Short Communication Open Access
Enhancing Visible Light Photocatalysis by N-doping
1Department of Environmental Sciences, Environment and Arid Land Agriculture, King Abdulaziz University, Jeddah, Saudi Arabia
2Center of Excellence in Environmental Studies (CEES), King Abdulaziz University, Jeddah
3Central Metallurgical R&D Institute, Helwan, Cairo, Egypt
- *Corresponding Author:
- Barakat MA
Department of Environmental Sciences
Faculty of Meteorology, Environment and Arid Land Agriculture
King Abdulaziz University, Jeddah, 21589, Saudi Arabia
Tel: 966544910070
E-mail: mabarakat@gmail.com
Received Date: September 22, 2015; Accepted Date: October 06, 2015; Published Date: October 13, 2015
Citation: Barakat MA (2015) Enhancing Visible Light Photocatalysis by N-doping. J Powder Metall Min 4:138.doi:10.4172/2168-9806.1000138
Copyright: © 2015 Barakat MA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
This article describes the role of N-doping with TiO2 nanoparticles in enhancing visible light photo catalysis. Anion doping with nitrogen ions plays an important role in enhancing effectiveness of TiO2 by introducing trapping sites. Thus, doped nanostructured TiO2-xNx films have been synthesized, characterized, and investigated as visible light photo catalysts for the degradation of pollutants in water. The synthesized nanoparticles can be investigated by suitable technique such as Raman and/or IR spectra in order to make evidence of the fixed amount of nitrogen. Photo degradation of chloro-phenols, as a model for hazardous pollutants, has been studied.
Keywords
Visible light; Photocatalysis; N-doping
Introduction
In light of the increasing contamination of the environment by hazardous chemicals, there is great interest to develop innovative technologies for the safe destruction of toxic pollutants. The processes must be cost-effective, easy to operate, and capable of achieving total or near total mineralization. This has prompted researchers to investigate innovative chemical oxidation technologies. Photocatalytic oxidative destruction of pollutants in wastewater provides the ultimate solution for the treatment of hazardous wastes. Photocatalysis would be a promising simple technique which can be used for both the degradation of organic pollutants and the removal of metals in onepot systems. The process requires ultra violet (UV) light which can be artificial or natural sources.
Among the most promising compounds for photo catalysis applications is titanium dioxide. TiO2, as efficient and stable catalyst, is one of the least expensive semiconductors. However, the major impediment to its wide spread application, particularly indoor, resides in the fact that TiO2 absorbs near-UV light (Eg=3.2 eV for anatase). This band gap does not match very well with solar spectrum. Therefore, a visible-light activated catalyst is desired that can take advantage of a larger fraction of the solar spectrum and would be much more effective in environmental cleanup. Nanostructured (~20- 30 nm) particles provide the optimal balance between volumetric and surface recombination and are thus best suited for photocatalysis. The photoelectronic properties of TiO2 can be strongly influenced by the dopants that introduce new electronic energy levels inside the band gap. Substitutional doping of nitrogen in TiO2 has revealed an improvement in visible light photocatalytic activity.
In the recent years, N-doped TiO2 nanoparticles were prepared by different sol-gel methods. The first adopted method was that one reported by Sato [1]. The obtained yellow powder was characterized in terms of particle size distribution, specific surface area by BET, band gap energy, and UV-VIS absorbance spectrum. Some of the relevant data, together with those relevant to P25- TiO2, are here after reported (Table 1).
| ID | Mean size [nm] | BET [m2/g] | Bandgap [eV] |
|---|---|---|---|
| N-TiO2 | 20 | 45 | 2.5 |
| P25 | 59 | 80 | 3.3 |
Table 1: Relevant measurements of both N-dopped TiO2 and undopped P-25.
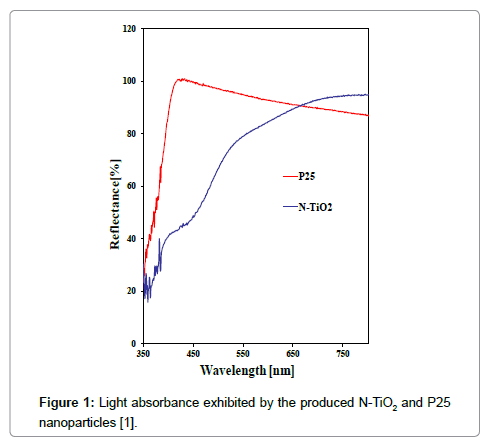
Figure 1 shows the wavelength range reflectance of the produced doped titania and pure TiO2 supplied by Degussa, for comparison. A significant light absorbance in the visible wavelength was achieved by doped titania.
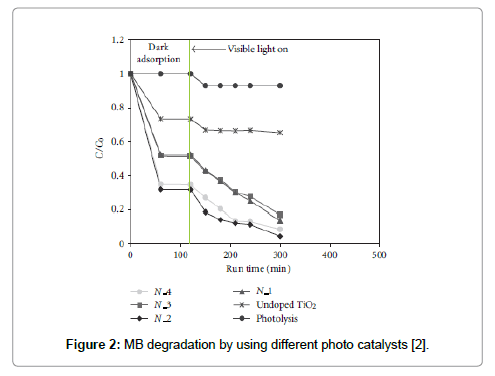
In a typical activity test a defined amount of photocatalyst was suspended in 100 ml of MB solution [2] in a pyrex cylindrical photoreactor (ID = 2.5 cm) equipped with an air distributor device. The photoreactor was irradiated by a strip composed by white light LEDs (nominal power: 6W) with wavelength emission in the range 400-800 nm or by a similar number of blue light LEDs (nominal power: 6W) with wavelength emission in the range 400-550 nm. The obtained results, reported in Figure 2, showed that only the N-doped TiO2 is a photocatalyst effective under visible light.
The drawback of this TiO2 N-doping procedure is the necessity to operate during the nanoparticles production at a very low temperature, near to 0°C, in order to fix the nitrogen. To overcome such a difficulty a second more suitable operating procedure, applied by Tushar [3], has been adopted by using diethyl ammine solution as nitrogen source. Such a method was performed at ambiance temperature and allowed the production of very effective photocatalysts under visible light irradiation.
Many studies [4-9] have verified that N-doping in oxide semiconductors could improve their photocatalytic activity because a portion of O atoms are replaced by N atoms to form a new donor energy level on top of the VB, which generates a red shift due to E(O2 p) = −14.8 eV and E(N2 p) = −13.4 eV. For the composite materials, an investigation of the presence of N in the photocatalysts is important because N is present in the urea, which is used as a fuel. In the combustion process, a significant amount of heat was released, which caused a high reaction temperature. The N derived from the gaseous products, such as N2 and NO, was doped into the photocatalysts to replace O.
Conclusions
N-doped-TiO2 nanoparticles can greatly enhance visible light photocatalysis of pollutants in wastewater. This can be due the tendency of N to form intermediate energy levels within the TiO2 matrix narrowing the band gap and enables the semiconductor to harness more photons of solar radiation.
References
- Chen X, Mao Y (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications and applications. Chemical Reviews 107: 2891-2959.
- Sacco O, Stoller M, Vaiano V, Ciambelli P, Chianese A, et al. (2012) Photocatalytic Degradation of Organic Dyes under Visible Light on N-Doped TiO2 Photocatalysts. International Journal of Photoenergy 2012: 1-8.
- Jagadale TC,Takale SP, Sonawane RS, Joshi HM,Patil SI, et al. (2008) N-Doped TiO2 Nanoparticle Based Visible Light Photocatalyst by Modified Peroxide Sol-Gel Method. J PhysChem C 112: 14595-14602.
- Wang DH, Jia L, Wu XL, Lu LQ, Xu AW (2012) One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity. Nanoscale 4: 576-584.
- Wang DS, Xiao LB, Luo QZ, Li XY, An J, et al. (2011) Highly efficient visible light TiO2 photocatalyst prepared by sol–gel method at temperatures lower than 300 ºC. J Hazard Mater 192: 150-159.
- Wang Y, Feng CX, Zhang M, Yang JJ, Zhang ZZ (2010) Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. ApplCatal B Environ 100: 84-90.
- Wang XP, Lim TT (2010) Solvothermal synthesis of C N codoped TiO2 and photocatalytic evaluation for bisphenolA degradation using a visible-light irradiated LED photoreactor. ApplCatal B Environ 100: 355-364.
- D’Arienzo M, Siedl N, Sternig A, Scotti R, Morazzoni F, et al. (2010) Solar light and dopant-induced recombination effects: photoactive nitrogen in TiO2 as a case study. J PhysChem C 114: 18067-18072.
- Hayat K, Gondal MA, Khaled MM, Yamani ZH, Ahmed S (2011) Laser induced photocatalytic degradation of hazardous dye (Safranin-O) using self synthesizednanocrystalline WO3. J Hazard Mater 186: 1226-1233.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 11231
- [From(publication date):
December-2015 - Aug 19, 2025] - Breakdown by view type
- HTML page views : 10256
- PDF downloads : 975