Engineering the Composition of Coffee to Potentially Improve its Health Benefits
Received: 13-Nov-2017 / Accepted Date: 08-Jan-2018 / Published Date: 11-Jan-2018
Abstract
This paper describes current views on the association of coffee consumption with various health factors, and illustrates how the composition of coffee can be engineered through manipulation of the roasting process; the possibility of using this information to improve health-related properties of the beverage is then discussed. It is also noted that although several bioactive compounds have been identified in coffee, there are other poorly defined compounds that may be involved in health effects associated with coffee consumption, and which are difficult to measure and define. In this short review, we consider specifically the antioxidant properties of the beverage, for which a large number of assays have been published. However, such assays produce values that are dependent on the chemical basis of the methodology, and it is considered important to use a platform of analytical assays to obtain a true picture of the antioxidant components the beverage; one such platform based on our own research is described and applied to demonstrating the influence of roasting conditions on different types of antioxidant in the coffee beverage. The contents of other bioactive molecules are also influenced by the roasting conditions, and with appropriate control, the roasting process can be used to optimize the balance of beneficial components and minimize the contents of undesirable components. Unfortunately, at the present time we lack important information that would allow us understand the effects of varying the balance of the various bioactive components, since reports of the health effects of the beverage generally neglect details of both the bean genetics and the various processes that contribute to the beverage composition. Thus although we now have the ability to refine the composition of the coffee beverage to generate products with optimized contents of various specific components, we still lack the basic knowledge of their biological consequences that is needed to develop products with improved abilities to act as functional foods.
Keywords: Coffee; Health; Antioxidants; Roasting; Chlorogenic acids; Melanoidins
Introduction
Links between coffee consumption and health
Over the past century a vast amount of literature has been generated on the links between coffee consumption and health. Some studies claim beneficial effects, whilst others claim that it is detrimental, and there has been overall an element of confusion concerning the health effects of regular consumption of the beverage. However, several recent books and reviews on the impact of coffee and health [1-4], show growing evidence from epidemiological studies that regular consumption of moderate amounts of coffee can provide a degree of protection against several diseases and physiological disorders, although there are some health problems that are directly associated with coffee consumption. Examples from the mainly recent literature are presented in Table 1. Notable amongst these are links with decreased development of various types of liver disease [5,6], Type 2 diabetes [7-9], neurodegeneration [10,11], and cancer [12]. It is perhaps significant that most recent papers on the links to cardiovascular disease show beneficial effects [13-15], even though coffee consumption is associated with elevated blood pressure [16] and cholesterol levels [17]. Furthermore, coffee has anti-inflammatory properties [18], and has been linked with decreased incidence of bronchial asthma [19], increased alertness [20] and a lower incidence of suicide [21]. Also, although coffee decreases the risk of gout in men [22], it is associated with an increased risk of rheumatoid arthritis [23]. Other detrimental associations with coffee consumption include sleep disorders [24], an increased risk of miscarriage [25], and low birth weight of babies [26]. Although associations between coffee consumption and some types of cancer were made in the older scientific literature, recent studies tend to indicate either a beneficial effect or no effect. Examples of benefical effects include lower incidents of cancers of the mouth/pharynx [27,28], oesophagus [29,30], stomach [31,32], colon/rectum [33,34], liver [6,35,36], pancreas [29,37], bladder [38], prostrate [39,40], endometrium [41], and brain [42]. Green coffee (i.e. a drink made from unroasted coffee beans) has also been considered for use in weight loss [43]. However, it should be noted that as with other aspects of life, different people are likely to respond differently to consumption of the beverage, depending on their genetic make-up and other aspects of their diet and lifestyle. Furthermore, it is often inappropriate to attribute biological effects to a single compound, except when it is seriously detrimental (i.e. toxic), and the beneficial effects of individual components of a balanced diet/lifestyle usually cannot be quantified, except when they contribute to an essential function that would be impaired in their absence (i.e. behaving as functional foods). As an example, small-to-moderate alcohol consumption is known to benefit the cardiovascular system, whilst having a detrimental effect on liver disease, and cancers of the upper and lower digestive system [44]. However, these are diseases to which coffee exhibits a degree of protection, and it has been suggested that it could be beneficial to balance the consumption of these beverages [45].
| Organ or health problem | Effect | Reference |
|---|---|---|
| Liver | Lowered levels of liver carcinoma and disease in general | [5,6] |
| Type 2 diabetes | Decreased incidence of Type 2 diabetes | [7-9] |
| Neurodegeneration | Decreased risk of Parkinson’s disease | [10] |
| Decreased risk of dementia | [11] | |
| Cancer | Overall risk of cancer decreased | [12] |
| CVD & blood pressure | Moderate coffee consumption associated with decreased CVD risk | [13] |
| Increased plasma antioxidant capacity | [14] | |
| Decreased risk of heart failure and stroke | [15] | |
| Coffee consumption raises blood pressure | [16] | |
| Coffee increases cholesterol levels | [17] | |
| Anti-inflammatory properties | Lower levels of systemic immune and inflammatory markers in serum | [18] |
| Asthma | Decreased incidence of bronchial asthma. | [19] |
| Mental state | Increased alertness | [20] |
| Lower risk of suicide | [21] | |
| Arthritis | Decreased risk of gout | [22] |
| Increased risk of rheumatoid arthritis | [23] | |
| Sleep | Increased in sleep disorders | [24] |
| Miscarriage/low birth rate | Increased risk of miscarriage | [25] |
| Maternal consumption linked to low birth weight in babies | [26] |
Table 1: Selected papers describing the association of coffee consumption with various disease risks and functional disorders.
Coffee components associated with its health effects
Coffee beans contain several specific compounds that have been shown individually to have bioactivity, including various alkaloids, such as caffeine (1,3,7-trimethylxanthine), trigonelline (1- methylpyridinium-3-carboxylic acid), and nicotinic acid (pyridine-3- carboxylic acid), which is also known as niacin or Vitamin B3, the chlorogenic acids (CGA), which are esters of quinic acid and various trans-cinnamic acids, and the diterpenes, kahweol and cafestol [46]. The contents of these compounds vary according to the plant genetics; for example Robusta contains higher levels of caffeine than Arabica coffee, whereas Arabica coffee generally contains more trigonelline [47]. Furthermore, cafestol occurs in both Arabica and Robusta beans, whereas kahweol is mainly in Arabica beans, and 16-O-methylcafestol is found only in Robusta [46]. Methods for quantifying these groups of compounds in a coffee matrix have been reviewed recently [48,49]. However, it is important to recognize that consumption of bioactive compounds in a coffee matrix may produce different physiological effects than in pure solutions [50], and the complexities of the chemical composition of whole foods suggest that it may often be inappropriate to extrapolate the health effects of individual chemical compounds to those experienced by their consumption in food where there may be interactions between various other dietary components in addition to those with the digestive system. Thus in the context of physiological and health effects on consumers, data on exposure to bioactive compounds during consumption of the brews are most relevant.
Many changes in the composition of coffee beans occur during processing, storage, and especially roasting. Furthermore, the composition of the beverage is also influenced by the method of extraction from the roasted and ground beans. Consequently, there is a question as to the extent to which changes in coffee composition determined by the roasting and preparation conditions of the beverage can be linked to specific health effects of coffee consumption. This is further complicated by the fact that roasting affects various bioactive compounds differently in Robusta and Arabica coffees [51], and new bioactive compounds are generated during the roasting process. These include 1-methyl pyridinium, and the dimethyl pyridiniums which are formed by the breakdown of trigonelline, and acrylamide which is formed from a Maillard reaction between asparagine and reducing sugars [52]. The dimethyl pyridiniums show mild antithrombotic properties [53] 1-methyl pyridinium may have anticancer properties [54] whereas acrylamide is considered to be a potential carcinogen [55]. In addition to the discrete molecules mentioned above, coffee also contains many poorly defined compounds which may be bioactive. An example is the melanoidins, which are formed by the Maillard and caramelization reactions during the roasting process, and have been associated with antimicrobial, anticarcinogenic, anti-inflammatory, antihypertensive, and antiglycative activities [56].
It is clear from the discussion above that the sources of the beneficial health effects of coffee consumption are chemically complex and multifactorial. However, one important contribution to health is associated with antioxidant activity, which controls potentiallydamaging oxidative processes. Since coffee makes a major contribution to the dietary antioxidant intake in many populations [57,58], we consider that improving our knowledge of the antioxidant properties of coffee represents an important step in understanding its contribution to a healthy lifestyle. This is addressed in the following sections.
Some background information on antioxidants in coffee
• First it is important to clarify some definitions.
• An antioxidant either prevents or inhibits oxidation processes.
• A pro-oxidant stimulates oxidation processes.
• A free radical is a molecule that contains an unpaired electron.
Various types of chemical assay are used to measure antioxidant properties, and these are discussed in more detail in the following section. However, it is important to recognize that in a biological system an antioxidant can function either by inhibiting the formation of an oxidizing agent, or by scavenging reactive oxygen species (ROS), and thereby breaking oxidative chain reactions. In the latter mechanism an oxygen-derived radical abstracts a hydrogen atom from the antioxidant, and produces an antioxidant-derived radical which is much less reactive than the original ROS. Such relatively stable radicals then have the ability to react more specifically, and could themselves also be bioactive. In biology, antioxidant behavior is often equated to free radical scavenging activity, because of the roles played by O2- derived free radicals such as O2-, HO2., and HO. in biological oxidation processes. However, not all reactive oxygen species (ROS) are free radicals, and not all free radicals are oxidizing agents, so caution must be exercised in the use of these terms.
The major antioxidants in unroasted (green) coffee beans are the CGAs, which account for ∼10% of the dry weight. These phenolic acids are highly bioavailable [59], and their health effects have been reviewed recently [60], specifically in the context of the implications of coffee consumption. Consumption of CGAs has been linked to weight loss through their effects on glucose absorption, and extracts of unroasted coffee beans or CGA-supplemented beverages could be used for treating obesity [61]. However, it should be noted that the name chlorogenic acid corresponds to a wide range of compounds; around 70 different derivatives have been described in green coffee beans [62], and it is likely that not all of these will exhibit similar bioactivity.
The CGAs are not stable to high temperatures, and during coffee roasting they are transformed into various low and high molecular mass products. Thus CGA contents of coffee beans are decreased as a result of roasting, but their diversity increases, and around 200 such compounds have been identified in roasted coffee beans [62]. As mentioned in the previous section, roasting also results in the formation of melanoidins, which have poorly defined structures that are mainly made up of fragments from polysaccharides and proteins, but which may also incorporate some CGA molecules [63]. The CGA and melanoidin components account for most of the total antioxidant potential of roasted coffee, but whereas the CGA contents decline as a result of roasting, the melanoidins are produced specifically during the roasting process. Furthermore, although both groups of molecule are classified as antioxidants, their chemical properties are distinctly different, and thus it seems probable that their biological properties will also differ. Consequently, as we attempt to refine our knowledge of the mechanisms through which coffee consumption has an impact on health, it is important to be able to discriminate between these two groups of antioxidant compound in analyses of the beverage.
Assays for the determination of antioxidant properties
The scientific literature contains a plethora of assays for measuring antioxidant properties of biological materials and food products. However, all are based on chemical reactions carried out in vitro, which immediately raises questions about their biological relevance, since they give no consideration to bioavailability, in vivo stability, retention by tissues, and reactivity in tissues [64]. Furthermore, reactions may occur with digestive fluids, other components of a product that is consumed alone, such as milk or sugar in the case with coffee, or other foodstuffs that make up a meal.
Antioxidant assays can involve either competitive or non-competitive reactions [65]. In competitive reactions, the target species and the antioxidant compound compete for reaction with an oxidizing agent, whereas in non-competitive assays, the antioxidant reacts directly with oxidizing agent. Thus in competitive antioxidant assays, the values for the antioxidant capacity are dependent on the relative concentrations of the antioxidant and target species, and on their reaction rates with the oxidizing agent. Furthermore, in order to select appropriate assays for antioxidant measurements, it is important to understand their chemistry and relevance to the application; for health-related investigations assays should be biologically relevant wherever possible. Unfortunately, this is not the case in many commonly-used assays based on electron transfer reactions, such as the in vitro quenching of stable radicals (e.g. DPPH, ABTS, TEMPO, DMPD), where the chemistry is fundamentally different from that of the small oxygen-derived radicals that initiate oxidative reactions in vivo . Nevertheless, such assays could have some relevance to the scavenging of the relatively stable secondary radicals that are produced after primary reactions involving highly reactive species, such as the hydroxyl radical. Also, methods based on the reduction capacity of a food product (e.g. Folin-Ciocalteu, FRAP, CUPRAC) are far too crude to provide a direct correlation with health effects, although they may have some biological relevance. Other methods that may be more biologically relevant (e.g. ORAC, TRAP) are time consuming, and still far removed from reactions in living systems.
It should also be noted that different assays show different sensitivities towards different types of antioxidant compound. Even different compounds in the same class can produce distinctly different values in antioxidant assays, as seen for example for the polyphenols gallic acid and caffeoylquinic acid [66]. Consequently, the use of a single assay can produce misleading conclusions. A combination of a few assays that involve different types of chemical reaction provides a more realistic overview of the antioxidant properties of a complex sample such as a food product. Even then such data should be used with caution, and it should be borne in mind that there is no such thing as a single antioxidant value that can be linked directly to biological properties.
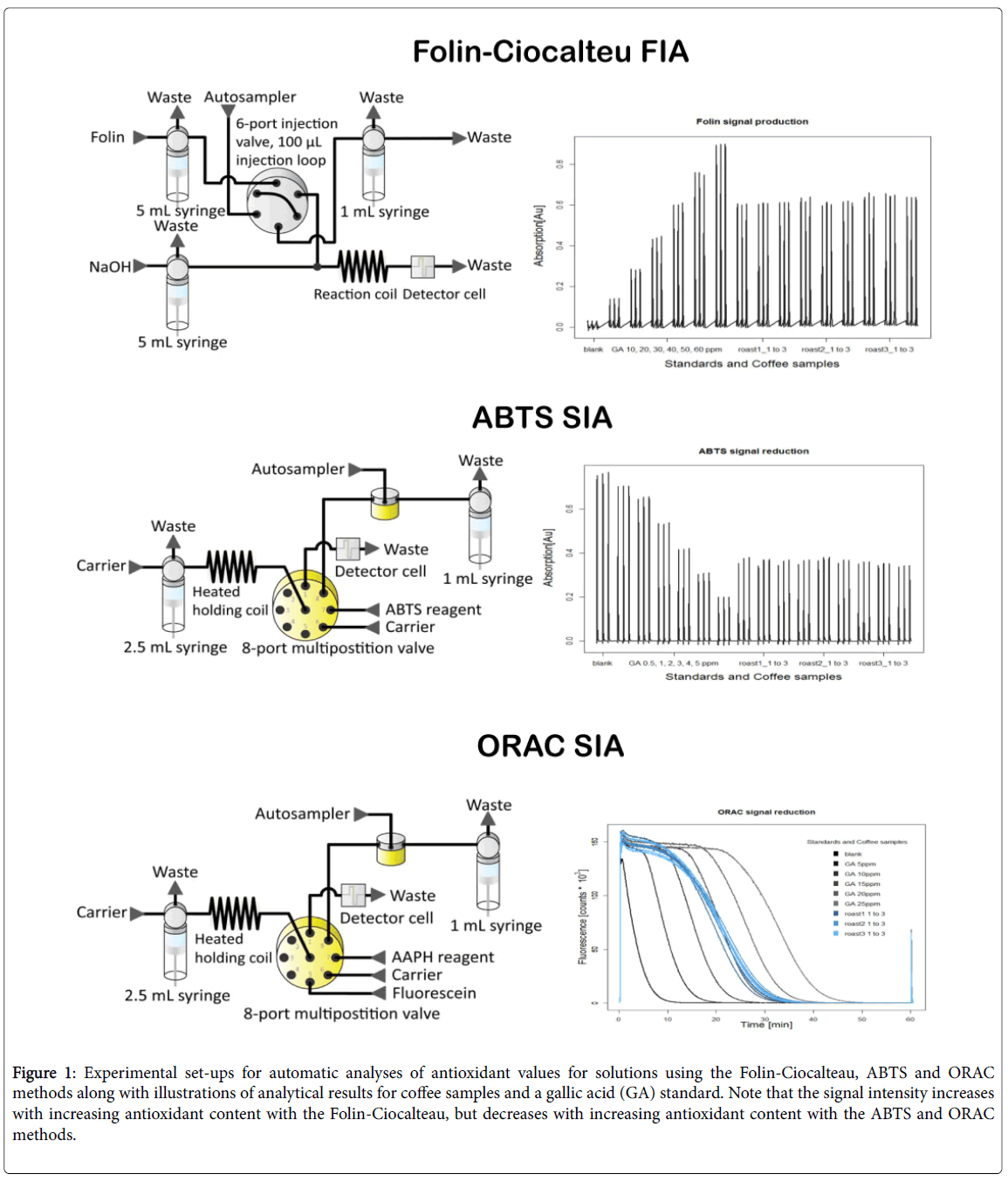
The adaption and validation of three complementary assays (Folin- Ciocalteu (FC), ABTS and ORAC) for the routine assessment of antioxidant capacity of beverages has been described recently by Opitz et al. [66,67], and examples are presented in the following sections of their applications to understanding the effects of roasting conditions on the antioxidant properties of coffee samples. The experimental set-ups are illustrated in Figure 1, which also shows the measurement of experimental values relative to those of a gallic acid standard.
Figure 1: Experimental set-ups for automatic analyses of antioxidant values for solutions using the Folin-Ciocalteau, ABTS and ORAC methods along with illustrations of analytical results for coffee samples and a gallic acid (GA) standard. Note that the signal intensity increases with increasing antioxidant content with the Folin-Ciocalteau, but decreases with increasing antioxidant content with the ABTS and ORAC methods.
Determining the effects of roasting on coffee antioxidants
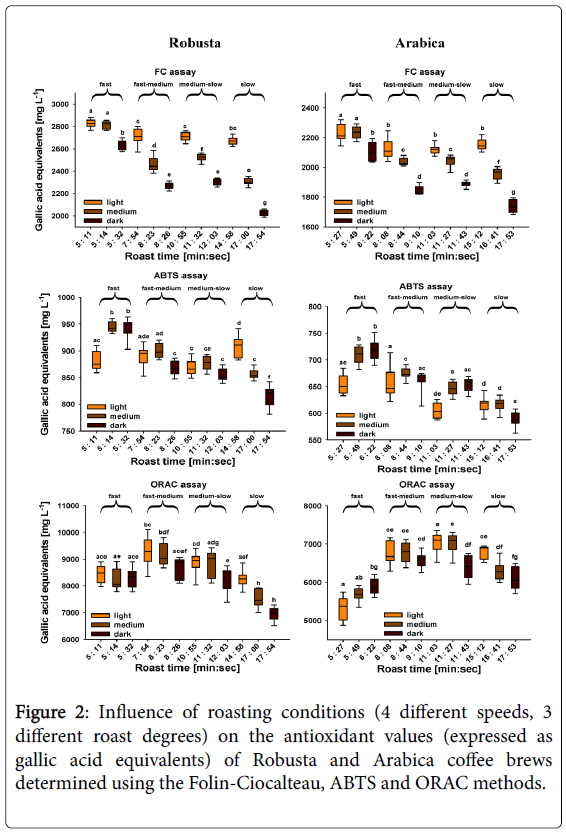
The antioxidant values for samples of unroasted Robusta coffee are usually higher than those from Arabica samples, and Opitz et al. [66] used the antioxidant platform described above to investigate extracts from coffee beans at different stages in the roasting process in order to understand the evolution of the antioxidant properties. They observed a progressive increase in antioxidant capacity during roasting to a light roast state, but with darker roasts the antioxidant values decreased towards slower and darker roasting degrees. These results were interpreted as showing that in the early stages of roasting the production of melanoidins had a higher antioxidant effect than that lost by degradation of CGAs, whereas with the darker roasts and slower roasting times, degradation of CGAs had the biggest effect on the overall antioxidant properties. In this work, the antioxidant values for brews from Robusta beans were consistently higher than those prepared from Arabica beans under equivalent roasting conditions, but as illustrated in Figure 2, major differences were observed in both the absolute antioxidant values and the trends with roasting conditions according to the method used.
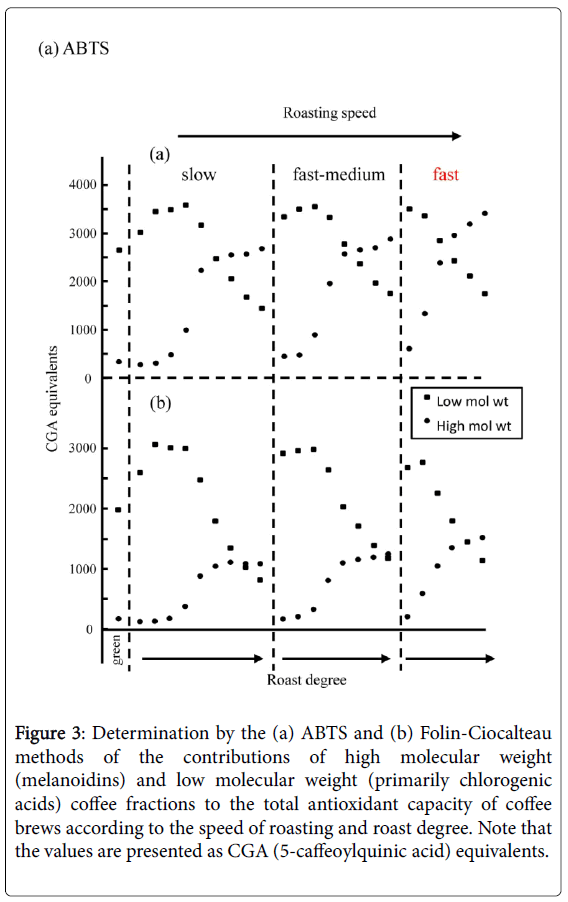
More direct information on the relative contributions of the CGAs and melanoidins to the overall antioxidant properties of the beverage can be obtained by coupling assays on-line to high performance size exclusion chromatography (HPSEC). This then allows the contributions of the high (melanoidin) and low (CGAs) mass products to be determined separately within the same brews [68-70]. As illustrated in Figure 3, the high molecular weight fraction consistently increased with roast degree, although there was a small loss with the darkest roasts. In contrast, the low molecular weight fraction decreased progressively starting at relatively mild roasting conditions, but some was still present even in the darkest roasts. Thus by combining chromatographic procedures with on-line assays, we are now developing a more differentiated view of the antioxidant properties of coffees, which is necessary for future engineering of special coffee products.
Figure 3: Determination by the (a) ABTS and (b) Folin-Ciocalteau methods of the contributions of high molecular weight (melanoidins) and low molecular weight (primarily chlorogenic acids) coffee fractions to the total antioxidant capacity of coffee brews according to the speed of roasting and roast degree. Note that the values are presented as CGA (5-caffeoylquinic acid) equivalents.
Free radical processes in coffee
It is unfortunate that there is a commonly-held view that free radicals are universally damaging for health. All aerobic organisms have a metabolism that is based on O2, the most stable form of which, 3O2, is a diradical, and its 1-electron reduction results in the formation of free radicals, such as .O2-, .OOH, and .OH. These free radicals are highly reactive and play many important roles – some beneficial and some detrimental - in biological processes. Indeed, in the functioning of the immune system, free radicals are used in the killing of invading pathogens, and one of the most important reactions in all of biology, photosynthesis, proceeds via a cascade of free radical reactions.
Furthermore, free radical centres are not restricted to oxygen atoms, and radicals occur in which the unpaired electron is based on C, N, or other atoms. It should also be noted that a huge range of reactivities is exhibited by different types of free radical; for example, although HO. has a half-life in vivo of ~10-9 s [71], stable radical centres are observed in minerals [72] and have been used for dating in archaeology [73]. Nevertheless, many important reactions in food processing proceed via oxidative free radical processes, an example being the formation of the melanoidin component during coffee roasting [74], and appropriate control of such free radical reactions should in principle influence the formation of both beneficial and detrimental compounds. However, the free radical processes that occur during the roasting of food products are exceedingly complex, and at the present time we still have difficulty in understanding even the simplest systems, but there is clearly the potential to manipulate the composition of coffee products in a desirable way, as we gain more knowledge of the reactions involved.
One approach that can be used for samples in which free radicals are stable, or have sufficiently long half-lives is to characterize their electronic structures by electron paramagnetic resonance (EPR) spectroscopy. One example, is the investigation of the free radical dynamics in real time during the roasting process [75]. Large differences were observed for different types of bean, and there were appreciable differences in different beans from the same batch, which illustrates the point made by Illy [76] about the importance of green bean selection on the quality of the final coffee product. It should also be noted that the environmental conditions during coffee roasting are essentially anaerobic, because of the release of CO2 during thermallyinduced breakdown of various components of the green bean, and the roasting induced radicals have EPR spectra consistent with C-centres; however, access to O2 during the cooling phase results in a huge increase in free radical generation [75], thus illustrating the importance of O2 to the free radical content of the melanoidin component. At present we do not understand the significance of this observation, but the presence of small amounts of O2 in the atmosphere during roasting could be the reason for differences in the product properties from fast and slow roasting protocols described in the previous paragraph. EPR has also made some other important contributions to our understanding of free radical processes in coffee products, including variations in free radical signal intensities in coffee solutions as a function of temperature [77], and changes in the intensities of the free radical EPR signal during the storage of roasted coffees and coffee extracts [78-80] which illustrate the role of O2 in storage-induced changes in coffee products.
The EPR measurements described in the previous paragraph were only able to detect free radicals with half-lives that are sufficiently long to allow their signals to be recorded, and it is not possible to detect very short-lived radicals directly using this technique. However, the generation and identification of short-lived radicals can sometimes be achieved by combining EPR spectroscopy with spin trapping technology. An example of the use of this approach is the observation of a temperature-dependent turnover of short-lived radicals (probably involving HO.) in soluble coffee solutions [77]. Reactions of such radicals may be the explanation for the deterioration in taste during storage of hot brewed coffee prior to consumption.
Controlling production to produce coffees with specific properties
As described briefly in this article, we are gaining more knowledge of the chemical and biochemical reactions that occur during the various stages of coffee production, and it is now possible to alter the concentrations of specific components, or groups of component by controlling various processing conditions. As illustrated in Figure 2, both the time and temperature of roasting, in addition to the darkness of the final roast, influence the antioxidant contents of prepared coffee brews. We also know that the roasting conditions can be manipulated to influence the relative concentrations of the CGA and/or melanoidin components (Figure 3). Since considerable knowledge is also being generated on the influence of coffee bean genetics, environmental conditions, agronomic practices, and coffee fruit processing procedures on the composition of green coffee beans, we are now establishing a foundation from which products can be prepared with specific ranges of concentrations of bioactive compounds or their precursors. Essentially, we have the potential to generate a family of novel functional food products. However, a major obstacle at the present time is a lack of basic knowledge of the influence of the brewing and preparation processes on beverage composition (including the effects of additives, such as milk and sugar) and on the mechanisms of action of various potentially bioactive compounds, especially in the context of whole meals. This complex problem is especially difficult for poorly defined components that may yield bioactive compounds when broken down in the digestive tract. Yet this is precisely the type of information that is needed for the development of functional food products, and there is a danger that commercial pressures will bring about the introduction of new products before obtaining a proper understanding of their effects.
Information needed for future developments
Although we have the potential to control various aspects of coffee production, including the genetic composition of the plants, cultivation conditions, harvesting and extraction of the green beans, post-harvest storage, roasting conditions, and storage of the roasted products, in addition to blending and extraction procedures to produce the beverage, we still lack much important information on their impacts on the properties of the beverage that relate to consumer health. Seldom is any attention given to these aspects of coffee production in studies of relationships between coffee consumption and health. Such investigations need to recognize that coffee is indeed a complex product, and that clinical studies should provide full descriptions of the beverage used, including its source, method of preparation, and timing of consumption. Over time such information may be useful for identifying the potential impact of various factors that have not hitherto been considered. In cohort studies, questionnaires should seek more detailed information on coffee consumption habits, though it is recognized that these are likely to be quite variable. However, the lack of information on the composition of coffee products may account for some of the conflicting evidence in the scientific literature, since there have been marked changes in coffee production technology and consumer habits over the past half century, in addition to there being distinct regional preferences for different coffee products.
More knowledge is also needed on the chemistry of individual components or simplified fractions of the beverage, because much of the scientific literature is based on informed speculation rather than hard facts. As an example consider the chemical behavior of polyphenols, which are generally considered to represent important dietary antioxidants. With transition metals, polyphenols can react in various ways depending on the pH of the medium, and result in complexation, or redox reactions which could be followed by polymerization of the metal and either polymerization of breakdown of the polyphenol. In addition, when polyphenols react with transition metal ions in reduced oxidation states, they can produce .OH radicals via the Fenton reaction [81], and thus behave as pro-oxidants. Quite clearly, a number obtained in a simple chemical assay falls far short of providing an understanding how polyphenols might behave in a complex biological system, and as observed for the different physiological effects of caffeine taken from pure solution compared to coffee, it is important to study reactions in appropriate matrices. A specific chemical example taken from the literature is the reaction of Cu(II) with solutions of the green tea polyphenols [82] where the products are different from those observed with actual tea extracts [83], even though such polyphenols are generally assumed to be responsible for much of the biological activity of green teas. Furthermore, the generally assumed redox reaction between Cu(II) and these polyphenols has been shown to be unimportant at physiological pH values [82], so clearly even some of the most fundamental chemical reactions of bioactive compounds in foods and beverages are still not fully understood. In the case of coffee, there is little evidence of the actual chemical mechanisms in which the individual CGAs may be involved after consumption. However, CGAs also occur in a wide range of plant products that are used in traditional medicines, so understanding their biological reactions has wider implications beyond coffee chemistry, and it may be significant that caffeoylquinic acid derived free radicals have been identified during antioxidant reactions of Ilex latifolia and Ilex kudincha [84]. In addition, it must be borne in mind that there are other components of coffee brews, many of which are not currently characterized, that could be bioactive, and thus responsible for some of the observed effects of the beverage.
Finally, in addition to considering the conditions for optimizing the contents of bioactive compounds with beneficial properties, it is important not to ignore the production of compounds that are potentially detrimental to health, and minimizing their production needs to receive particular attention when engineering coffee products for improved health properties. One molecule that has received considerable attention during the past decade is acrylamide which is formed by a Maillard reaction between asparagine and reducing sugars in the early stages of coffee roasting [52]. Acrylamide levels tend to be higher for Robusta than Arabica coffees [85], and decrease with increasing temperature and time of roasting for a similar roast degree. Thus they roughly parallel the CGA levels and illustrate the types of compromise that will need to be undertaken when developing functional foods from coffee. At present the production of high quality coffee is regarded as an art, but with increased knowledge of the relevant reactions it can be increasingly more of a science, and this science should lead to the development of beverages that make increasingly valuable contributions to health as well as having pleasant sensory properties.
Acknowledgements
BG acknowledges Guangxi University for a Guest Professor position. The Commission for Technology and Innovation (CTI) of Switzerland, the Zurich University of Applied Sciences (ZHAW) and Bühler AG are acknowledged for financial support (Project no. 13897.1 PFIW-IW). We also acknowledge Dr. Stefan Schenker and Marco Wellinger for assistance in this project.
References
- Goodman BA (2012) Coffee Consumption and Health. Nova Science Publishers, Inc., Hauppauge, NY.
- Chu YF (2012) Coffee: Emerging Health Effects and Disease Prevention. Wiley-Blackwell, Hoboken.
- Ludwig IA, Clifford MN, Lean MEJ, Ashihara H, Crozier A (2014) Coffee: biochemistry and potential impact on health. Food Funct 5: 1695-1717.
- Preedy VR (2015) Coffee in Health and Disease Prevention. Elsevier, Amsterdam.
- Saab S, Mallam D, Cox GA 2nd, Tong MJ (2014) Impact of coffee on liver disease a systematic review. Liver Int 34: 495-504.
- Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C (2017) Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev 26: 368-377.
- Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, et al. (2009) Coffee, decaffeinated coffee, and tea consumption in relation to incident Type 2 diabetes mellitus. Arch Intern Med 169: 2053-2063.
- Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, et al. (2015) The evaluation of inflammatory and oxidative stress biomarkers on coffee–diabetes association: results from the 10-year follow-up of the ATTICA Study (2002–2012). Eur J Clin Nutr 69: 1220-1225.
- Santos RM, Lima DR (2016) Coffee consumption, obesity and type 2 diabetes: a mini review. Eur J Nutr 55: 1345-1358.
- Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, et al (2000) Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283: 2674-2679.
- Eskelinen MH, Kivipelto M (2010) Caffeine as a protective factor in dementia and Alzheimer’s disease. J Alzheimers Dis 20: S167-S174.
- Alicandro G, Tavani A, La Vecchia C (2017) Coffee and cancer risk: a summary overview. Eur J Cancer Prev 26: 424-432
- Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB (2014) Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 129: 643-659.
- Aqudelo-Ochoa GM, PulgarÃn-Zapata IC, Velásquez-Rodriguez CM, Duque-RamÃrez M, Mauricio Naranjo-Cano M, et al. (2016) Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J Nutr 146: 524-531.
- Stevens L, Görg C, Kao D (2017) Coffee intake affects heart failure and stroke survival and is significant in predicting heart failure and stroke risk. Circulation 136: A21081.
- Jee SH, He J, Whelton PK, Suh I, Klag MJ (1999) The effect of chronic coffee drinking on blood pressure: a meta-analysis of controlled clinical trials. Hypertension 33: 647-652.
- Correa TA, Rogero MM, Mioto BM, Tarasoutchi D, Tuda VL, et al. (2013) Paper filtered coffee increases cholesterol and inflammation biomarkers independent of roasting degree. Nutrition 29: 977-981.
- Loftfield E, Shiels MS, Graubard BI, Katki HA, Chaturvedi AK, et al. (2015) Associations of coffee drinking with systemic immune and inflammatory markers. Cancer Epidemiol Biomarkers Prev 24: 1052-1060.
- Pagano R, Negri E, Decarli A, La Vecchia C (1988) Coffee drinking and prevalence of bronchial asthma. Chest 94: 386-389.
- Agostoni CV, Bresson JL, Fairweather-Tait S, Flynn A, Golly I, et al. (2011) Scientific Opinion on the substantiation of health claims related to Lactobacillus fermentum ME-3 and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 3025) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA journal 9: 2232.
- Lucas M, O’Reilly EJ, Pan A, Mirzaei F, Willett WC, et al. (2014) Coffee, caffeine, and risk of completed suicide: Results from three prospective cohorts of American adults. World J Biol Psychiatry 15: 377-386.
- Choi HK, Willett W, Curhan G (2007) Coffee Consumption and risk of incident gout in men: a prospective study. Arthritis Rheum 56: 2049-2055.
- Heliövaara M, Aho K, Knekt P, Impivaara O, Reunanen A, et al. (2000) Coffee consumption, rheumatoid factor, and the risk of rheumatoid arthritis. Ann Rheum Dis 59: 631-635.
- Clark I, Landolt HP (2016) Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep Med Rev 31: 70-78.
- Weng X, Odouli R, Li DK (2008) Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol 198: 279.e1-279.e8.
- Sengpiel V, Elind E, Bacelis J, Nilsson S, Grove J, et al. (2013) Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC Med 11: 42.
- Al-Dakkak I (2011) Tea, coffee and oral cancer risk. Evid Based Dent 12: 23-24.
- Hildebrand JS, Patel AV, McCullough ML, Gaudet MM, Chen AY, et al. (2013) Coffee, tea, and fatal oral/pharyngeal cancer in a large prospective US cohort. Am J Epidemiol 177: 50-58.
- Yu X, Bao Z, Zou J, Dong J (2011) Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer 11: 96.
- Zheng JS, Yang J, Fu YQ, Huang T, Huang YJ, et al. (2013) Effects of green tea, black tea, and coffee consumption on the risk of esophageal cancer: a systematic review and meta-analysis of observational studies Nutr Cancer 65: 1-16.
- Li L, Gan Y, Wu C, Qu X, Sun G, et al. (2015) Coffee consumption and the risk of gastric cancer: a meta-analysis of prospective cohort studies. BMC Cancer 15: 733.
- Xie Y, Huang S, He T, Su Y (2016) Coffee consumption and risk of gastric cancer: an updated meta-analysis. Asia Pac J Clin Nutr 25: 578-588.
- Michels KB, Willett WC, Fuchs CS, Giovannucci E (2005) Coffee, tea, and caffeine consumption and incidence of colon and rectal cancer. J Natl Cancer Inst 97: 282-292.
- Schmit SL, Rennert HS, Rennert G, Gruber SB (2016) Coffee consumption and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 25: 634-639.
- Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C (2013) Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol 11: 1413-1421.
- Wadhawan M, Anandt AC (2016) Coffee and liver disease. J Clin Exp Hepatol 6: 40-46.
- Dong J, Zou J, Yu XF (2011) Coffee drinking and pancreatic cancer risk: a meta-analysis of cohort studies. World J Gastroenterol 17: 1204-1210.
- Sugiyama K, Sugawara Y, Tomata Y, Nishino Y, Fukao A, et al. (2017) The association between coffee consumption and bladder cancer incidence in a pooled analysis of the Miyagi cohort study and the Ohsaki cohort study. Eur J Cancer Prev 26: 125-130.
- Wilson KM, Kasperzyk JL, Rider JR, Kenfield S, van Dam RM, et al. (2011) Coffee consumption and prostate cancer risk and progression in the health professionals follow-up study. J Natl Cancer Inst 103: 876-884.
- Pounis G, Tabolacci C, Costanzo S, Cordella M, Bonaccio M, et al. (2017) Reduction by coffee consumption of prostate cancer risk: evidence from the Moli-sani cohort and cellular models. Int J Cancer 141: 72-82
- Je Y, Giovannucci E (2012) Coffee consumption and risk of endometrial cancer: findings from a large up-to-date meta-analysis. Int J Cancer 131: 1700-1710.
- Holick CN, Smith SG, Giovannucci E, Michaud DS (2010) Coffee, tea, caffeine intake, and risk of adult glioma in three prospective cohort studies. Cancer Epidemiol Biomarkers Prev 19: 39-47.
- Onakpoya I, Terry R, Ernst E (2011) The use of green coffee extract as a weight loss supplement: a systematic review and meta-analysis of randomised clinical trials. Gastroenterol Res Pract pii: 382852.
- Nelson DE, Jarman DW, Rehm J, Greenfield TK, Rey G, et al. (2013) Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health 103: 641-648.
- Goodman BA (2014) Can coffee ameliorate the cancer risks associated with low-moderate alcohol consumption in men? J Food Nutr Disor 3.
- Oestreich-Janzen S (2013) Chemistry of Coffee. Elsevier, Amsterdam 1-28.
- Caprioli G, Cortese M, Maggi F, Minnetti C, Odello L, et al. (2014) Quantification of caffeine, trigonelline and nicotinic acid in espresso coffee: the influence of espresso machines and coffee cultivars. Int J Food Sci Nutr 65: 465-469.
- Rodrigues NP, Bragagnolo N (2013) Identification and quantification of bioactive compounds in coffee brews by HPLC–DAD–MSn. J Food Compost Anal 32: 105-115.
- Nuhu AA (2014) Bioactive micronutrients in coffee: recent analytical approaches for characterization and quantification. ISRN Nutr 2014: 384230.
- Graham TE, Hibbert E, Sathasivam P (1998) Metabolic and exercise endurance effects of coffee and caffeine ingestion. J Appl Physiol 85: 883-889.
- Vignoli JA, Viegas MC, Bassoli DG, Benassi MT (2014) Roasting process affects differently the bioactive compounds and the antioxidant activity of Arabica and Robusta coffees. Food Res Int 61: 279-285.
- Stadler RH, Blank I, Varga N, Robert F, Hau J, et al. (2002) Food chemistry: acrylamide from Maillard reaction products. Nature 419: 449-450.
- Kalaska B, Piotrowski L, Leszczynska A, Michalowski B, Kramkowski K, et al. (2014) Antithrombotic effects of pyridinium compounds formed from trigonelline upon coffee roasting. J Agric Food Chem 62: 2853-2860.
- Boettler U, Volz N, Pahlke G, Teller N, Kotyczka C, et al. (2011) Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo. Mol Nutr Food Res 55: 798-802.
- Friedman M (2003) Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem 51: 4504-4526.
- Borrelli RC, Visconti A, Mennella C, Anese M, Fogliano V (2002) Chemical characterization and antioxidant properties of coffee melanoidins. J Agric Food Chem 50: 6527-6533.
- Pulido R, Hernández-GarcÃa M, Saura-Calixto F (2003) Contribution of beverages to the intake of lipophilic and hydrophilic antioxidants in the Spanish diet. Eur J Clin Nutr 57: 1275-1282.
- Svilaas A, Kaur Sakhi A, Frost Andersen L, Svilaas T, Ström EC, et al. (2004) Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr 134: 562-567.
- Farah A, Monteiro M, Donangelo CM, Lafay S (2008) Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr 138: 2309-2315.
- Tajik N, Tajik M, Mack I, Enck P (2017) The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur J Nutr 56: 2215-2244.
- Thom E (2007) The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J Int Med Res 35: 900-908.
- Kuhnert N, Jaiswal R (2012) Occurrence and identification of chlorogenic acids in green and roasted coffee beans. In: Goodman BA Ed Coffee Consumption and Health, Nova Science Publishers, New York, 65-97.
- Moreira ASP, Nunes FM, Domingues MR, Coimbra MA (2012) Coffee melanoidins: structures, mechanisms of formation and potential health impacts. Food Funct 3: 903-915.
- Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53: 1841-1856.
- Magalhaes LM, Segundo MA, Reis S, Lima JL (2008) Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta 613: 1-19.
- Opitz SEW, Smrke S, Goodman BA, Keller M, Schenker S, et al. (2014) Antioxidant generation during coffee roasting: a comparison and interpretation of the results from three complementary assays. Foods 3: 586-604.
- Opitz SEW, Smrke S, Goodman BA, Yeretzian C (2014) Methodology for the measurement of antioxidant capacity of coffee. In: Processing and Impact on Antioxidants in Beverages. 253-264.
- Opitz SEW, Goodman BA, Keller M, Smrke S, Wellinger M, et al. (2017) Understanding the effects of roasting on antioxidant components of coffee brews by coupling on-line ABTS assay to high performance size exclusion chromatography. Phytochem Anal 28: 106-114.
- Smrke S, Opitz SEW, Petrozzi S, Yeretzian C (2012) Characterization of coffee brews made from different roasting degrees of coffee using size exclusion chromatography and antioxidant assays. Chimia 66: 7/8.494.
- Smrke S, Opitz SEW, Vovk I, Yeretzian C (2013) How does roasting affect the antioxidants of coffee brew: exploring the antioxidant capacity of coffee via on-line antioxidant assays coupled to size exclusion chromatography. Food Funct 4: 1082-1092.
- Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215: 213-219.
- Toyoda S, Nagashima K, Yamamoto Y (2016) ESR signals in quartz: applications to provenance research - a review. Quat Int 397: 258-266.
- Carvajal E, Montes L, Almanza OA (2011) Quaternary dating by electron spin resonance (ESR) applied to human tooth enamel. Earth Sci Res J 15: 115-120.
- Goodman BA, Yeretzian C (2014) Free radical processes in coffee I. Solid samples. In: Preedy VR Ed Food Processing and Impact on Active Components: A Modern Approach, Elsevier, Amsterdam, 559-566.
- Goodman BA, Pascual EC, Yeretzian C (2011) Real-time monitoring of free radical processes during the roasting of coffee beans using electron paramagnetic resonance spectroscopy. Food Chem 125: 248-254.
- Pascual EC, Goodman BA, Yeretzian C (2002) Characterisation of free radicals in soluble coffee by electron paramagnetic resonance spectroscopy. J Agric Food Chem 50: 6114-6122.
- Yeretzian C, Pascual EC, Goodman BA (2012) Effect of roasting conditions and grinding on free radical contents of coffee beans stored in air. Food Chem 131: 811-816.
- Yeretzian C, Pascual EC, Goodman BA (2013) Probing free radical processes during storage of extracts from whole roasted coffee beans: impact of O2 exposure during extraction and storage. J Agric Food Chem 61: 3301-3305.
- Yeretzian C, Pascual EC, Goodman BA (2013) Effects of O2 during various processing steps on free radical concentrations in hot aqueous extracts of R&G coffee and their changes during storage. Proceedings of the Pure and Applied Chemistry International Conference 355-358.
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). (1997). Compiled by McNaught AD, Wilkinson A Blackwell Scientific Publications, Oxford.
- Pirker KF, Baratto MC, Basosi R, Goodman BA (2012) Influence of pH on the speciation of copper(II) in reactions with the green tea polyphenols, epigallocatechin gallate and gallic acid. J Inorg Biochem 112: 10-16.
- Goodman BA, Severino JF, Pirker KF (2012) Reactions of green and black teas with Cu(II). Food Funct 3: 399-409.
- Pirker KF, Goodman BA (2010) Caffeoylquinic acid derived free radicals identified during antioxidant reactions of Bitter tea (Ilex latifolia and Ilex kudincha). Food Funct 1: 262-268.
- Soares CMD, Alves RC, Oliveira MBPP (2015) Acrylamide in coffee: influence of processing. Processing and Impact on Active Components in Food, Academic Press 575-582.
Citation: Goodman BA, Opitz SEW, Smrke S, Yeretzian C (2018) Engineering the Composition of Coffee to Potentially Improve its Health Benefits. J Nutr Diet 1: 101.
Copyright: © 2018 Goodman BA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 7126
- [From(publication date): 0-2018 - Nov 11, 2025]
- Breakdown by view type
- HTML page views: 6066
- PDF downloads: 1060