Epidemiology of Acute Kidney Injury (AKI) among Hospitalized and Outpatients Frequent to Al-Lieth Kidney Unit (AKU)
Received: 29-Jul-2017 / Accepted Date: 08-Aug-2018 / Published Date: 18-Aug-2017
Abstract
Background: Acute kidney injury (AKI) is a syndrome characterized by decreased glomerular filtration. The spectrum of AKI ranges from minimal elevations in serum creatinine (SCr) to complete anuric kidney failure. Despite ample knowledge of the biologic basis of AKI, descriptions of the incidence, risk factors, sequelae, and outcomes of AKI remain relatively limited or have been based upon older descriptions without reflecting the most current definitions or practice patterns.
Aim of the study: Our study aims to assess the epidemiology of AKI among Saudi Patients attending to Al- Lieth Kidney Unit (AKU) that will contribute to define incidence and prevalence of the disease in Al-Lieth specific area via a structured Questionnaire that includes demographical data, medical information and measured diagnostic tests.
Materials and methods: The study was carried out on fifty patients in Al-Lieth Kidney Unit (AKU) compared with twenty five healthy control volunteers of matched age and sex. They were classified in accordance with KDIGO criteria.
Results: The mean age of patients in the AKI group was 53.44 ± 6.98 years while in control group was 51.08 ± 6.89 years, there was a significant increase in age in AKI group more than in the control group. eGFR was significantly decreased in AKI group than the control group. The most significant risk factors if present together in same person may lead to AKI disease, the risk factors were age, female sex, decreased eGFR, and decreased water consumption per day, diabetes mellitus and increase serum creatinine.
Keywords: Epidemiology; Acute kidney injury; Serum creatinine; Egfr; Al-Lieth Kidney Unit; Kingdom Saudi Arabia
165731Abbreviations
AKI: Acute Kidney Injury; SCr: Serum Creatinine; AKU: Al-Lieth Kidney Unit; eGFR: Estimated Glomerular Filtration rate; CPB: Cardiopulmonary bypass; RAAS: Renin-Angiotensin Aldosterone System; GPF: Glomerular Plasma Flow; GP: Glomerular Pressure; GFSA: Glomerular Filtration Surface Area; TGF: Tubuloglomerular Feedback; BUN: Blood Urea Nitrogen; SN-GFR: Single nephron GFR; RIFLE: Renal Injury Failure Loss of Function End-stage; ADQI: Acute Dialysis Quality Initiative consensus; UO:Urine Output; CKD: Chronic Kidney Disease; ICU: Intensive Care Unit; AKIN: Acute Kidney Injury Network; KDIGO: Kidney Disease Improving Global Outcomes; MDRD: Modification of Diet in Renal Disease; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; DM: Diabetes mellitus; BMI: Body Mass Index; RRT: Renal Replacement Therapy
Introduction
Acute Kidney Injury (AKI) is a common complication after cardiovascular surgery that is associated with adverse patient outcomes and severe drop in renal functions manifested by sharp rise of serum creatinine levels and abrupt decline in urine output [1,2]. An increasing number of Cardiovascular surgeries are being performed every year all over the world and usually associated with cardiopulmonary bypass (CPB) and reduced cardiac output, which results in activation of the renin-angiotensin-aldosterone system (RAAS) and renal vasoconstriction that increase the risk of developing AKI among operated patients [3,4].
The occurrence of AKI postoperatively comprises around 5-40% with increased morbidity and mortality rates among patients and extends the hospitalization period [5,6]. The possible etiologies of AKI may be classified into three categories: prerenal, renal (intrinsic) and postrenal. The prerenal AKI, termed sometimes as “prerenal azotemia” occur secondary to renal hypoperfusion due to systemic changes associated with cardiorenal syndrome, cirrhosis, hepatorenal syndrome, hypovolemia, hypotension, sepsis, renal artery stenosis, renal vein thrombosis and use of antihypertensive drugs resulted in glomerular filtration rate (GFR) drop without any influences on the renal parenchyma. Renal AKI or Intrinsic AKI is the result of renal parenchymal damage due to glomerulonephritis, acute tubular necrosis, interstitial nephritis, and nephrotoxicity. The last one, postrenal AKI is caused by urinary tract obstruction without prominent parenchymal pathology [7].
In spite of incompletely understood pathogenesis of AKI, typical pathophysiological mechanisms could be explained as a result of hemodynamic changes including reduction of afferent arteriolar blood flow to the kidney and mesangial contraction that minimize glomerular plasma flow (GPF), glomerular pressure (GP), glomerular filtration surface area (GFSA) and finally leading to diminished GFR. There is growing evidence that renal vascular resistance is a result of coming from preceding disturbances in the balance of intrarenal vasoactive mediators via stimulating vasoconstrictors (Angiotensin II, adenosine, thromboxane, leukotrienes, endothelin and platelet activating factor) versus vasodilators (endothelial-derived nitric oxide and prostacyclin) contributing to a persistent medullary hypoxia [8,9].
Furthermore, renal tubular impairment and apoptosis contribute to diminished GFR by 3 different routes:
• Disruption of sodium chloride reabsorption by proximal tubules then, transferred to distal tubules would stimulate tubuloglomerular feedback (TGF) mechanism via purinergic signaling leading to increased afferent vascular resistance and a concomitant decrease in GFR [10,11].
• Glomerular backleak of filtrates causes hypoproteinemia, interstitial edema due to albuminuria and increases the levels of blood urea nitrogen (BUN) and creatinine [12,13].
• Renal tubular obstruction elevates renal tubular pressure, reduces the single nephron GFR (SN-GFR), upset renal tubular recovery to potentiate cell apoptosis [14].
Risk Injury Failure Loss of function and End stage (RIFLE) is a package of standards for interpretation of kidney function developed by The Acute Dialysis Quality Initiative consensus (ADQI) [15,16]. The RIFLE classification is based on serum creatinine (SCr) and urinary output (UO) to classify the prognostic seriousness of deteriorating kidney function into three stages (Risk, Injury and Failure). Many reviews concluded that the beneficial role of RIFLE was hindered by the accompanying considerable drawbacks:
• Estimated SCr baseline is more specific for chronic kidney disease (CKD) rather than AKI.
• SCr can be impacted by nonspecific variables and consequently was problematic.
• Using UO is influenced by diuretics and must be measured by bladder catheter in an ICU.
• SCr was considered to be a marker for renal function, not kidney injury [17].
The Acute Kidney Injury Network (AKIN) is considered to be another modified version of RIFLE criteria that dependably utilizes two measurements of serum creatinine and urinary output disregarding GFR measurement [18,19]. AKIN allowed the identification and stratification of AKI in a large proportion of hospitalized patients and was independently associated with the outcome however, it failed to exhibit a better prognostic acuity in terms of in-hospital mortality because of moderate and insignificant changes in serum creatinine values over time [20]. The latest tool for AKI classification, Kidney Disease Improving Global Outcomes (KDIGO) was approved for AKI staging and severity as it includes almost all focal points of both RIFLE and Associated criteria in a fit way [21].
Although, most epidemiological studies of AKI are still primitive and requiring more efforts, they have the ability to contribute to early detection, improve therapeutic interventions and reduce patient adverse outcomes. This limitation reflects that worldwide incidence rates of AKI are calculated by imprecise methods on account of tinny numbers of case report studies, missing gaps in data collection from patients and discrepancies in standardized definitions of AKI between developed and developing countries [22-24]. Late reviews conducted in the USA and Spain demonstrated that incidence rates of AKI were roughly 23.8 cases per 1000 hospital discharge and 209 cases for each million population, respectively [25,26]. A current population-based study led in the UK revealed high frequency of AKI, of 1811 cases for every million in 2003 [27]. A report from Kuwait showed a rate of 4.1 cases for each 100,000 population for each year [28]. Moreover, the annual incidences for AKI in Brazil and northern India were 7.9 and 6.4 cases for each 1000 admissions [29,30]. However, Saudi Arabia incidence for AKI is not well known and mysterious. Surprisingly, developed counties incidences were high among elderly people but in developing counties, children and young adults are the most severely affected which is a result of critical shortages in healthcare professionals and systematic evaluation of other related health problems like malaria, obstetric procedures and hemolytic uremic anemia [31,32]. The median age of patients suffering from Acute Kidney Injury (AKI), is increasing due to rising life expectancy of aging population [33].
When addressing the difference between CKD in men and women, we must take note of the fact that eGFR (commonly used in studies) is based on a patient’s sex, among other variables. The two most common equations for assessing eGFR, the MDRD (Modification of Diet in Renal Disease) and CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equations, both use sex as a variable. They are based on the assumption that for a given creatinine level, men will have higher levels of kidney function than women due to higher muscle mass and increased creatinine generation among men [34].
Diabetes mellitus (DM) is the single largest contributor to the growing prevalence of chronic kidney disease (CKD); 35% of adults who have diabetes also have CKD [35]. When associated with kidney disease, diabetes poses a triple threat. It not only leads to ESRD, but also increases the risk for hospitalization, and is one of the major risk factors for development of acute kidney injury (AKI) in hospitalized patients [1,3-6]. There is limited information regarding the effect of AKI on the risk of advanced kidney disease in the setting of DM [36]. Our study aims to assess the epidemiology of AKI among Saudi Patients attending to Al- Lieth Artificial Kidney Unit (AKU) that will contribute to define incidence and prevalence of the disease in Al-Lieth specific area via a structured Questionnaire that includes demographical data, medical information and measured diagnostic tests.
Materials and Methods
Study design
The study was conducted after approval of the study protocol by “The Ethics Committee of Al-Lieth General Hospital”, informed consents were obtained from all study participants prior to data collection. Our study was carried out on fifty patients in Al-Lieth Kidney Unit (AKU) compared with twenty five healthy control volunteers of matched age and sex. They were classified in accordance with KDIGO criteria in (Table 1) [21].
| Stages | Serum creatinine criteria | Urine output criteria |
|---|---|---|
| 1 | Serum creatinine increase ≥ 26.5 μmol/l within 48 hours. Increase ≥ 1.5–1.9 -fold from baseline. |
<0.5 ml/kg/h for ˃6 h |
| 2 | Serum creatinine increase >2.0–2.9-fold from baseline. | <0.5 ml/kg/h for ˃12 h |
| 3 | Serum creatinine increase >3.0-fold from baseline. or serum creatinine ≥ 354 μmol/l or need for RRT. |
<0.3 ml/kg/h for ˃24 h or anuria for 12 h |
Table 1: KDIGO criteria for classification of acute kidney injury (AKI) patients.
Inclusion criteria: Patients with ages from 20–80 years old and who suffered from cardiovascular disease, liver disease, benign prostatic hypertrophy, acute interistial nephrosis, rhabdomyolysis, and urinary tract obstruction.
Exclusion criteria: Patients with a history of kidney diseases, chronic autoimmune diseases, malignancies, diabetic nephropathy, overlapping syndromes, renal transplants, and pregnant were excluded.
Materials and laboratory investigations
Using a structured questionnaire that would be filled in according recorded patient files that encompass the disease history, demographical data and measured diagnostic tests. Moreover, Estimated GFR (eGFR) was calculated by using Modification of Diet in Renal Disease (MDRD) formula GFR (mL/min/1.73 m2)=175 × (Scr)-1.154 × (Age)-0.203 × (0.742 if female) × (1.212 if African) [37] (Table 1).
Statistical analysis
Our results were statistically analyzed by IBM SPSS (version 22) software and expressed as mean ±SD or median with interquartile range. Data will be analyzed using Student’s t-test for mean comparisons between AKI and normal control groups. Correlations between measurable variables were assessed using Pearson correlation analysis, where significance was set at p<0.05. Multi variant analysis was done to predict the most significant risk factors for AKI.
Sample size
Sample size was calculated by using Med Calc statistical software. Assuming area under ROC to be 0.80, an alpha of 0.05 and power of study 90.0%. A minimum sample size required to evaluate this study was 50 patients and 25 control subject.
Results
Our study encompassed two groups; Patient group (50) and Control group (25). By using KDIGO criteria for classification of AKI cases dependent on serum creatinine values. General demographical data of all patients were summarized in (Table 2).
| Items | AKI group | Control group | P* |
|---|---|---|---|
| Number of cases | 50 | 25 | |
| Gender (Male/ Female) | 10/40 | 11/14 | 0.012* |
| Age (years) | 53.44 ± 6.98 | 51.08 ± 6.89 | 0.033* |
| Smoker | 20 (40.0%) | 5 (20.0%) | 0.016* |
| BMI (kg/m) | 28.59 ± 3.20 | 25.34 ±3.04 | 0.002* |
| eGFR (mL/min/1.73 m2) | 92.64 ± 17.54 | 99.68±14.39 | 0.007* |
| eGFR <60 ml/min/1.73 m2 (number of patients) | 7 | 1 | 0.002* |
| Serum creatinine | 96.3 ± 39.2 | 60.0 ± 14.9 | 0.001* |
| Drinking Water (Ground, Tape, Bottled) | 0.98 ± 0.23 | 2.01 ± 0.31 | 0.019* |
| Medical comorbidity | |||
| DM | 20 (40.0%) | 5 (20.0%) | 0.017* |
| Hypertension | 22 (44%) | 7 (28.05) | 0.011* |
| Presence nearby factories | 11 (22 %) | 2 (8 %) | 0.022* |
| Residence | |||
| Rural | 17 (34.0%) | 14 (56.0%) | 0.06 |
| Urban | 33 (66.0%) | 11 (44.0%) | |
P*: Data are represented as mean ± SD
Table 2: A summary of patient’s demographics.
Regarding gender, females represent 80.0% of cases in the AKI group, while representing 56.0% of the control group, there was a significant increase in female in AKI group more than in the control group. The mean age of patients in the AKI group was 53.44 ± 6.98 years while in control group was 51.08 ± 6.89 years, there was a significant increase in age in AKI group more than in the control group (p<0.05). 40.0% of the AKI patients were smokers, while 20.0% of the control group patients were smokers, there was a significant increase in smokers in AKI group more than control group.
BMI was significantly higher in AKI group than control group; in AKI, it was 28.59 ± 3.20, while in the control group, it was 25.34 ± 3.04. eGFR was significantly decreased in AKI group than the control group (92.64 ± 17.54 in AKI group and 99.68 ± 14.39 in the control group). Serum creatinine shows a significant increase in AKI group more than the control group (p]<0.05).
Water drinking per day shows a highly significant decrease in AKI group than the control group; in the AKI group, it was 0.98 ± 0.23 L/day while in the control group, it was 2.01 ± 0.31 L/day. The comorbidity (DM and hypertension) was significantly increased in AKI group more than control group. Regarding the residence, 22.0% of AKI group patients are living near factories, while there was no significant difference between the two groups regarding the residence (rural or urban), (Table 3) show the multivariate analysis of different risk factors which may affect AKI disease, it was found that the most significant risk factors if present together in same person may lead to AKI disease, the risk factors were age, female sex, decreased eGFR, decreased water consumption per day, diabetes mellitus and increase serum creatinine.
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| (Constant) | 1.198 | 0.413 | 2.9 | 0.005 | |
| Age | -0.007 | 0.004 | -0.110 | -2.048 | 0.045* |
| Sex | -0.071 | 0.07 | -0.068 | -2.009 | 0.031* |
| Smoking | -0.023 | 0.067 | -0.023 | -0.350 | 0.728 |
| BMI | -0.020 | 0.011 | -0.143 | -1.727 | 0.089 |
| eGFR | 0.007 | 0.003 | 0.235 | 2.654 | 0.035* |
| eGFR <60 | -0.019 | 0.141 | -0.013 | -0.136 | 0.893 |
| Amount of drinking water | 0.59 | 0.064 | 0.686 | 9.186 | 0.0001* |
| Presence nearby factories | -0.026 | 0.066 | -0.021 | -0.391 | 0.697 |
| Residence | -0.024 | 0.05 | -0.025 | -0.483 | 0.631 |
| D.M. | 0.025 | 0.054 | 0.025 | 2.06 | 0.047* |
| Hypertension | 0.001 | 0.053 | 0.001 | 0.012 | 0.99 |
| Serum creatinine | 0.002 | 0.001 | 0.154 | 2.617 | 0.027* |
*:Dependent Variable of AKI.
Table 3: Multivariate analysis of different risk factors of AKI.
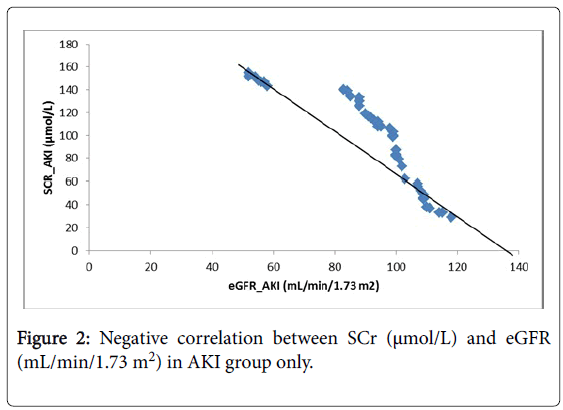
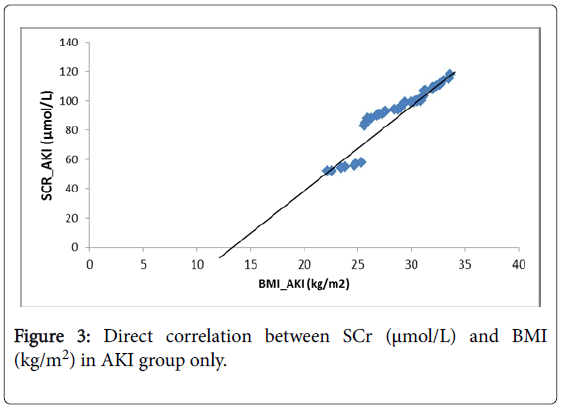
As well, a regression model was plotted in (Figure 1). The (Figure 2), shows that there was a negative significant correlation between SCr (μmol/L) and eGFR (mL/min/1.73 m2) in AKI group only, while (Figure 3) showed a positive significant correlation between SCr (μmol/L) and BMI (kg/m2) in AKI group only.
Discussion
The application of KDIGO criteria is successful to discriminate between AKI and non-AKI groups dependent on serum creatinine values. As shown in figures that serum creatinine values were increased nominally in AKI group than non-AKI which is an indicator of renal degeneration over time by one of the aforementioned reasons.
Many previous studies pointed that serum creatinine despite a good tool but influenced badly by non-renal factors [38]. This observation was clear in our study as serum creatinine concentrations were positively proportional with Body mass index (BMI) and negatively proportional with eGFR. Additionally, relationships with both gender and age were direct but without any significant difference. Surprisingly, serum creatinine levels showed an inverse proportional with types of drinking water and direct one with the presence of nearby factories.
We suppose that from the expectable ongoing reasons for AKI among AL-Lieth area were types of drinking water and/or nearby factories. We tested other factors including; drug abuse, medications, smoking, and congenital anomalies according to the structured questionnaire but gave a null statistical meaning which might be explained due to the increase awareness due to noticeable activities of Kafa organization in this area against drug abuse and smoking, strict procedures of Saudi Ministry of Health restrict narcotic medications release. Congenital anomalies in our study comprised 1% of proper causes of AKI.
In this study, it was found that there was a significant effect of age on AKI, the AKI patients were older than the control cases. This result was in agreement with Antoni et al. study (2012), Elderly patients with Chronic Kidney Disease (CKD) who develop AKI are at high risk for mortality, non-recovery from AKI and progression to more advanced stages of CKD and even to end stage renal disease. As a consequence, the challenge for nephrologists is to find strategies to either prevent AKI or prevent the transition from AKI to CKD [39].
The gender in our study shows a significant effect as a risk factor of AKI, female’s percent was higher than male, Nitsch et al. demonstrate that as kidney disease progresses in women, the elevation in mortality risk increases. This effect has not been shown in men, and further research is needed to understand the role of gender in the association between renal disease severity and mortality. Diabetes mellitus was significant risk factor in our study, also Charuhas et al . (2011) provide that AKI episodes are associated with a cumulative risk for developing advanced CKD in diabetes mellitus, independent of other major risk factors of progression.
Conclusion
Our study concluded that etiology of AKI is still vague and could be potentiated by comorbidities like types of drinking water and being near factories that should be further studied in more cases. Relying on serum creatinine and/or GFR is satisfactory for classification of AKI but a future challenge developed towards novel biomarkers with prominent sensitivity /specificity against AKI situation in the earlier timings before the unwanted irreversible reactions.
AKI is a problem, but the true incidence is unknown. From a worldwide perspective, there is a obvious need to realize the epidemiology of AKI more carefully. Use of standardized definitions and descriptions of existing at-risk and high-risk populations, both in community and institutional settings in developing and underdeveloped countries, are the first steps to improve outcomes.
The knowledge about the risk factors associated with AKI and early prediction of these complications in patients may be useful in clinical practice, particularly to implement early preventive measures.
Recommendation
Despite of lack of comparable studies in that area, we recommend starting a cohort study on AKI epidemiology on large scale among western Saudi regions focusing on coastal areas to list possible causes of AKI that augment CKD and ESRD as a rapid development of uncontrolled disease.
References
- Rewa O, Bagshaw SM (2014) Acute kidney injury-epidemiology: Outcomes and economics. Nat Rev Nephrol 10: 193-207.
- Hamzic Mehmedbasic A, Rebic D, Balavac M, Muslimovic A , Dzemidzic J (2015) Clinical analysis of etiology: Risk factors and outcome in patients with acute kidney injury. Mater Sociomed 27: 70-74.
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, et al. (2005) Beginning, Ending Supportive Therapy for the Kidney I: Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818.
- Bellomo R, Auriemma S, Fabbri A, D'Onofrio A, Katz N, et al. (2008) The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). Int J Artif Organs 31: 166-178.
- Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, et al. (2006) Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol 1: 43-51.
- De Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, et al. (2000)Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26: 915-921.
- Holley JL (2009) Clinical approach to the diagnosis of acute renal failure. In: Primer on Kidney Diseases. Greenberg A, Cheung AK (5th edn) National Kidney Foundation, Philadelphia.
- Iglesias J, Liberthal W (2000) Clinical evaluation of acute renal failure. In: Comprhensive clinical nephrology. Johnson RJ, Feehally I, Mosby, London.
- Bonventre JV, Weinberg JM (2003) Recent advances in the pathophysiology of ischemic acute renal failure.J Am Soc Nephrol 14: 2199-210.
- Burnstock G, Evans LC, Bailey MA (2014) Purinergic signalling in the kidney in health and disease. Purinergic Signal 10: 71-101.
- Osswald H, Vallon V, Mühlbauer B (1996) Role of adenosine in tubuloglomerular feedback and acute renal failure. J Auton Pharmacol 16: 377-380.
- Solez K (1983) Acute renal failure. In: Pathophysiology of the kidney. Heptinstall R, Little, Brown and Company, Toronto.
- Basile DP, Anderson MD, Sutton TA (2012) Pathophysiology of Acute Kidney Injury. Comp Physiol 2: 1303-1353.
- Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334: 1448-1460.
- Bagga A, Bakkaloglu A, Devarajan P, Mehta RL, Kellum JA, et al. (2007) Improving outcomes from acute kidney injury: Report of an initiative. Pediatr Nephrol 22:1655-1658.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, et al. (2004) Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Crit Care 8: R204-212.
- Lopes JA, Jorge S (2013) The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin Kidney J 6: 8-14.
- McCullough PA, Kellum JA, Mehta RL, Murray PT, Ronco C (2013) ADQI Consensus on AKI Biomarkers and Cardiorenal syndromes. Karger, Switzerland.
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, et al. (2007) Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31.
- Englberger L, Suri RM, Li Z, Casey ET, Daly RC, et al. (2011) Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care 15: R16.
- Lameire N, Van Biesen W, Vanholder R (2006) The rise of prevalence and the fall of mortality of patients with acute renal failure: what the analysis of two databases does and does not tell us. J Am Soc Nephrol 17: 923-925.
- Cerdá J, Lameire N, Eggers P, Neesh P, Sigehiko U, et al. (2008) Epidemiology of acute kidney injury. Clin J Am Soc Nephrol 3: 881-886.
- Lameire N, Van Biesen W, Vanholder R (2006) The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol 2: 364-377.
- Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, et al. (2006) Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135-1142.
- Liaño F, Pascual J (1996) Epidemiology of acute renal failure: A prospective, multicenter, community-based study, Madrid Acute Renal Failure Study Group. Kidney Int 50: 811-818.
- Ali T, Khan I, Simpson W, Prescott G, Townend J, et al. (2007) Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol 18:1292-1298.
- Abraham G, Gupta RK, Senthilselvan A, Van Der Meulen J, Johny KV, et al. (1989) Cause and prognosis of acute renal failure in Kuwait: A 2-year prospective study. J Trop Med Hyg 92: 325-329.
- Noronha IL, Schor N, Coelho SN, Jorgetti V, Romão Júnior JE, et al. (1997) Nephrology, dialysis and transplantation in Brazil. Nephrol Dial Transplant 12: 2234-2243.
- Srivastava RN, Bagga A, Moudgil A (1990) Acute renal failure in north Indian children. Indian J Med Res 92: 404-408.
- Arora P, Kher V, Rai PK, Singhal MK, Gulati S, et al. (1997) Prognosis of acute renal failure in children: a multivariate analysis. Pediatr Nephrol 11: 153-155.
- Abdel-Kader K, Palevsky PM (2010) Acute kidney injury in the elderly. Clin Geriatr Med 25: 331-58.
- Pounds LL, Teodorescu VJ (2013) Chronic kidney disease and dialysis access in women. J Vasc Surg 57: 49-53S.
- Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, et al. (2009) United States Renal Data System 2008 Annual Data Report. Am J Kidney Dis 53: S1-374.
- Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, et al. (2005) Chronic kidney disease awareness, prevalence, and trends among US adults, 1999 to 2000. J Am Soc Nephrol 16: 180-188.
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, et al. (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247-254.
- Perrone RD, Madias NE, Levey AS (1992) Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 38: 1933-1953.
- Del Giudice A, Piemontese M, Valente G, Prencipe M, Di Giorgio C, et al. (2012) Acute Kidney Injury in the Elderly: Epidemiology, Risk Factors and Outcomes. J Nephrol Therapeut 2: 1-8.
- Nitsch D, Grams M, Sang Y, Black C, Cirillo M, et al. (2013) Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: A meta-analysis. BMJ 346: f324.
- Thakar CV, Christianson A, Himmelfarb J, Leonard AC (2011) Acute Kidney Injury Episodes and Chronic Kidney Disease Risk in Diabetes Mellitus. Clin J Am Soc Nephrol 6: 2567–2572.
Citation: Mosa OF, Fouad MA, Zafar TA, Fahmy AM, Alyazidi F, et al. (2017) Epidemiology of Acute Kidney Injury (AKI) among Hospitalized and Outpatients Frequent to Al-Lieth Kidney Unit (AKU). Epidemiology (Sunnyvale) 7:317.
Copyright: © 2017 Mosa OF, et.al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3889
- [From(publication date): 0-2017 - May 01, 2025]
- Breakdown by view type
- HTML page views: 3048
- PDF downloads: 841