Letter to Editor Open Access
First Report of Histological Evaluation of Human Tissue-Engineered Vasculature
Goki Matsumura1* and Toshiharu Shinoka21Department of Cardiovascular Surgery, The Heart Institute of Japan, Tokyo Women’s Medical University, Shinjuku, Tokyo 162-8666, Japan
2Department of Cardiothoracic Surgery, The Heart Center, Nationwide Children’s Hospital, 700 Children’s Drive, T2294, Columbus, OH 43205, USA
- Corresponding Author:
- Goki Matsumura, MD, PhD
Department of Cardiovascular Surgery
The Heart Institute of Japan
Tokyo Women’s Medical University
Shinjuku, Tokyo 162-8666, Japan
Tel: +81-3-3353-8111
Fax: +81-3356-0441
Email: smatumur@hij.twmu.ac.jp
Received date: September 07, 2015; Accepted date: September 21, 2015; Published date: September 27, 2015
Citation: Matsumura G, Shinoka T (2015) First Report of Histological Evaluation of Human Tissue-Engineered Vasculature. J Biotechnol Biomater 5:200. doi:10.4172/2155-952X.1000200
Copyright: © 2015 Matsumura G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
From September 2001 to December 2004, 25 patients underwent a Fontan procedure using tissue-engineered vasculature (TEV) under informed consent and institutional review board approval [1]. Six of these 25 patients died, although no graft-related mortality occurred. The case presented herein is the first case in which the family agreed to the performance of an autopsy to elucidate the late-term histological characteristics of human TEV.
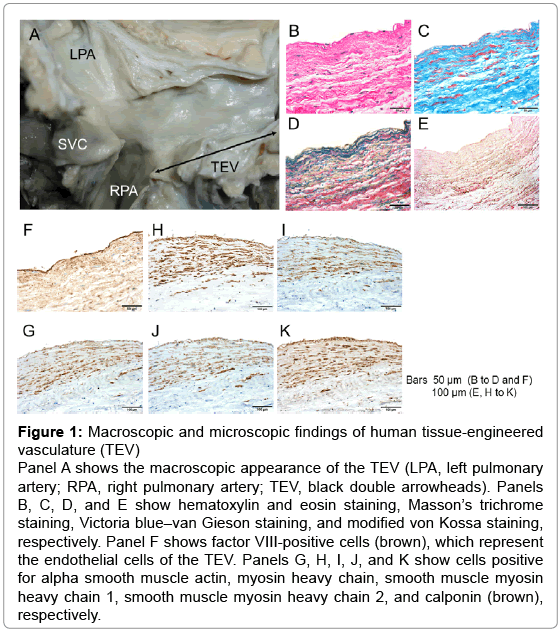
A 16-year-old girl had a history of heart murmur and cyanosis at birth, followed by diagnoses of heterotaxy syndrome (polysplenia), a single right ventricle, pulmonary stenosis, bilateral superior vena cava, a hemiazygos connection, and a common atrium with mild to moderate regurgitation from a common atrioventricular valve. She underwent a Fontan procedure with common atrioventricular valve plasty following staged operations at the age of 4 years. A 12-mm-diameter biodegradable scaffold seeded with mononuclear bone marrow cells was interposed between the hepatic vein and pulmonary artery to accomplish the Fontan procedure [1]. At the age of 16 years, she died of multiple organ failure, low cardiac output syndrome, disseminated intravascular coagulation, pancytopenia, and brain abscessation. An autopsy of the patient was performed, and the tissue samples were evaluated either macroscopically or microscopically for histological analyses. Macroscopic examination showed well-formed tissueengineered vessels connected to the pulmonary artery (Figure 1A).
Figure 1: Macroscopic and microscopic findings of human tissue-engineered vasculature (TEV) Panel A shows the macroscopic appearance of the TEV (LPA, left pulmonary artery; RPA, right pulmonary artery; TEV, black double arrowheads). Panels B, C, D, and E show hematoxylin and eosin staining, Masson’s trichrome staining, Victoria blue–van Gieson staining, and modified von Kossa staining, respectively. Panel F shows factor VIII-positive cells (brown), which represent the endothelial cells of the TEV. Panels G, H, I, J, and K show cells positive for alpha smooth muscle actin, myosin heavy chain, smooth muscle myosin heavy chain 1, smooth muscle myosin heavy chain 2, and calponin (brown), respectively.
The tissue samples were then fixed in 10% formaldehyde, embedded in paraffin, and sectioned at 4 to 5 μm. Some sections were subjected to hematoxylin and eosin, Masson’s trichrome, Victoria blue–van Gieson, or modified von Kossa staining (Figure 1B–E), which showed components of the four basic vascular layers (endothelial cells, vascular smooth muscle cells, elastic fibers, and collagen fibers) 12 years after implantation of the TEV. Calcified lesions (black-spots lesions) were not observed in any samples (Figure 1E). Immunostaining of the remaining sections was performed as previously described [2-4] using antibodies to factor VIII, alpha smooth muscle actin, myosin heavy chain, smooth muscle myosin heavy chain 1, smooth muscle myosin heavy chain 2, and calponin. Endothelialization (Figure 1F) and mature vascular smooth muscle cell proliferation (Figure 1G–K) were observed in the human TEV.
In conclusion, this is the first report of tissue generation of TEV in human samples, suggesting the usefulness of the TEV strategy in the repair of congenital heart disease. Previous studies have involved only animals [2-4]. This technique allows for the development of a biological conduit with adequate histological maturation in vivo and good longterm results.
Acknowledgements
The authors thank Dr. Motoko Kawaguchi, Department of Pathology 1, Tokyo Women’s Medical University, for help in sample collection and sample preparation. Dr. Shinoka has received grant support from Gunze Ltd.
References
- Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, et al. (2010) Late-term results of tissue-engineered vascular grafts in humans. See comment in PubMed Commons below J ThoracCardiovascSurg 139: 431-436, 436.
- Matsumura G, Nitta N, Matsuda S, Sakamoto Y, Isayama N, et al. (2012) Long-term results of cell-free biodegradable scaffolds for in situ tissue-engineering vasculature: in a canine inferior vena cava model. PLoS One 7, e35760
- Matsumura G, Isayama N, Matsuda S, Taki K, Sakamoto Y, et al. (2013) Long-term results of cell-free biodegradable scaffolds for in situ tissue engineering of pulmonary artery in a canine model. See comment in PubMed Commons below Biomaterials 34: 6422-6428.
- Matsumura G, Miyagawa-Tomita S, Shin'oka T, Ikada Y, Kurosawa H (2003) First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. See comment in PubMed Commons below Circulation 108: 1729-1734.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14443
- [From(publication date):
September-2015 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 9829
- PDF downloads : 4614