proportion of plantlets formed from intermediate-size immature embryosranged from less than 10% in Ada, Balami, Enatite and Magna (direct embryogenesis)as well as in Ada, Variegata, Tsedey and Magna (indirect embryogenesis) to more than 70% inAddisie, Beten, Manyi and Variegata (direct embryogenesis), andGommadie, Manyi and Rosea (indirect embryogenesis) (Figure 3B). Some inconsistencies were observed in some genotypes between the capacity of plantlet formation and somatic embryogenesis. For instance, in Alba cultivar, although the percentage of direct embryogenesis from the intermediate-size embryo was only 2% (Figure 2), the plantlet formation reached 100% for same size of embryos (Figure 3) indicating high capacity of plantlet formation from extremely low rate of somatic embryo development. Regarding the regeneration capacity from large embryos, variations revealed in the other two embryo sizes were also observed among different genotypes (Figure 3C). Three landraces (Ada and Beten from direct embryogenesis, and Manyifrom indirect embryogenesis) gave exceptionally high plantlet formation. |
| Culture efficiency which refers to proportions of initial immature embryos resulting in plantlets was also investigated. Significant variability was observed among the tef genotypes and the sizes of immature embryos used as an explant (Table 1). The performance of some landraces was high when small starting material was used while in others large explants gave superior results. The large explants from Addise, Gea Lamie and Manyi had extremely high efficiency. Although inconsistencies in performance were observed for the three sizes of embryos, Manyi gave exceptionally high efficiency for all sizes of embryos. Irrespective of the size of immature embryos, all three improved tef varieties performed extremely poor indicating their low application in regeneration and transformation related studies. The main reason for the culture efficiencies above 100% is due to the friable nature of somatic embryos which resulted in the generation of several viable pieces generated from a single somatic embryo. |
| Correlations among steps of in vitro regeneration |
| Pearson correlation coefficients were used to investigate the relationship among the three parameters of regeneration (embryogenesis-, regeneration- and culture-efficiency) and three sizes of immature embryos used as an explant (small, intermediate and large). Very significant and positive correlations (p<0.01) were observed among the three parameters and three sizes of immature embryos as well as their interaction (Table 2). The only non-significant correlation (p=0.108) was between the embryogenesis from the small explant and the regeneration from the large explant. |
| Determination of morphological, phenotypic and yield related traits |
| The existence of substantial variability in diverse morphological and phenotypic traits were earlier reported for the same 18 tef ecotypes derived from seed [22,28]. In Table 3, comparisons were made for selected traits between those obtained from the seed [22] and those developed through in vitro regeneration (the current study). Tef lines generated through in vitro method were more robust than those from seeds for key morphological traits especially in the numbers of tillers and numbers of panicles per plant which have positive impact on the productivity of the crop. While most landraces derived from seeds hada maximum of two tillers per plant, those from the in vitro had up to seven tillers per plant. Astonishingly, three landraces (namely, Alba, Balami and Variegata) which did not develop a single tiller when generated from seeds were able to form 3-5 tillers when generated in vitro. Number of panicles which is also dependent on the form of the panicle is normally low for plants with compact panicles (e.g. Gommadie) and high for those with loose panicle (e.g. GeaLamie). Compared to those developed from seeds, up to 3-fold increase in the number of panicles was obtained for those from in vitro regenerated plants. Although substantial variability was obtained among the tef genotypes for the number of internodes per plant, culm length and plant height, differences between those generated from seeds and those from in vitro were not obvious for the three traits. |
| Significant diversity was also observed for phenotypic and yield related traits among the 18 tef genotypes generated from the seed or from in vitro method (Table 4). However, the differences between those from seed and those from immature embryos were inconsistent for most of the traits. Amazingly, all the three improved tef varieties developed from immature embryos were superior over those from the seed for all traits investigated. This positive effect from tissue cultured plants was revealed on grain yield (up to 8-fold) and harvest index (up to 3-fold) over those from seed. Although improved varieties were mainly selected based on high grain yield and harvest index, the three improved varieties in the current study were inferior to some land races for these two valuable traits. |
| Discussion |
| The diversity in regeneration capacity among tef genotypes indicates the existence of a genetic control of this process which differs among diverse tef lines as earlier reported for Arabidopsis [9] and rice [29]. This variation among genotypes might be related to the level of endogenous hormones [30] or the effect of exogenous growth regulators on the level of endogenous hormones through influencing their biosynthesis and distribution [31], which subsequently alters the in vitro regeneration responses [32-34]. Interestingly, all three improved tef varieties used in the current study had extremely poor regeneration capacity. This was mainly due to the development of tef varieties in the past focused on selecting genotypes with superior grain yield without considering their in vitro regeneration efficiency. |
| The size and/or age of an explant is also responsible for controlling the frequency and speed of regeneration in plants. Although not a single albino plant or plantlet was observed in the current study, up to 50% of shoots of barley derived from large immature embryos were albinos unlike those from small embryos [14]. The absence of albinos in tef plants developed through in vitro regeneration increases the acceptability of the technique by researchers as it has positive impact on the viability and productivity of the crop. Large immature embryos of tef tend to initially form a callus before differentiating into somatic embryo. This indirect somatic embryogenesis requires an additional one week to the direct embryogenesis to form somatic embryos. This makes large embryos less favorable to use as an explant. As earlier reported for Sudan grass and tef, large embryos are more determined to germinate than to produce a callus [15,21]. The strong positive correlation among the three steps of the in vitro regeneration suggests the existence of general internal/genetic mechanism which controls the response of a particular genotype to the tissue culture, irrespective of the size of the explant or the step of the in vitro regeneration process. This means, a highly-responsive genotype performs better than a low responsive one for all sizes of explant and steps of the tissue culture. In winter wheat, only culture efficiency tended to increase as regeneration capacity was enhanced [35]. |
| The comparison between the 18 tef genotypes developed from the seed [22] and those generated via the tissue culture (the current study) revealed significant differences for major morphological, phenotypic and yield related traits. Plants developed through in vitro regeneration were more vigorous and productive than those from seed especially in the number of tillers and panicles. |
| Since tissue culture or in vitro regeneration could result in useful stable somaclonal variations, thorough investigation of progenies needs to be done as some of the changes could be used in developing tef cultivars with improved traits. Using somaclonal variation, potato cultivar with reduced plant height, and sorghum and rice varieties tolerant to drought were developed [36-38]. |
| In conclusion, based on the regeneration efficiency, the intermediate-size explants are the best. Among the germplasms, the Manyi landrace provides the highest culture efficiency. Since the three improved tef varieties were inferior in the efficiency of regeneration, they are not suggested for use especially with the technique and type of the explant indicated in the current study. |
| Acknowledgements |
| Financial and technical support was provided by Syngenta Foundation for Sustainable Agriculture, SystemsX.ch and University of Bern. We would like to thank Christopher Ball and Jasmin Sekulovski for taking care of our plants. |
| References |
References
- value="1" id="Reference_Titile_Link">CSA (2014) Agricultural Sample Survey for 2013/14. Addis Ababa, Ethiopia.
- value="2" id="Reference_Titile_Link">Assefa K, Yu JK, Zeid M, Belay G, Tefera H, et al. (2011) Breeding tef [Eragrostis tef (Zucc.) trotter]: conventional and molecular approaches. Plant Breeding 130: 1-9.
- value="3" id="Reference_Titile_Link">MoA (2014) Crop Variety Register Issue No. 15. Addis Ababa, Ethiopia.
- value="4" id="Reference_Titile_Link">Assefa K, Aliye S, Belay G, Metaferia G, Tefera H, et al. (2011) Quncho: the first popular tef variety in Ethiopia. International Journal of Agricultural Sustainability 9: 25-34.
- value="5" id="Reference_Titile_Link">Cannarozzi G, Plaza-Wuthrich S, Esfeld K, Larti S, Wilson YS, et al. (2014) Genome and transcriptome sequencing identifies breeding targets in the orphan crop tef (Eragrostis tef). BMC genomics 15:581.
- value="6" id="Reference_Titile_Link">Garcia-Gonzales R, Quiroz K, Carrasco B, Caligari P (2010) Plant tissue culture: Current status, opportunities and challenges. Ciencia E Investigacion Agraria 37: 5-30.
- value="7" id="Reference_Titile_Link">Larkin PJ, Scowcroft WR (1981) Somaclonal Variation - a Novel Source of Variability from Cell-Cultures for Plant Improvement. Theoretical and Applied Genetics 60: 197-214.
- value="8" id="Reference_Titile_Link">Jain SM (2001) Tissue culture-derived variation in crop improvement. Euphytica 118: 153-166.
- value="9" id="Reference_Titile_Link">Candela M, Velazquez I, De la Cruz R, Sendino AM, De la Pena A (2001) Differences in in vitro plant regeneration ability among four Arabidopsis thaliana ecotypes. In Vitro Cellular & Developmental Biology-Plant 37: 638-643.
- value="10" id="Reference_Titile_Link">Haliloglu K, Baenziger PS (2005) Screening wheat genotypes for high callus induction and regeneration capability from immature embryo cultures. Journal of Plant Biochemistry and Biotechnology 14: 155-160.
- value="11" id="Reference_Titile_Link">Dodig D, Zoric M, Mitic N, Nikolic R, Surlan-Momirovic G (2008) Tissue culture and agronomic traits relationship in wheat. Plant Cell Tissue and Organ Culture 95: 107-114.
- value="12" id="Reference_Titile_Link">Maddock SE, Lancaster VA, Risiott R, Franklin J (1983) Plant-Regeneration from Cultured Immature Embryos and Inflorescences of 25 Cultivars of Wheat (Triticum-Aestivum). Journal of Experimental Botany 34: 915-926.
- value="13" id="Reference_Titile_Link">Ge XJ, Chu ZH, Lin YJ, Wang SP (2006) A tissue culture system for different germplasms of indica rice. Plant cell reports 25: 392-402.
- value="14" id="Reference_Titile_Link">Chang Y, von Zitzewitz J, Hayes PM, Chen THH (2003) High frequency plant regeneration from immature embryos of an elite barley cultivar (Hordeum vulgare L. cv. Morex). Plant cell reports 21: 733-738.
- value="15" id="Reference_Titile_Link">Gupta S, Khanna VK, Singh R, Garg GK (2004) Identification of in vitro responsive immature embryo size for plant regeneration in Sudan grass (Sorghum sudanenses Piper). Indian Journal of Biotechnology 3: 124-127.
- value="16" id="Reference_Titile_Link">Bekele E, Klock G, Zimmermann U (1995) Somatic embryogenesis and plant regeneration from leaf and root explants and from seeds of Eragrostis tef (Gramineae). Hereditas 123: 183-189.
- value="17" id="Reference_Titile_Link">Mekbib F, Mantell SH, BuchananWollaston V (1997) Callus induction and in vitro regeneration of Tef [Eragrostis tef (Zucc.) Trotter] from leaf. Journal of Plant Physiology 151: 368-372.
- value="18" id="Reference_Titile_Link">Kebebew A, Gaj MD, Maluszynski M (1998) Somatic embryogenesis and plant regeneration in callus culture of tef, Eragrostis tef (Zucc.) Trotter. Plant cell reports 18: 154-158.
- value="19" id="Reference_Titile_Link">Tefera H, Chapman GP (1992) Invitro Normal and Variant Development of Tef (Eragrostis-Tef) Spikelets. Plant Cell Tissue and Organ Culture 31: 233-237.
- value="20" id="Reference_Titile_Link">Gugsa L, Sarial AK, Lorz H, Kumlehn J (2006) Gynogenic plant regeneration from unpollinated flower explants of Eragrostis tef (Zuccagni) Trotter. Plant cell reports 25: 1287-1293.
- value="21" id="Reference_Titile_Link">Gugsa L, Kumlehn J (2011) Somatic Embryogenesis and Massive Shoot Regeneration from Immature Embry Explants of Tef. Biotechnology Research International: 7.
- value="22" id="Reference_Titile_Link">Plaza-Wüthrich S, Cannarozzi G, Tadele Z (2013) Genetic and phenotypic diversity in selected genotypes of tef [Eragrostis tef (Zucc.)] Trotter. African Journal of Agricultural Research 8: 1041-1049.
- value="23" id="Reference_Titile_Link">USDA A, National Genetic Resources Program. Germplasm Resources Information Network - (GRIN). [Online Database] National Germplasm Resources Laboratory, Beltsville, Maryland.
- value="24" id="Reference_Titile_Link">Deutsch F, Kumlehn J, Ziegenhagen B, Fladung M (2004) Stable haploid poplar callus lines from immature pollen culture. Physiologia Plantarum 120: 613-622.
- value="25" id="Reference_Titile_Link">Kumlehn J, Serazetdinova L, Hensel G, Becker D, Loerz H (2006) Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with Agrobacterium tumefasciens. Plant Biotechnology Journal 4: 251-261.
- value="26" id="Reference_Titile_Link">Zale JM, Borchardt-Wier H, Kidwell KK, Steber CM (2004) Callus induction and plant regeneration from mature embryos of a diverse set of wheat genotypes. Plant Cell Tissue and Organ Culture 76: 277-281.
- value="27" id="Reference_Titile_Link">Hundera F, Nelson LA, Baenziger PS, Bechere E, Tefera H (2000) Association of lodging and some morpho-agronomic traits in tef [Eragrostis tef (Zucc.) Trotter]. Tropical Agriculture 77: 169-173.
- value="28" id="Reference_Titile_Link">Ebba T, editor (1975) Tef cultivars. Part II. Dire Dawa, Ethiopia: Addis Ababa University, College of Agriculture.
- value="29" id="Reference_Titile_Link">Khalequzzaman M, Haq MD, Hoque E, Aditya TL (2005) Regeneration efficiency and genotypic effect of 15 indica type Bangladeshi rice (Oryza sativa L.) landraces. Plant Tissue Cult 15: 33-42.
- value="30" id="Reference_Titile_Link">Hess JR, Carman JG (1998) Embryogenic competence of immature wheat embryos: Genotype, donor plant environment, and endogenous hormone levels. Crop Science 38: 249-253.
- value="31" id="Reference_Titile_Link">Kaminek M, Motyka V, Vankova R (1997) Regulation of cytokinin content in plant cells. Physiologia Plantarum 101: 689-700.
- value="32" id="Reference_Titile_Link">Valdes AE, Ordas RJ, Fernandez B, Centeno ML (2001) Relationships between hormonal contents and the organogenic response in Pinus pinea cotyledons. Plant Physiology and Biochemistry 39: 377-384.
- value="33" id="Reference_Titile_Link">Klems M, Slamova Z, Motyka V, Malbeck J, Travnickova A et al. (2011) Changes in cytokinin levels and metabolism in tobacco (Nicotiana tabacum L.) explants during in vitro shoot organogenesis induced by trans-zeatin and dihydrozeatin. Plant Growth Regulation 65: 427-437.
- value="34" id="Reference_Titile_Link">Yasmin S, Mensuali-Sodi A, Perata P, Pucciariello C (2014) Ethylene influences in vitro regeneration frequency in the FR13A rice harbouring the SUB1A gene. Plant Growth Regulation 72: 97-103.
- value="35" id="Reference_Titile_Link">Ozgen M, Turet M, Altinok S, Sancak C (1998) Efficient callus induction and plant regeneration from mature embryo culture of winter wheat (Triticum aestivum L.) genotypes. Plant cell reports 18: 331-335.
- value="36" id="Reference_Titile_Link">Duncan RR, Waskom RM, Nabors MW (1995) In-Vitro Screening and Field-Evaluation of Tissue-Culture-Regenerated Sorghum (Sorghum-Bicolor (L) Moench) for Soil Stress Tolerance. Euphytica 3: 373-380.
- value="37" id="Reference_Titile_Link">Adkins SW, Kunanuvatchaidach R, Godwin ID (1995) Somaclonal viariation in rice-drought tolerance and other agronomic characters. Aust J Bot 43: 201-209.
- value="38" id="Reference_Titile_Link">Valkonen JPT, Moritz T, Watanabe KN, Rokka VM (1999) Dwarf (di)haploid pito mutants obtained from a tetraploid potato cultivar (Solanum tuberosum subsp tuberosum) via anther culture are defective in gibberellin biosynthesis. Plant Science 149: 51-57.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 15688
- [From(publication date):
August-2015 - Sep 02, 2025] - Breakdown by view type
- HTML page views : 10998
- PDF downloads : 4690
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
