Head and Neck Abscesses in Complicated Acute Otitis Media-Pathways and Classification
Received: 02-Mar-2018 / Accepted Date: 19-Mar-2018 / Published Date: 26-Mar-2018 DOI: 10.4172/2161-119X.1000345
Abstract
Objective: To investigate the pathways of inflammatory spread of extratemporal otogenic complications and present the classification including extratemporal spread of otitis media and mastoiditis. Study design: A retrospective case series at an academic referral center, observational study.
Methods: Literature review of rare emergency cases in the course acute otitis media with the development of head and neck abscess. Presentation of different pathways on the lateral and inferior wall of the temporal bone found in computed tomography and during surgery.
Results: Inflammatory spread to head and neck following acute otitis media may lead through the mastoid cells (with mastoiditis), external auditory canal, or Eustachian tube. Bezold abscess is the most common extratemporal otogenic neck abscess, but the inflammation may pass through any area of either inferior or lateral wall of the temporal bone, leading to extratemporal complications.
Conclusion: Head and neck abscess may complicate the course of AOM and emerges when destruction of lateral or inferior temporal bone surface develops. We can distinguish pathways of inflammatory spread from middle ear to extracranial compartment what should find place in classification of AOM complications.
Keywords: Otogenic complications; Mastoiditis; Head and neck abscess; Bezold abscess; Temporal bone
Introduction
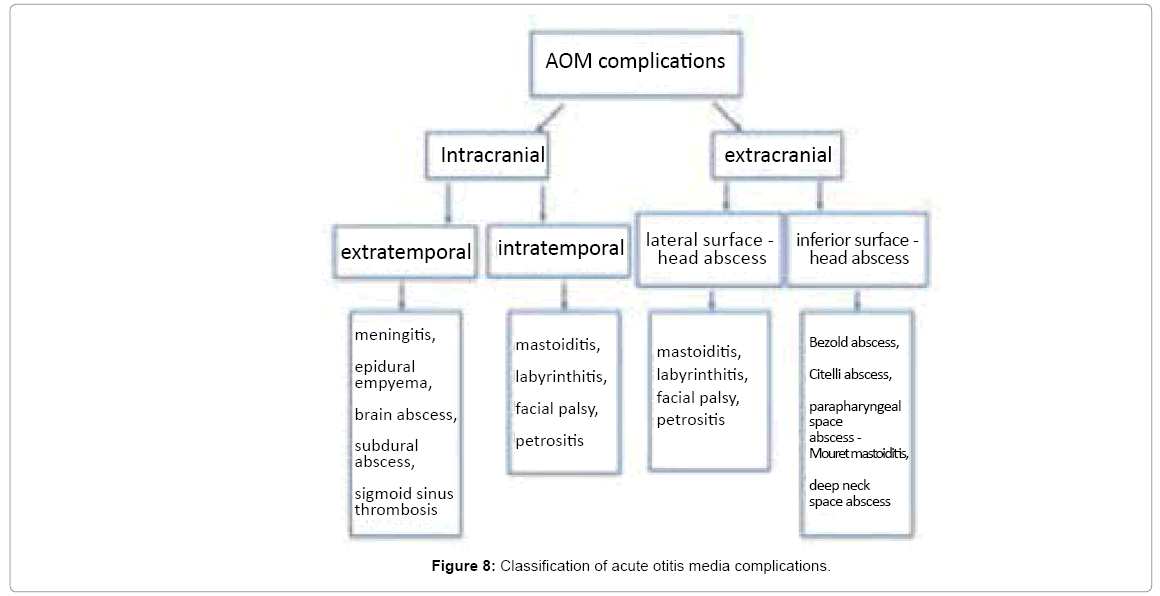
Acute otitis media (AOM) complications are commonly divided into intratemporal and intracranial complications. Intratemporal complications of AOM include mastoiditis, labyrinthitis, facial nerve paresis and petrous apicitis. Mastoiditis, which is the most common AOM complication, can lead to the development of subperiosteal abscess. Subperiosteal abscesses may be formed in the following directions from temporal bone:
· Laterally to mastoid process, in retroauricular area-the retroauricular subperiosteal abscess [1,2],
· Inferiorly, through apex of mastoid process, in the neck Bezold abscess [3-6],
· Posteriorly to mastoid process, heading towards occipital bone and developing in the neck area Citelli abscess [7,8],
· Superior and anterior, through external auditory canal, to zygoma Luc abscess [9-12],
· Medial and inferior from mastoid process-parapharyngeal space abscess/retropharyngeal abscess [13,14].
Abscesses due to otitis media can also develop intracranially, where they are most common in petrous apex (epidural abscess or empyema), in middle cranial fossa (temporal lobe abscess), or posterior cranial fossa (cerebellar abscess).
Methods
We performed a literature review of complications in the course AOM with formation of an extratemporal complication. We searched PubMed database, Google and Google Books for the key words such as Bezold abscess, Citelli abscess, Luc abscess, Mouret mastoiditis and otogenic abscess. Next we present four patients with different pathways of inflammatory spread on the lateral and inferior wall of temporal bone that reported to our Emergency Department between 2008 and 2017 and were then admitted our Otorhinolaryngology Department. We present a thorough analysis of computed tomography and intraoperative findings. Finally we present of a systematized division of otogenic complications including extratemporal complications [15-20].
Results
A literature review showed various pathways of head and neck abscesses due to AOM including Bezold abscess, Citelli abscess, Luc abscess, or parapharyngeal space abscess. Mastoid pneumatic cell tracts closure during AOM plays major role in the pathogenesis of otogenic head and neck abscesses. Osteomyelitis underlying the bone damage with perforation of the mastoid walls is most commonly observed.
Mastoid apex disease and neck abscess was first described by German otologist Friedrich Bezold in 1881 as a complication of otitis media. Contemporarily Bezold abscess occurs with a frequency of 6% among mastoid process abscesses [21]. Among the predispositions of developing otogenic abscesses he mentioned: superficial cells in the mastoid process, thin bony cellular septations and single large cells. In addition, he mentioned that step-by-step, mastoiditis can spread outside mastoid borders in every direction. Retroauricular subperiosteal abscess is the most common complication of mastoiditis and it is usually found in children. Such coincidence also complies with Bezold thesis in children the largest cell is antrum and thus most frequently occurring abscess is the one in the antrum projection on the lateral surface of the temporal bone. Bezold abscess is usually described running down the neck through the apex and medial wall of the mastoid process. It extends along the sternocleidomastoid muscle or along the posterior belly of digastric muscle inferiorly [22].
Citelli abscess is way less frequently mentioned in literature than Bezold abscess and was first described in 1901 by Citelli [23]. He described cells in posterior and superior part of mastoid process that may create a corridor for pus heading the neck during mastoiditis. This abscess is located posteriorly to mastoid process – between the mastoid and occipital bone [24]. Another hypothesis of Citelli abscess genesis involves mastoid emissary vein route or occipitomastoid suture pathway [9].
The gravitational abscesses spread from mastoid process either inferiorly or posteriorly along the sternocleidomastoid or digastric muscle. Moreover, the space between those two muscles contains the attachment of splenius and longissimus capitis muscle, which may contribute to the spread of an abscess along these structures. The simplest division of these abscesses is based on their location: Bezold abscess emerges under the mastoid process, while Citelli posteriorly to mastoid process. Decision whether Bezold or Citelli does not change the scope of surgical treatment but may direct the surgeon within the mastoid.
Jules Mouret, in 1914 pointed out a complication he called cervicodigastric inflammation of the mastoid process [25]. Mouret mastoiditis progresses through the subtympanic and perifacial cells medial to jugular process of occipital bone. Mouret described a region within mastoid process, called digastric triangle corresponding to digastric bulla in posterior two-thirds (a small hump separated laterally from mastoid process with digastric fossa) and to lateral sinus in anterior one-third (bony base of mastoid antrum). Described route of infection through medial wall of mastoid process (osteomyelitis), can lead to parapharyngeal abscess formation.
In 1913, Henri Luc described his experience of subperiosteal temporal bone abscess and identified cases with no mastoid involvement when performing antrotomy. He found that middle ear infection can spread above the notch of Rivinius, or along the branches of profound auricular artery and external auditory canal thus leading to extratemporal and subperiosteal pus formation under the temporal muscle [10,11,26].
Case series
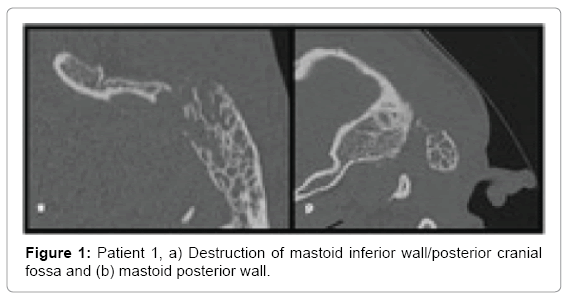
Patient 1: A 27 year old male admitted to hospital due to increasing pain in the area of mastoid process and neck on the left side. History revealed upper respiratory tract infection about month earlier, followed by left ear pain with purulent ear discharge. Patient received treatment with amoxicillin and clavulanic acid for 7 days. Shortly after treatment, symptoms returned, with pain and mastoid and neck swelling on the same side with torticollis. He started topical course of ciprofloxacin with fluocinolone. Complaints have decreased but he felt some discomfort and he showed up at Emergency Department. Physical examination revealed swelling and tenderness in retroauricular space, towards the occiput, with local skin reddening and protruding ear on the left side. Otoscopy showed red and thickened tympanic membrane with irregular surface and central perforation.
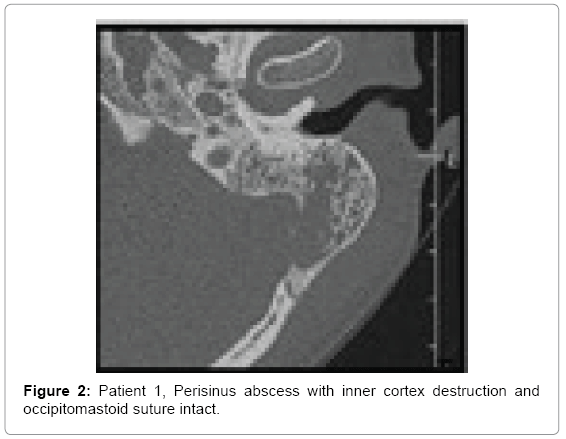
Computed tomography of temporal bones revealed bone destruction and soft tissue edema above the mastoid (Figures 1 and 2). Immediate wide antromastoidectomy with abscess drainage alltogether with intravenous antibiotic therapy was implemented. The culture taken intraoperatively led to isolation of Streptococcus pyogenes. Initial treatment with cefuroxime and clindamycin i.v was continued for 9 days and then oral cefuroxime axetil for another 7 days. Tympanic membrane perforation healed with no residual disease and no necessity of myringoplasty.
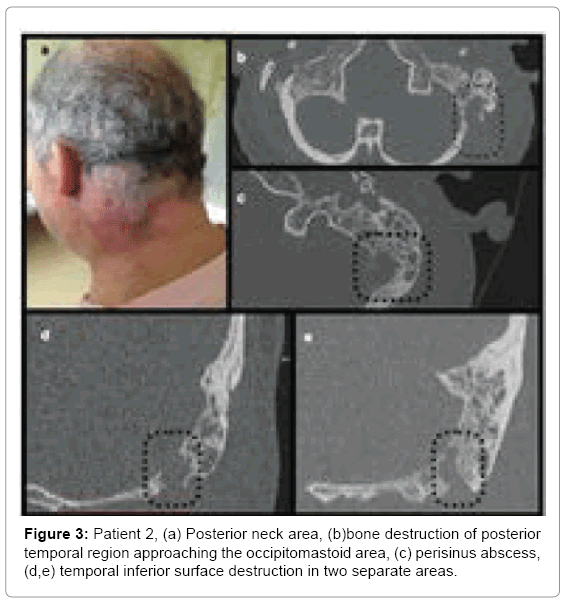
Patient 2: A 56 year old male admitted to hospital due to AOM with otorrhoea and swelling/redness in the postauricular area and neck on the left side (Figure 3a), with torticollis and lockjaw. History revealed left ear pain that started 10 days prior to admission. Two days after onset the swelling appeared in postauricular and neck area on the left side and gradually exacerbated. After 4 days patient noticed purulent discharge. In the outpatient clinic general practitioner prescribed amoxicillin and noticed improvement pain decreased and ear discharge resolved. Neck ultrasound performed 4 days before hospitalization, showed reactive lymph nodes on the left side, in the mandible angle down the supraclavicular area without any visible pathological reservoirs but consulting laryngologist referred him to hospital immediately after examination.
Patient’s history also revealed total thyroidectomy due to follicular thyroid cancer many years earlier followed by two courses of radiotherapy.
Computed tomography of the neck and temporal bones without contrast showed opacification of all mastoid cells and tympanic cavity (Figure 3). Below the mastoid a large infiltration with possible fluid spaces was found, extending to the neck muscles.
Patient underwent left side antromastoidectomy with neck abscess drainage. Culture results were negative. Pharmacological treatment included: ceftriaxone with metronidazole (7 days) followed by clindamycin (14 days). His follow-up was uneventful.
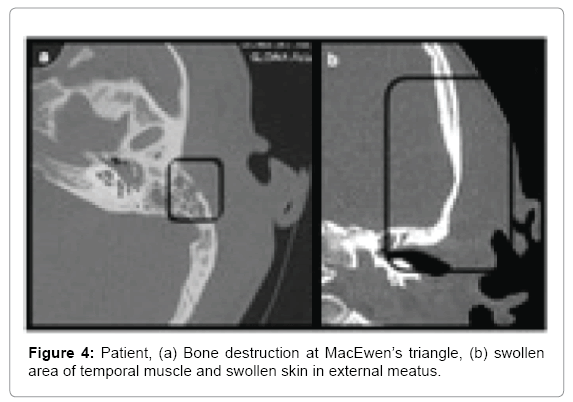
Patient 3: A 29 year old male admitted to hospital due to hemifacial left side pain. Prior to admission treated with ciprofloxacin (for 7 days) due to AOM with high fever. Physical examination revealed swelling on the left side of the face (cheek, upper and lower eyelid) reaching retroauricular area and neck. On palpation, there was lymphadenopathy with painful, but movable lymph nodes on the left side. Otoscopy revealed moderate edema of the external auditory canal with granulation tissue inferiorly in external auditory canal with present purulent discharge in this area. The tympanic membrane was thickened and reddened. Patient’s history and examination showed severe scoliosis, resulting in significant deformation of the body. Computed tomography revealed minor bone loss in the area of planum mastoideum on the left side with swelling of the temporal muscle region (Figure 4).
Immediately, antromastoidectomy with ear drainage was performed. Simultaneously a large subperiosteal abscess formed under the temporal muscle was drained. It was extending from the planum mastoideum over the entrance to external auditory canal, up to zygomatic arch anteriorly. Cultures enabled isolation of Streptococcus pneumoniae.
Patient was treated with clindamycin and ceftriaxone for 9 days, followed by cefuroxime axetil for 7 days.
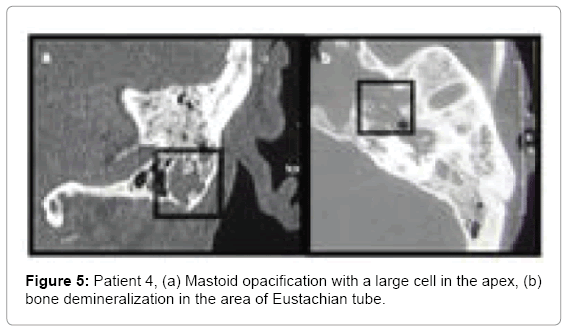
Patient 4: A 61 year old female with coronary artery disease, hypertension and insulin-dependent diabetes was admitted to hospital with symptoms: permanent and severe headaches for about 2 months, radiating to the temporal area and forehead, resistant to analgesics, without fever. Examination showed edema of the posterior wall of external auditory canal with a pulsating discharge through the perforation in the tympanic membrane; polyp in the left nasal cavity; asymmetry of the glossopharyngeal arches, anteriorly pushed left tonsil with simultaneous immobilization of the left glossopharyngeal arch. Facial nerve function was normal. History of ear diseases was negative. Before admission to hospital she was treated with ceftazidime and ciprofloxacin for 3 weeks. Cultures taken prior to admission revealed methicillin-susceptible Staphylococcus aureus and coagulasenegative Staphylococcus spp. Hospital taken cultures led to isolation of Pseudomonas aeruginosa and Pseudomonas putida.
Computed tomography images showed inflammatory edema in the left parapharyngeal space. Bone of the auditory tube was eroded (Figure 5). Initially patient was treated empirically with amoxicillin with clavulanic acid and metronidazole and after swab results with ciprofloxacin (20 days) and ceftriaxone for 14 days.
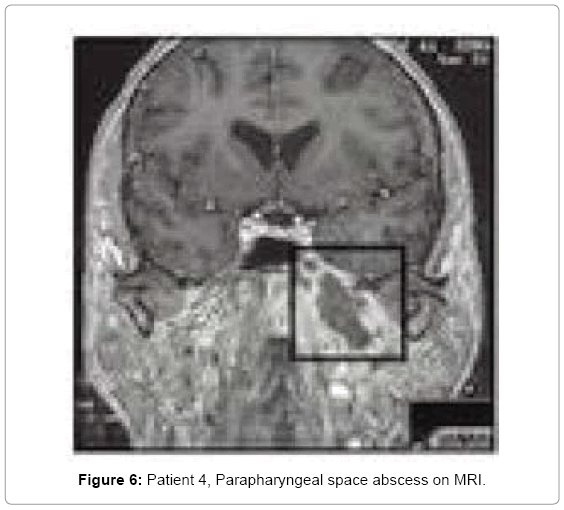
Due to poor glycaemia control, her diabetes treatment was modified. After 48 h of culture directed antibiotic therapy with no improvement, an anastromastoidectomy was performed. Constant ear discharge and no change in the neck area, led to MRI of the head and neck a polycystic parapharyngeal space abscess was found (Figure 6). It was drained through the external approach. Drainage was left for 3 days. After ear surgery only minor discharge continued through tympanic membrane perforation. Headaches decreased but did not cease. A week after parapharyngel abscess drainage, ear was completely dry and headaches ceased.
Discussion
Otogenic complications most frequently occur in children and adults up to the age of 30 [21]. Our material included only adults two patients had complications in the third decade of life, while the remaining two were in 6 and 7 decade of life. Younger patients did not present significant comorbidities. On the other hand, our patients in the 6-7th decade of life were carrying extra burden of radiotherapy in the neck area due to thyroid cancer (patient 2) and diabetes (patient 4).
Their history of ear disease was short and the most prominent sign leading to diagnosis was the recurrence of symptoms or their exacerbation after initial treatment. In three cases (patients 1-3), head and/or neck swelling with simultaneous coexisting swelling of posterior wall of external auditory canal was present. In two cases torticollis (patients 1 and 2) and lockjaw (patients 1 and 3) was observed. Among the symptoms the most commonly described are fever (not necessarily high), ear pain and neck swelling, redness of the swollen skin, otorrhoea with tympanic membrane perforation, hearing deterioration, torticollis, neck pain and less often facial nerve paralysis on the same side as ear infection [1,2,21].
The risk of complications in AOM is higher in people with immunosuppression (HIV, after radiotherapy), diabetes [6,19,27] or after mastoidectomy [15,16]. In our case series, patients 1 and 3 presented no risk factors and still developed head and neck abscess in the course of AOM.
Imaging is the most important diagnostic tool when dealing with otogenic complications. Recently ultrasonography is being suggested as helpful in differentiating soft tissues changes. Ultrasonography is a valuable and rapid test that allows visualizes and assess the pathological neck reservoir. It is especially useful for those abscesses, which are not palpable. This study may help to differentiate whether we are dealing with lymphadenopathy or with fluid reservoir [18].
Computed tomography is the method of choice allowing evaluation of bone destruction, enabling differentiating the suspected abscess formation with other pathologies such as neoplasms or coexistence of other complications. It may help recognizing the coexistence of other complications, but also allows surgeon planning the surgery and its risk [1,3,15-17,20]. Diagnostic accuracy of this method reaches 87-100%. Images usually present inflammatory tissue or fluid filling mastoid process and tympanic cavity, but also demineralization and destruction of temporal bone and its surroundings. CT with contrast helps assess abscess borders in soft tissues [16].
In our case series, three males had CT scan performed without contrast in our Emergency Department prior to admission. Image results altogether with physical examination and history allowed the diagnosis of mastoiditis with strong suspicion of an abscess. Ear surgery was performed immediately in all cases. CT results were the following:
• Patient 1-large antral cell within mastoid process, destruction of postero-inferior portion of mastoid process heading occipito-mastoid suture and posterior cranial fossa; spread of inflammation into the neck tissues through the medial wall of the mastoid process within its postero-inferior portion changes suggest typical Bezold abscess (Figures 1 and 2).
• Patient 2-antrum divided with Koerner’s septum, large cell with cortical layer destruction in postero-superior portion of mastoid process (Figure3b), continuity of inflammatory changes between neck and mastoid in two sites: one located medially and posteriorly to incisura digastrica changes suggesting Citelli’s abscess (Figures 3c and 3d) and another one at incisura digastrica (Figure 3e).
• Patient 3-bone destruction above postero-superior wall of external auditory canal, at MacEwen’s triangle; swelling of external auditory canal skin running into the tissues superior to the auricle, swelling in the area of temporal muscle and zygomatic arch (Figure 4). Except for a small destruction at MacEwen’s triangle, continuity of inflammatory changes suggested Luc abscess.
• Patient 4 mastoid process partially opacified, with large cells at the apex of mastoid process. Eustachian tube and sphenopetrous fissure area with local bony destruction (osteomyelitis) (Figure 5). Parapharyngeal abscess in the MRI study (Figure 6).
Computed tomography in our first patient, performed without contrast, did not show an evident abscess, but bone destruction was obvious. Superficially located neck abscess was diagnosed and drained intraoperatively (Bezold abscess).
Physical examination in patient 2 suggested an abscess but CT without contrast did not show it clearly. Intraoperative abscess location differed from the first one as it was found deeper in the neck tissues and suggested spread along the splenius capitis muscle (Citelli abscess).
First two cases similarly present with inflammation in the mastoid with evident bone destruction. In both patients, we see large cells in mastoid process separated with smaller ones from the antral cell, damage of cortical layer and presence of abscesses in the neck area in continuity with these cells. The cells were located either superoposteriorly or infero-posteriorly in the mastoid. They also shared thin bony septations and close connection with thin cortical bone layer, also mentioned by Bezold. Both patient 1 and 2 have developed perisinus abscess with bone destruction of mastoid process adjacent to posterior cranial fossa and sigmoid sinus (Figure 3c). Decision whether it is Bezold or Citelli does not change the scope of surgical treatment but can direct the surgeon within the mastoid.
Third case was tricky in evaluation because we can see bone destruction in the area of MacEwen’s triangle and opacification of the mastoid process altogether with changes along the superior wall of external auditory canal running into soft tissues above superior attachment of the auricle. The second route reflects Luc abscess while the first one subperiosteal retroauricular abscess. Intraoperative finding of purulent compartment superior to the attachment of the auricle, elevating the periosteum beneath temporal muscle and heading towards zygoma leans us rather towards Luc abscess, but still both pathways are possible. The patient underwent immediate antromastoidectomy due to mastoiditis with a suspicion of an abscess based on examination bulging superiorly and posteriorly to the auricle with auditory canal wall collapse. CT performed without contrast did not show the abscess clearly but bone destruction. Intraoperative pus reservoir was opened underneath the temporal muscle and was heading zygoma (as Luc abscess).
In three patients who had CT routinely performed without contrast in ED, ultrasonography could be a valuable addition towards more precise preoperative diagnosis.
In fourth case the pathway of inflammatory spread was completely different from others. Here, the middle ear infection led to osteomyelitis of the skull base in the Eustachian tube area, at the fusion of temporal and sphenoid bone. Osteomyelitis usually occurs due to malignant external otitis and most commonly is diagnosed in patients with diabetes [28]. It involves the bone of external auditory canal. In our patient the same pathogen as in malignant external otitis was found P. aeruginosa. Computed tomography with contrast showed middle ear changes with bone demineralization around Eustachian tube and large inflammatory infiltration of skull base. Even though patient underwent ear surgery and pharmacotherapy was culture-guided, inflammatory infiltration of skull base processed into an abscess in parapharyngeal space. Here, we benefited from MRI that showed us location of carotid sheath and enabled planning abscess drainage from external approach. Our fourth case was different from others and treatment was more challenging. Patient was diagnosed with otitis media and skull base osteomyelitis. She underwent antromastoidectomy with mastoid drainage and as the parapharyngeal space inflammatory infiltration progressed into an abscess it was subsequently drained from external approach. Pharmacotherapy changed during treatment due to isolation of P. aeruginosa. Treatment difficulties comprised unstable diabetes with poor toleration of steroids enrolled initially in order to decrease edema.
Surgery in otogenic complications with head and neck abscess usually requires wide antromastoidectomy enabling removal of the inflammatory tissues and opening the temporal bone pneumatic tracts. Neck abscess should be drained altogether with ear surgery. In isolated cases of Luc abscess the abscess drainage is reported to be sufficient [10-12].
Analysis of presented case series, our experience and published literature put forward the following routes of ear inflammatory spread of head and neck:
1. Through mastoid cells (mastoiditis) patients 1,2 and 3
2. Through external auditory canal (Luc abscess) patient 3
3. Through the Eustachian tube patient 4.
On the other hand extratemporal otogenic complications can be divided according to direction they develop:
• Through lateral surface of temporal bone with formation of:
• Retroauricular, subperiosteal abscess
• Luc abscess (through external auditory canal)
• Through inferior surface of temporal bone with formation of:
• Bezold abscess inferiorly to lateral portion of mastoid
• Citelli abscess postero-inferiorly to mastoid
• Deep neck space abscess, parapharyngeal space abscess through Mouret mastoiditis (jugulo-digastric mastoiditis) inferiorly to the medial portion of mastoid, or through Eustachian tube area.
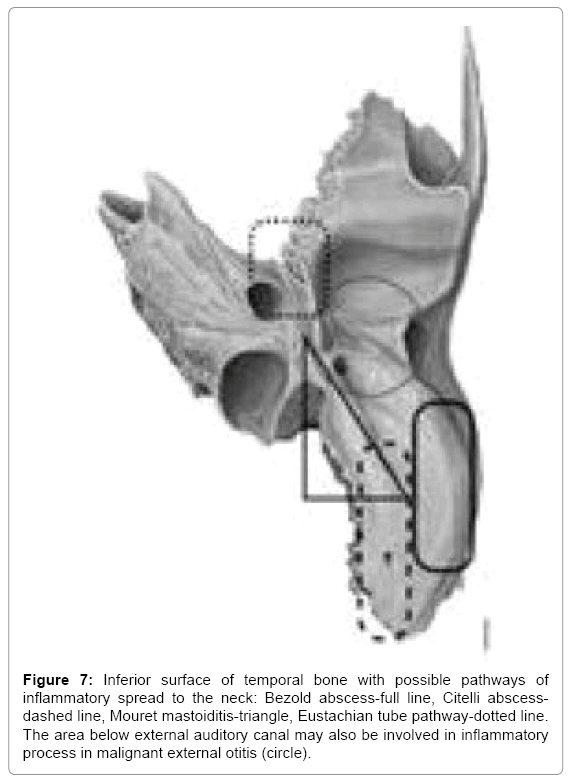
We might also look at these abscesses as head abscesses emerging on the lateral surface of temporal bone or neck abscesses when they develop through inferior surface of temporal bone. More challenging they get when we set them in order from lateral to medial starting with Luc abscess and retroauricular subperiosteal on the lateral surface through Bezold abscess (along sterno-cleido-mastoid muscle) and Citelli abscess (laterally to digastric muscle attachment at skull base, along splenius capitis or longissimus capitis muscle) and finally the most medial deep neck space abscess, parapharyngeal space abscess (medially to digastric muscle attachment jugulo-digastric area or Eustachian tube area) (Figure 7). Last pathway is usually referred to in literature as skull base osteomyelitis [29,30].
Figure 7: Inferior surface of temporal bone with possible pathways of inflammatory spread to the neck: Bezold abscess-full line, Citelli abscessdashed line, Mouret mastoiditis-triangle, Eustachian tube pathway-dotted line. The area below external auditory canal may also be involved in inflammatory process in malignant external otitis (circle).
Classification of otogenic complications
Contemporary classification of otogenic complications mark out intratemporal complications (mastoiditis, labyrinthitis, facial palsy, petrositis) and intracranial complications (meningitis, epidural empyema, brain abscess, subdural abscess and sigmoid sinus thrombosis). Such division completely ignores extratemporal head and neck abscesses. Thus we propose widening the spectrum of otogenic complications present in our practice, described in literature but somehow neglected in classification. Figure 8 presents the division of otogenic complications into intracranial (including extratemporal and intratemporal) and extracranial (including lateral surface and inferior surface complications).
As the presented classification is based on anatomical background, it seems worth underlining that the more medial the complication gets the more difficult it may become to treat. The classification does not exclude coexistence of a few complications.
Conclusion
Head and neck abscess may complicate the course of AOM and emerges when destruction of lateral or inferior temporal bone surface develops. For the last decade we have observed in adults more atypical localizations of otogenic head and neck abscesses compared to most typically reported retroauricular subperiosteal abscess.
We can distinguish the following direct pathways of inflammatory spread from middle ear (intratemporal) to extracranial compartment:
1. Mastoid cells
• Lateral surface of temporal bone retroaricular subperiosteal abscess
• Inferior surface of temporal bone Bezold abscess, Citelli abscess, skull base osteomyelitis with Mouret mastoiditis
2. External auditory canal
• Luc abscess
3. Auditory tube
• Skull base osteomyelitis
We observe that the more medial is the pathway of inflammatory spread, the more difficult the treatment becomes. Challenging cases have comorbidities such as diabetes or past radiotherapy in our case series.
References
- Ghadersohi S, Young NM, Smith-Bronstein V, Hoff S, Billings KR (2016) Management of acute complicated mastoiditis at an urban, tertiary care pediatric hospital. Laryngoscope 127: 2321-2327.
- Krajewska A, S´miechura M, Struzycka M, Makowska-Piontek A, Konopka W (2012) Acute mastoiditis with subperiosteal abscess in children. Otorynolaryngologia 11: 22-26.
- Nelson D, Jeanmonod R (2013) Bezold abscess: A rare complication of mastoiditis. Am J Emerg Med 31: 1626.
- McMullan B (2009) Bezold's abscess: A serious complication of otitis media. J Paediatr Child Health 45: 616-618.
- Â Doan NM, Levy C (2018) Bezold abscess: A compication of mastoiditis.
- Spiegel JH, Lustig LR, Lee KC, Murr AH, Schindler RA (1998) Contemporary presentation and management of a spectrum of mastoid abscesses. Laryngoscope 108: 822-828.
- Quoraishi S, George J, Farboud A, Conor M (2017) Atypical presentation of Bezold's and Citelli's abscesses, with recollection following an incomplete postoperative course of antibiotics. BMJ Case Rep 17: bcr2016218072.
- Sahoo A, Preetam Ch, Samal D, Sarkar S (2017) Citelli’s abscess following otitis media: A case report. Iran J Otorhinolaryngol 29: 161-163.
- Scrafton DK, Qureishi A, Nogueira C, Mortimore S (2014) Luc's abscess as an unlucky complication of mastoiditis. Ann R Coll Surg Engl 96: e28-e30.
- Knappe MV, Gregor RT (1997) Luc's abscess--a rare complication of middle-ear infection. J Laryngol Otol 111: 461-464.
- Gan JY, Kiaang Tan HK, Koh LH (2016) Luc’s abscess masquerading as severe otitis externa. Int J Pediatr Otorhinolaryngol 16: 10e13.
- Bihani A, Dabholkar JP (2015) A rare case of Bezold’s abscess presenting as parapharyngeal abscess. Int J Otorhinolaryngol Head Neck Surg 1: 48-51.
- Mydam J, Thiagarajan P (2009) A nine month old child with retropharyngeal abscess sec-ondary to mastoid abscess presenting as torticollis: A case report. Cases J 2: 6460.
- Dimatos SC, Neves LR, Ramos HL, Dimatos OC (2009) Bezold's abscess: Case report and literature review. Einstein 7: 369-371.
- Castillo M, Albernaz VS, Mukherji SK, Smith MM, Weissman JL (1998) Imaging of Bezold's abscess. AJR Am J Roentgenol 171: 1491-1495.
- Pradhananga R (2014) An unusual complication of chronic suppurative otitis media: Bezold abscess progressing to scapular abscess. Int Arch Otorhinolaryngol 18: 412-414.
- Secko M, Aherne A (2013) Diagnosis of Bezold Abscess Using Bedside Ultrasound. J Emerg Med 44: 670-672.
- Patel N, Goodman J, Singh A (2010) Bezold's abscess in the setting of untreated HIV infection. Laryngoscope 120 Suppl 4: S134.
- Lazim NM (2011) An Extensive Cholesteatoma with Bezold’s Abscess. IJCM 2: 292-294.
- Wu JF, Jin Z, Yang JM, Liu YH, Duan ML (2012) Extracranial and intracranial complications of otitis media: 22-year clinical experience and analysis. Acta Otolaryngol 132: 261-265.
- http://www.treccani.it/enciclopedia/salvatore-citelli_(Dizionario-Biografico)/
- Naumann HH, Martin F, Scherer H, Schorn K (1993) Differential Diagnosis in Otorhinolaryngology: Symptoms, Syndromes and Interdisciplinary Issues. Thieme.
- Laumans EP (1961) Mastoiditis according to Mouret. Pract Otorhinolaryngol (Basel) 23: 329-337.
- Dhingra PL, Shruti D (2014) Diseases of Ear, Nose and Throat. Elsevier Health Sciences.
- Mascarinas CA, Singer MC, Hanson MB (2010) Bezold's Abscess in the Setting of Radiation Induced Mastoiditis. Laryngoscope 120: 211.
- Ostfeld E, Rubinstein E (1980) Acute gram-negative bacillary infections of middle ear and mastoid. Ann Otol Rhinol Laryngol 89: 33-36.
- Prasad SC, Prasad KC, Kumar A, Thada ND, Rao P, et al. (2014) Osteomyelitis of the temporal bone: terminology, diagnosis and management. J Neurol Surg B Skull Base 75: 324-331.
- Dudkiewicz M, Livni G, Kornreich L, Nageris B, Ulanovski D, et al. (2005) Acute mastoiditis and osteomyelitis of the temporal bone. Int J Pediatr Otorhinolaryngol 69: 1399-1405.
Citation: Brożek-Mądryz E, Waniewska-Łęczycka M, Robert B, Krzeski A (2018) Head and Neck Abscesses in Complicated Acute Otitis Media-Pathways and Classification. Otolaryngol (Sunnyvale) 8: 345. DOI: 10.4172/2161-119X.1000345
Copyright: © 2018 Brożek-Mądryz E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15161
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 13849
- PDF downloads: 1312