Case Report Open Access
Endoscopic Management of Pancreatic Pseudo cyst Complicated with Obstructive Jaundice: Case Report and Literature Review
Kalpit Devani1*, Tarvinder Gilotra1, Pranav Patel2, Dhara Chaudhari2, Parag Brambhatt2, Puneet Goenka2 and Mark Young2
1Department of Internal Medicine, East Tennessee State University, Quillen College of Medicine, USA
2Department of Gastroenterology, East Tennessee State University, Quillen College of Medicine, USA
- Corresponding Author:
- Kalpit Devani
MD., Department of Internal Medicine
East Tennessee State University
325 N State of Franklin, Johnson city, TN 37604, USA
Tel: +1 856-400-4763
E-mail: Kalpit_Devani@yahoo.com
Received Date: March 26, 2016; Accepted Date: April 12, 2016; Published Date: April 18, 2016
Citation: Devani K, Gilotra T, Patel P, Chaudhari D, Brambhatt P, et al. (2016) Endoscopic Management of Pancreatic Pseudo cyst Complicated with Obstructive Jaundice: Case Report and Literature Review. J Gastrointest Dig Syst 6:414. doi:10.4172/2161-069X.1000414
Copyright: © 2016 Devani K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Obstructive jaundice due to pancreatic pseudocyst is not common. Local Complications of Pancreatic pseudocyst can be managed with Endoscopic drainage, achieved through transmural approach via stomach or duodenal wall into the cyst, or transpapillary/transductal stent placement. Endoscopic drainage of pancreatic pseudocyst is becoming more favorable nowadays because of shorter hospital stay, lower cost, and lower mortality rate. EUSguided drainage is preferred over conventional endoscopic drainage because of higher technical success rate and because of higher success rate, technical feasibility and quick resolution of pancreatic pseudocyst, fully covered fully covered self-expandable metal stents are recently gaining popularity.
Keywords
Endoscopy; Obstructive jaundice; Pancreatic pseudocyst
Introduction
Pancreatic pseudocyst is a collection of mature fluid outside the pancreas with well-defined wall without necrosis or solid material. It usually develops at least four weeks after the acute pancreatitis episode. Management of pancreatic pseudocyst is warranted only when it is symptomatic or associate with local complications such as infection, hemorrhage, rupture, obstruction of adjacent structure (obstructive jaundice, gastric outlet obstruction). Drainage of pseudocyst can be done via surgical, percutaneous, and endoscopic approach. Endoscopic drainage has been gaining more popularity due to less morbidity, lower cost, shorter hospital stay, and post-procedural quality of life. Here in, We present a case of pancreatic pseudocyst presenting with obstructive jaundice and underwent endoscopic cyst drainage with transmural cystogastrostomy. We discuss different approach to drainage and review of current literature regarding double pigtail stent, plastic stent and fully covered metal stent.
Case Report
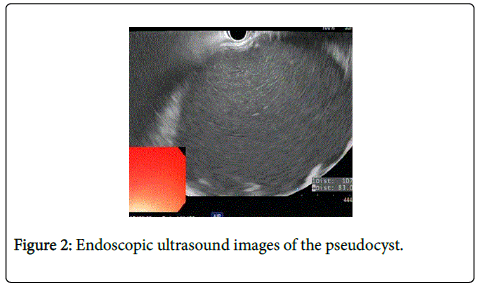
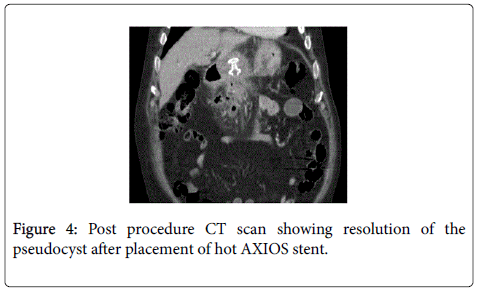
Eighty One year old male presented with painless jaundice. Patient had biliary pancreatitis 6 weeks before the presentation for which he underwent laparoscopic cholecystectomy. Physical exam showed stable vital signs, scleral icterus and overt jaundice. Initial laboratory work up revealed unremarkable CBC and basic chemistry panel, total bilirubin 21.3 mg/dl, direct bilirubin 12.4 mg/dl, alkaline phosphatase 847 IU/L, AST 343 IU/L, ALT 345 IU/L, amylase 92 IU/L, lipase 56 IU/L. CT abdomen showed a complex fluid collection throughout the pancreatic bed measuring 11.6 × 21.8 cm, causing external compression over the common bile duct producing biliary obstruction (Figure 1). MRI with MRCP confirmed the findings. Endoscopic ultrasound showed anechoic large fluid collection with well-defined wall consistent with pseudocyst (Figure 2). Cyst gastrostomy was performed with Hot AXIOS catheter system with drainage of milky fluid (Figure 3). Fluid analysis was negative for malignancy but culture grew Candida Albicans and he was treated with Fluconazole. Post procedure CT imaging confirmed the proper placement of the stent with cyst resolution (Figure 4). Patient improved clinically over the next few days with complete resolution of jaundice and liver function test completely normalized after 5 weeks. Patient remained asymptomatic and Hot AXIOS stent was removed after 8 weeks.
Discussion
Pancreatic Fluid Collection (PFC) is a fairly common complication of acute pancreatitis, and most of these collections were formerly known as pseudocysts. However, the new revised 2013 Atlanta classification has defined mainly 4 different types of PFC based on the pathophysiology, content and organization [1]. Acute PFC occurs fairly quickly (usually within 4 weeks of acute interstitial pancreatitis), and is defined as pure fluid collection with no solid or necrotic component, and no defined wall. Acute necrotic collection also occurs quickly and hence lacks a definable wall, but in contrast to acute PFC, it contains both solid and liquid components, as this is mainly a consequence of acute necrotizing pancreatitis.
Walled-off PFCs includes Pancreatic Pseudocyst (PPC) (PPC fits where in PFC-new Atlanta classification) and Walled-Off Pancreatic Necrosis (WOPN). PPC is a more mature cyst that typically forms more than 4 weeks after acute pancreatitis with clearly defined wall. However, as the name suggests, pseudocyst lacks the true epithelial lining, and is rather defined by granulation and fibrous tissue, which is further walled by adjacent structures such as stomach, colon, omentum and pancreas itself. Similar to acute PFC, pseudocyst is also purely fluid containing and lacks the solid necrotic material. Walled-Off Pancreatic Necrosis (WOPN) is another mature clearly demarcated PFC that usually builds up after 4 weeks of acute pancreatitis, and as the name suggests, it contains a mixture of both necrotic and liquid components.
Walled-off PFCs are formed by containment of the peripancreatic fluid collection that is a result of eventual liquefactive necrosis of inflamed pancreatic tissue, or progressive ductal obstruction leading to ductal disruption and consequent leakage of pancreatic fluid. Approximately 40% of walled-off PFCs can self-resolve, and hence can be managed conservatively [2].
Treatment of walled-off PFCs is warranted only when it is symptomatic or associate with local complications such as infection, hemorrhage, rupture causing pancreatic ascites, adjacent structure involvement (such as GI tract obstruction, biliary obstruction), erosion into adjacent vessel causing GI bleeding, fistulization with adjacent structures such as pancreatico-pleural fistula causing pleural effusions, GU tract fistula), or when pseudocyst itself becomes symptomatic with abdominal distention, pain, nausea/vomiting, or GI bleed [3].
Currently there are three major modalities of treatment available for drainage of pseudocyst: surgical intervention, percutaneous drainage and endoscopic drainage. Endoscopic drainage has been gaining more popularity over surgical drainage lately because of its non-inferiority in terms of morbidity, and its superiority in terms of lower cost, shorter hospital stay, and post-procedural quality of life [4-6]. Endoscopic drainage has significantly high success rate that ranges from 70% to 87% with lower complication rate of 11-34% [7-10]. Percutaneous drainage is another draining modality but it carries the risk of indwelling catheter providing a nidus for infection, and possible pancreatico-cutaneous fistula formation [11].
Endoscopic drainage can be achieved through transmural approach via stomach or duodenal wall into the cyst, or transpapillary/ transductal stent placement. The latter is more feasible when pseudocysts are small and are in direct communication with the pancreatic duct. Transductal stents are also recommended in combination with transmural drainage to bridge the causal main pancreatic duct leaks or a disrupted side branch to prevent recurrences [12-14]. Transmural endoscopic drainage is however more common and preferred mainly because it allows for the insertion of large and multiple stents [15]. It can either be done either in a conventional way of puncturing the apex of visible bulge in a “semi-blind” manner, or through endoscopic ultrasound (EUS)-guided trans-luminal drainage through the stomach or duodenum. Both procedures involve creating either a cystogastrostomy or a cystoduodenostomy, which is then secured with stent placement for cystic fluid to be drained internally.
Long-term success of the trans-duodenal route is better than the trans-gastric route (83% vs. 64%) which is thought to be due to longer durability of cystoduodenal over cystogastric fistula after stent removal [16]. EUS-guided drainage is preferred over conventional endoscopic drainage because of higher technical success rate (95% vs. 60%) [17,18]. Almost half of the PFCs are not obvious and bulging, increasing the risk of puncturing neighboring structures, bleeding, and perforation when draining with blind puncture [19-23]. Besides exactly locating PFC, EUS can also help differentiating the PFC from cystic tumors, gallbladder, and pseudo-aneurysm. EUS can also help determine content of the collection, which is important in case of WONC with significant necrotic content that might require endoscopic, interventional radiology, and surgical intervention [2,23-26].
Conventionally, plastic double pigtail stents have been used for almost 2 decades now with good success. European Society of Gastrointestinal Endoscopy (ESGE) recommends use of at least 2 double pigtail stents based on the better success rates observed with multiple vs single stent in various studies [27-29]. However, fully covered self-expandable metal stents (FCSEMSs) are recently gaining popularity because of their technical feasibility, quick resolution of PPC, and higher success rate.
Plastic stent placement is a multi-step procedure requiring puncture of the cyst, inserting a rigid guidewire, creation of fistula, and finally deployment of multiple stents. This can be lengthy process involving multiple device exchange over the guidewire, with chances of losing the wire access prolonging it even further. In contrast, FCSEMS deployment is a single-step, single-stent procedure, and hence time conservative and technically feasible procedure. Moreover, FCSEMS have a larger diameter that provides better drainage and faster resolution of the infected PPCs with thick content that are difficult to drain through multiple plastic stents [10,14,30,31]. Furthermore, metal stents might provide the radial force to tamponade the bleeding vessels in the cyst wall [31]. One limitation of FCSEMS is possible dislodgement, which not only requires intervention for removal, but can also cause bleeding from scratching the lumen wall [30,32]. Some successful attempts to overcome this limitation with an idea of inserting double pigtail stents within the lumen of FCSEMS have already been reported [31]. A newly designed dumbbell-shaped lumen-apposing FCSEMS (AXIOS; Xlumena Inc., Mountain View, CA, USA) was launched recently with anchor flanges at both ends to hold luminal tissues in apposition [32]. Furthermore, it shortens on deployment thereby reducing the risk of leak or perforation. In 2012, first report of successful use of AXIOS stent was published, including pancreatic necrosectomy which was made possible through the wider diameter of new stents, with only 1/15 stent migration, and complete resolution of PFC in all 15 patients with no recurrences during almost a year median follow-up [33].
Shah et al. [34] reported similar success rate in a large multi-centric AXIOS trial to date with successful placement in 31/33 patients, only 1/31 migration, 7% stent blockage, and 94% (31/33) cyst resolution at 2 month follow-up. Gornals et al. [35] reported technical feasibility with AXIOS stents in comparison to plastic double pigtail stents with no dislodgment (compared to 2/10 in pigtail stents), shorter mean stent retrieval time of 33 ± 40 days, and only 1 PFC recurrence 4 weeks after stent removal.
In our case, we used a novel catheter system to drain the PPC for the management of obstructive jaundice. This catheter system combines a cautery-enabled access catheter with the revolutionary AXIOS stent known as ‘Hot AXIOS™ Stent and Electrocautery-Enhanced Delivery System’ which represents a development of the previously used AXIOS stent (FCSEMS delivery system) combined with an electrocautery component at the distal tip. Unlike the previous AXIOS stent, the electrocautery at the distal tip in new Hot AXIOS delivery system allows direct penetration of the cyst cavity without need for the puncture with 19-gauge needle and a .035-mm guidewire placement. This approach is easy and faster compare to the previous one. As per the large retrospective multicenter study from Europe where Ninety-three patients with PFCs (where majorities had complex collections) underwent drainage using the Hot AXIOS devise had complete resolution of the PFC in 92.5% with no recurrence during follow-up.
Conclusion
Endoscopic management of pancreatic pseudocyst causing obstructive jaundice has been gaining more popularity over surgical drainage lately because of its non-inferiority in terms of morbidity, and its superiority in terms of lower cost, shorter hospital stay, and post-procedural quality of life and of all stents fully covered self-expandable metal stents (FCSEMSs) are recently gaining popularity because of their technical feasibility and higher success rate.
References
- Thoeni RF (2012) The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 262: 751-764.
- Bang JY, Varadarajulu S (2014) Endoscopic ultrasound-guided management of pancreatic pseudocysts and walled-off necrosis. Clin Endosc 47: 429-431.
- Zerem E, Hauser G, Loga-Zec S, Kunosia S, Jovanovia P, et al. (2015) Minimally invasive treatment of pancreatic pseudocysts. World J Gastroenterol 21: 6850-6860.
- Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, et al. (2013) Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 145: 583-590.
- Varadarajulu S, Lopes TL, Wilcox CM, Drelichman ER, Kilgore ML, et al. (2008) EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc 68: 649-655.
- Seewald S, Ang TL, Richter H, Teng KY, Zhong Y, et al. (2012) Long-term results after endoscopic drainage and necrosectomy of symptomatic pancreatic fluid collections. Dig Endosc 24: 36-41.
- Baron TH, Harewood GC, Morgan DE, Yates MR (2002) Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc 56: 7-17.
- Binmoeller KF, Seifert H, Walter A, Soehendra N (1995) Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 42: 219-224.
- Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, et al. (2005) Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy 37: 977-983.
- Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, et al. (2006) Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc 63: 635-643.
- Adams DB, Harvey TS, Anderson MC (1990) Percutaneous catheter drainage of infected pancreatic and peripancreatic fluid collections. Arch Surg 125: 1554-1557.
- Trevino JM, Tamhane A, Varadarajulu S (2010) Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol 25: 526-531
- Baron TH (2008) Endoscopic drainage of pancreatic pseudocysts. J Gastrointest Surg 12: 369-372.
- Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM (2011) Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg 15: 2080-2088.
- Beckingham IJ, Krige JE, Bornman PC, Terblanche J (1997) Endoscopic management of pancreatic pseudocysts. Br J Surg 84: 1638-1645.
- Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM (2008) Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc 68: 1102-1111.
- Park DH, Lee SS, Moon SH (2009) Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy 41: 842-848.
- Giovannini M (2007) EUS-guided pancreatic pseudocyst drainage. Tech Gastrointest Endosc 9: 32-38.
- Howell DA, Holbrook RF, Bosco JJ, Muggia RA, Biber BP (1993) Endoscopic needle localization of pancreatic pseudocysts before transmural drainage. Gastrointest Endosc 39: 693-698.
- Antillon MR, Shah RJ, Stiegmann G, Chen YK (2006) Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc 63: 797-803.
- Cortes ES, Maalak A, Le Moine O, Baize M, Delhaye M, et al. (2002) Endoscopic cystenterostomy of nonbulging pancreatic fluid collections. Gastrointest Endosc 56: 380-386.
- Kahaleh M, Shami VM, Conaway MR (2006) Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy 38: 355-359.
- Varadarajulu S, Wilcox CM, Tamhane A, Eloubeidi MA, Blakely J, et al. (2007) Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc 66: 1107-1119.
- Andrén-Sandberg A, Dervenis C (2004) Pancreatic pseudocysts in the 21st century. Part II: natural history. JOP 5: 64-70.
- Jacobson BC, Baron TH, Adler DG, Davila RE, Egan J, et al. (2005) ASGE guideline: The role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc 61: 363-370.
- Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, et al. (2012) Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 44: 784-800.
- Catalano MF, Linder JD, George S, Alcocer E, Geenen JE (2004) Treatment of symptomatic distal common bile duct stenosis secondary to chronic pancreatitis: comparison of single vs. multiple simultaneous stents. GastrointestEndosc 60: 945-952.
- Fabbri C, Luigiano C, Cennamo V, Polifemo AM, Barresi L, et al. (2012) Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: a case series. Endoscopy 44: 429-433.
- Singhal S, Rotman SR, Gaidhane M, Kahaleh M (2013) Pancreatic fluid collection drainage by endoscopic ultrasound: an update. Clin Endosc 46: 506-514.
- Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, et al. (2008) Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc 68: 1199-1203.
- Penn DE, Draganov PV, Wagh MS, Forsmark CE, Gupte AR, et al. (2012) Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 76: 679–684.
- Wrobel PS, Kaplan J, Siddiqui AA (2014) A new lumen-apposing metal stent for endoscopic transluminal drainage of peripancreatic fluid collections.Endoscopic Ultrasound 3: 203-204.
- Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, et al. (2012) Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc 75: 870-876.
- Shah RJ, Shah JN, Waxman I (2013) EUS-guided drainage of pancreatic pseudocysts (PP) utilizing a novel anchoring, covered self-expanding metal stent (ACSEMS): Results From a prospective, multi-center study. Gastrointestinal Endoscopy 77: AB128.
- Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, Loras C, Sánchez-Cantos AM, et al. (2013) Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. SurgEndosc 27: 1428-1434.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 12502
- [From(publication date):
April-2016 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11508
- PDF downloads : 994