Human Angiosarcoma: A Histological and Biological Phenotyping Using Xenografts in Nude Mice: Analysis of Five Cases
Received: 27-Jan-2015 / Accepted Date: 23-Feb-2015 / Published Date: 28-Feb-2015 DOI: 10.4172/2161-0681.1000213
Abstract
Human angiosarcoma (AS) is an infrequent soft tissue malignancy that may be difficult to diagnose, especially the solid, epithelioid or pleomorphic variant. A broad panel of antibodies is often necessary to determine the phenotype by Immunohistochemistry (IHC). To improve our knowledge of the morphology, immunophenotype, genetics and molecular biology, we constructed an in vivo xenograft platform using human soft tissue AS in nude mice. Tissue Microarrays (TMAs) were built from the original tumors and subsequent passages. The study included four original human AS, plus one secondary (a primary Malignant Peripheral Nerve Sheath Tumor (MPNST) which became an angiosarcoma), followed over successive tumor transfers for several generations. Endothelial marker expression (CD31, CD34, FVIII, CD105 (endoglin), Caveolin (CAV-1), D2-40, V-cadherin, FLI1 and ERG n-terminal) was evaluated by IHC. Molecular and cytogenetics studies were also performed. Only three out of the five AS (60%), grew in nude mice. Between 30 and 52 passages were achieved. Cytogenetically, the tumors showed complex karyotypes with many clonal numerical and structural abnormalities. Molecular biology showed p53 wild-type and KDR in all the cases. The WWTR1-CAMTA fusion was absent, which may be helpful in distinguishing epithelioid AS from epithelioid hemangioendothelioma (EHE), as it has been described as a hallmark in the latter. CD31, ERG, D2-40 and V-cadherin were the most sensitive, and ERG and FLI1 the most specific markers in the present series. This is the first study to combine nude mice xenografts and TMA studies in human AS, and describes for the first time an angiosarcomatous differentiation of an MPNST which occurred during the late passages generating a Kasabach-Merritt-like syndrome in the animal.
Keywords: Angiosarcoma; Immunohistochemistry; MPNST; Tissue microarray; Xenografts
312249Introduction
Angiosarcoma is a rare malignant neoplasm displaying vascular differentiation. It occurs frequently in subcutaneous tissue and deep soft tissue often associated with a previous history of radiotherapy, chronic lymphedema, or is of unknown etiology [1]. Rare locations such as the digestive tract or intracardial angiosarcomas have also been described [2-5].
Histologically, AS may be difficult to diagnose, especially the solid, epithelioid or pleomorphic variant. This neoplasm is characterized by the presence of atypical cells, ranging from prominent endothelia to highly pleomorphic cells, producing clefts and slits arranged in vascular-like channels, intermingled with solid areas of epithelioid or spindle cells [1,6]. The cells may show intracytoplasmic vacuoles with occasional erythrocyte emperipolesis. The mitotic index is high, with atypical figures (tripolar and explosive mitosis) [6,7]. Many vascular markers have been described; CD31 and CD34 are the most widely used, while ERG and FLI1 seem to be the most specific [6,8,9].
Xenograft models provide a valuable experimental platform, especially for tumors with negative cell cultures, or positive cultures that are nevertheless inadequate for assessing the tissue histology, relationship with stromal component, angiogenesis, invasive potential or metastatic capacity [10,11]. Furthermore, xenografted tumors provide a good source of abundant fresh tissue, and allow the characterization of a neoplasia using various procedures such as histopathology, Immunohistochemistry (IHC), Electron Microscopy (EM), cytogenetics (conventional and Fluorescence In-Situ Hybridization/ FISH) and molecular biology, due to the consistent preservation of the morphology over the generations [10-14].
Tissue Microarray (TMA) technology allows the assessment of histopathology and IHC in a large cohort of tumors on a single slide, saving in time, cost and tissue. As a result, several laboratories use TMAs to investigate archival samples in large series of tumors, although only one TMA study in original angiosarcomas, (without xenografts), has been reported previously [8]. The use of xenograft models of AS, including successive generations of mice passages in combination with TMA technology has not been reported so far. This study describes for the first time a new experimental model of AS based upon the experience gained from the study of 5 xenografted cases, including an angiosarcomatous differentiation of an MPNST which occurred during the late passages generating a Kasabach-Merritt-like syndrome in the animal.
Materials and Methods
Study subjects
Five angiosarcomas, four de novo and one secondary, derived from a Malignant Peripheral Nerve Sheath Tumor (MPNST) which transformed into an angiosarcoma during the passages in nude mice, were included. All the cases underwent surgery at the Hospital Clínico, Valencia (Table 1). After selecting tissue for diagnosis and freezing, all remaining material was inoculated into nude mice within 2 hours of surgery. A cytological evaluation (touch preparation) of the tumor was performed to assess the quality of material and certify the presence of tumor cells. The present study was performed in compliance with this institution’s ethics committee protocol for experimentation on animals.
| Case | Age (years) |
Gender | Site | Size | TREATMENT | RELAPSE | OFS (months) | DFS (months) |
DOD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | F | Right auricle | 5cm | Palliative Ifosfamide |
Progression | 3 | 0 | yes |
| 2 | 32 | F | Right auricle | 4cm | Palliative Surgery Ifosfamide |
Progression Yondelis |
13 | 0 | yes |
| 3 | 51 | F | Posterior thigh | 3cm | Surgery Adriamycin RT |
6 months relapse 9 months Lung Met +relapse |
10 | 6 | yes |
| 4 | 43 | M | Axillar fossa | 7cm | Surgery Adriamycin | 6 months Lung Met Yondelis | 9 | 6 | yes |
| 5 | 29 | F | Posterior thigh | 4cm | Surgery Adriamycin RT |
7 months Liver Met 36 months Lung Met |
43 | 7 | yes |
Table 1: Patient clinical data. OFS = overall free survival, DFS = disease free survival, DOD = Died of disease, Met = metastasis. Survival is expressed in months and age is expressed in years.
Sample size
Two mice were used for each inoculation. The aim was to prospectively follow the growth of the tumors in vivo over successive passages. The number of tumor transfers in vivo depended on the biology of the tumor growth and was based upon previous literature on the subject [10,11], focusing on grouping the passages into initial (1 to 4 transfers), middle (5 to 10) and late passages (more than 11) in order to assure continuity of the xenograft and to evaluate the phenotype over successive passages.
Study variables
The variables evaluated were tumor biology in the animal (size and growth rate), presence of metastasis at the time of sacrifice, morphology of the original tumor and throughout the passages, and characteristic and diagnostic immunohistochemical and molecular markers. The panel of diagnostic immunohistochemical markers for AS, markers related to tumor biology and growth and markers used for differential diagnosis are summarized in Table 2.
| Antibody | Clone | Laboratory | dilution |
|---|---|---|---|
| CD31 M0823 |
JC70A | DAKO | 1:50 |
| CD34 M7165 |
QBEnd/10 | DAKO | 1:50 |
| ERG CM421A |
9FY | BIOCARE MEDICAL | 1:200 |
| VEGF Receptors 1-3 R1 FLT-1(C-17)SC-316 R2 FLK-1(C-20)SC-315 R3 FLT-4(C-20)SC-321 |
POLYCLONAL RABBIT | SANTA CRUZ BIOTECHNOLOGY | 1:400 |
| ENDOGLINE M3527 |
SN6h | DAKO | 1:20 |
| V-CADHERIN SC-6458 |
POLYCLONAL GOAT |
SANTA CRUZ BIOTECHNOLOGY | 1:50 |
| CAVEOLIN-1 SC-894 |
POLYCLONAL RABBIT | SANTA CRUZ BIOTECHNOLOGY | 1:200 |
| FVIII | POLYCLONAL RABBIT | CONCEPTA | 1:200 |
| D2-40 IR072 |
D2-40 | DAKO CYTOMATION | PREDILUTED |
| KI67 M7240 |
MIB-1 | DAKO | 1:200 |
| p53 NCL-L-p53-DO7 |
DO7 | NOVOCASTRA/LEICA BYOSISTEMS | 1:50 |
| CK AE1/AE3 M3515 |
AE1/AE3 | DAKO | 1:100 |
| EMA M0613 |
E29 | DAKO | 1:200 |
| S100 Z0311 |
POLYCLONAL RABBIT | DAKO | 1:2000 |
| NF-200 NCL-NF200 |
RT97 | NOVOCASTRA/LEICA BYOSISTEMS | 1:100 |
| PGP9.5 NCL-PGP9.5 |
10 A 1 | NOVOCASTRA/LEICA BYOSISTEMS | 1:50 |
| FLI-1 MAD210407Q |
MRQ1 | MASTER DIAGNOSTICA | 1:40 |
Table 2: Antibodies used in the experience.
Methodology
Specific Pathogen Free (SPF) animals (Nude mice, Charles River, BCN) were employed. The animals were housed under special conditions in the animal facility at the Medical School, University of Valencia.
Tumor fragments between 2 and 3 mm were introduced subcutaneously into the animals’ backs under anesthesia. The animals were monitored daily to ensure their welfare and observe tumor growth. At the time of tumor transfer (once the tumor reached 2-3 cm in size), a standardized autopsy was carried out on the sacrificed mouse in search of metastasis, using both macroscopic and microscopic evaluation.
Likewise, the most representative areas for constructing the tissue microarrays were selected following thorough histological evaluation.
Arrays were constructed from paraffin blocks of the original and xenotransplanted tumors (all successful passages), including at least two cores from each using the “manual tissue arrayer” (Beecher Instruments, Sun Prairie, WI). For the immunohistochemical analysis, antigen retrieval was performed by pressure cooker boiling for 3 minutes in 10 mmol/L of citrate buffer (pH 6.0). The LSAB method (DakoCytomation) was used, followed by revelation with 3,3´-diaminobenzidine. All slides were evaluated blindly by three pathologists (EMA, IM and ALLB).
Molecular biology
Genomic DNA was isolated from frozen tumor tissue by phenol/ chloroform extraction and quality was confirmed by spectrophotometry. The mutational status of TP53 (exon 5 to exon 8), KDR (exons 15, 16, 24) was assessed by direct sequencing of genomic DNA. Protocols and primers are available on request. For reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, total RNA was extracted using conventional protocols for frozen tissue. WWTR1-CAMTA1 genomic amplification was analyzed by differential PCR (dPCR) with primers specific for the WWTR1-CAMTA1 and MGBR genes, adapted from the method described by Errani et al., 2011 [15].
Cytogenetics
Samples from the tumors were immersed in sterile RPMI 1640 medium (Gibco BRL, Grand Island, New York) supplemented with 2% antibiotics and 0.05 mg/ml of gentamicin sulfate. The fragments were disaggregated using enzymatic disintegration. The cells were seeded into 25-cm2 tissue culture flasks (Nunc, Roskide, Denmark) containing 5 ml of complete medium and maintained in a humidified atmosphere at 5% CO2 in air at 37° C. Cytogenetic analyses were made from a shortterm culture of the AS. Cells were prepared for karyotyping according to ISCN guidelines (2005).
Ultrastructure
The tumors (both original and every passage) were fixed in glutaraldehyde 9%, postfixed in osmium tetroxide and examined at 60- 80KV in a JEM 100B Jeol transmission electron microscope.
Results
Five AS were included in this study, two conventional (cases 1 and 2), one pleomorphic with abundant solid areas (case 3), one epithelioid (case 4) and one derived from an MPNST (case 5). Four patients were female and one male, with no superficial location and no previous history of radiotherapy in any of the cases. Two cases were intracardial, located in the right auricle (cases 1 and 2), and three were in deep soft tissue (axilla and posterior thigh). The tumor size ranged from 1.4 to 7 cm and none of the cases had received chemotherapy before excision. All clinical data for the patients are listed in Table 1.
Three of the five AS (60%) grew in nude mice. The two AS of the heart grew in short term cell culture, but not in nude mice. Over 20 serial passages were achieved in each of the successful cases.
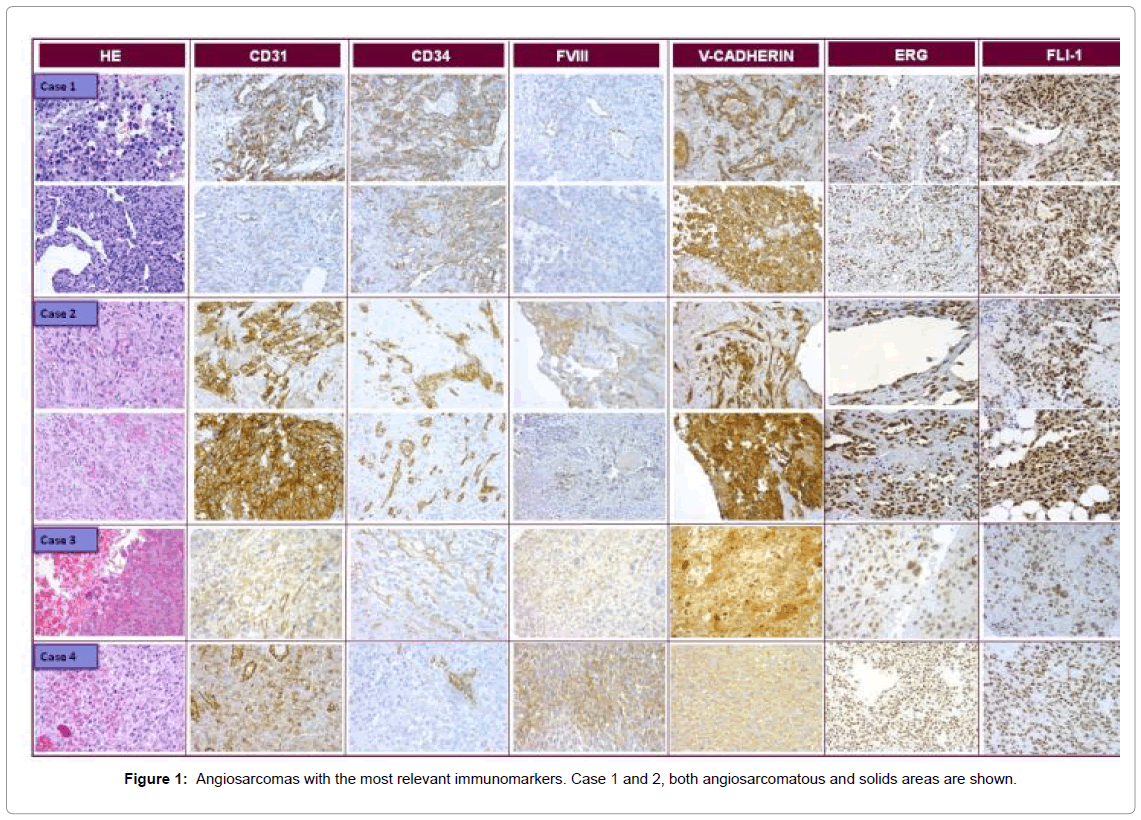
For tumor biology in the animals, the number of days between inoculation and sacrifice was used to calculate speed of growth (Table 3). The first passage was the slowest in all cases, taking up to 209 days to reach 1.5 cm. Once the experience was established the speed of growth became constant and was similar among the cases, achieving one passage every month and a half. Over time the growth rate decreased, at which point the experience was ended. The morphology as well as the immunophenotype of the cases was well preserved over the passages (Figure 1).
| Case | Nº passages | First passage | Mean time | Median time |
Total time |
|---|---|---|---|---|---|
| 3 | 41 | 209 | 62 | 66 | 2509 |
| 4 | 52 | 33 | 38 | 36 | 1967 |
| 5 | 38* | 136 | 36 | 67 | 2827 |
Table 3: Xenografted AS with their biological behavior in nude mice. First passage, Mean, Median and Total times are expressed in days.
Case 1 and 2
Both cases shared similar clinical and morphological features. Clinically, a large polypoid mass, located on the right auricle wall was resected with imprecise margins. Histologically an atypical proliferation of mesenchymal cells was observed that defined intermingled channels, optically empty or containing erythrocytes, with few dispersed solid areas. The cells were large, elongated with large atypical nuclei and intracytoplasmic vacuoles. Occasionally erythrocytic emperipolesis was observed.
The immunohistochemical analysis revealed intense and diffuse positivity for vascular markers, with some differences in expression between vascular and solid areas. Vascular areas were positive for CD31, CD34, ERG, FLI1, V-CAD and cav-1; while negative for D2- 40 and endoglin and only occasionally positive for FVIII, whereas solid areas were positive for ERG, FLI1, FVIII, V-CAD and cav-1, only focally positive for D2-40, and negative for CD31, endoglin and CD34.
Case 3
The histology showed few conventional AS areas similar to cases 1 and 2, intermingled with round epithelioid cells growing in a solid pattern. The immunohistochemical analysis revealed the same expression pattern as cases 1 and 2, apart from FVIII which was only occasionally positive. The immunophenotype was maintained over the passages, apart from CD34, which became positive in the xenograft.
Case 4
The histology showed a proliferation of large atypical cells, with epithelioid morphology and round moderately pleomorphic nucleus. The cells grew in a diffuse solid pattern with vascular channels. IHC revealed intense and diffuse positivity for V-CAD, ERG, FLI1 and VEGFR and intense positivity in isolated cells for CD31, CD34 and FVIII. CAV-1 and endoglin were negative. This phenotype was maintained over the passages. Positivity for CK (AE1-AE3) and EMA was maintained over the passages. The expression was focal but intense.
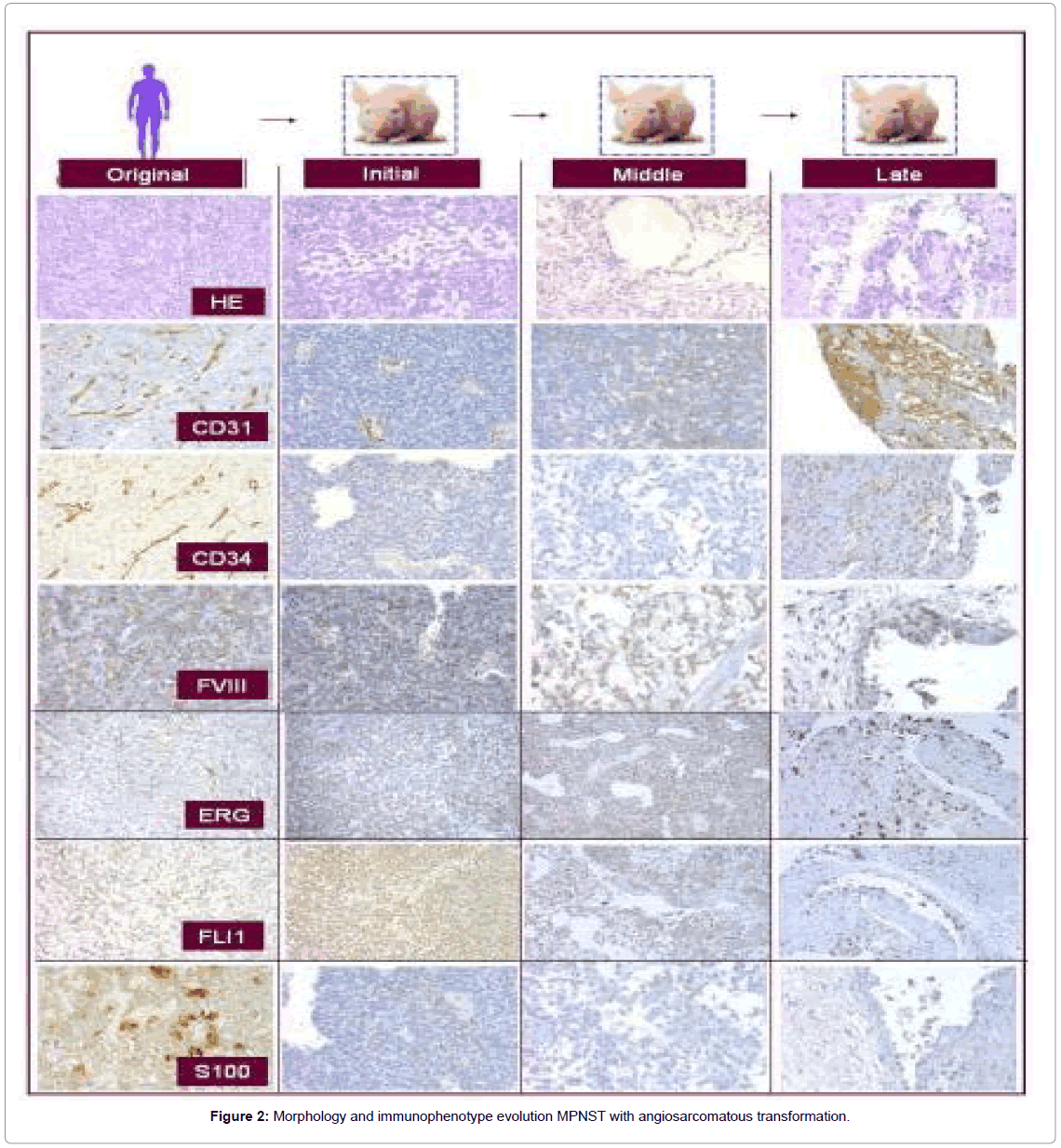
Case 5
The original MPNST tumor showed a fascicular, moderately cellular neoplasm with fusiform cells with poorly defined, elongated and eosinophilic cytoplasm and elongated nuclei with moderate pleomorphism. Angiosarcomatous differentiation initiated in passage 5, in which both solid and loose areas of tumor were progressively transformed into clefts or cavities containing occasional erythrocytes, covered by elongated, pleomorphic cells with endothelial appearance. In the late passages the tumor displayed clear vascular spaces. The endothelial cells of the vascular channels displayed Weibel–Palade bodies at the ultrastructural level.
Only VEGF receptors 1-3, and FVIII (in isolated cells) and FLI1 (diffusely), were positive in the original tumor; the remaining antibodies being negative. Over the passages, they became positive once the vascular morphology was present. Precisely the opposite occurred with the neural markers, which were positive in the original tumor and negative when the vascular morphology was present (Figure 2).
The results of the immunohistochemical analyses are listed in Tables 4 and 5.
| Atb | 1 | Original | 2 | Original | 3 | Original | Initial 0-4 |
Middle 5-9 |
Late Last 5 |
4 | Original | Initial 0-4 |
Middle 5-9 |
Late Last 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD31 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | ||||
| CD34 | 3 | 2 | 1 | 3 | 3 | 3 | 0 | 2 | 2 | 2 | ||||
| V-CAD | 2 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 2 | 2 | ||||
| ERG | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||
FLI1 |
3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||
| VEGFR | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||
| FVIII | 2 | 2 | 1 | 0 | 0 | 0 | 3 | 2 | 3 | 3 | ||||
| Caveolin | 2 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | ||||
| Endoglin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| S100 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| PGP9.5 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | ||||
| NF200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | ||||
| CkAE1-AE3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | ||||
| EMA | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||||
| p53 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | ||||
| ki67 | 2 | 2 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 3 |
Table 4: Expression of the antibodies in each case grouped as original tumor and passages in nude mice: Initial (from first inoculation to passage 4), Middle (from passage 5 to 9) and late (last 5 passages). Intensity is expressed from 0 to 3 (0,1: negative and 2,3: positive).
| Atb | Original | Initial 0-4 |
Medium 5-9 | Late Last 5 |
|---|---|---|---|---|
| CD31 | 0 | 2 | 2 | 3 |
| CD34 | 0 | 0 | 0 | 2 |
| V-CAD | 0 | 0 | 0 | 2 |
| ERG | 1 | 1 | 2 | 3 |
| FLI1 | 1 | 2 | 2 | 3 |
| VEGFR | 3 | 3 | 3 | 3 |
| Caveolin | 0 | 0 | 0 | 2 |
| Endoglin | 0 | 0 | 0 | 0 |
| FVIII | 2 | 2 | 2 | 3 |
| D2-40 | 0 | 0 | 0 | 0 |
| S100 | 2 | 2 | 0 | 0 |
| PGP9.5 | 2 | 0 | 0 | 0 |
| NF200 | 2 | 0 | 0 | 0 |
| CkAE1-AE3 | 2 | 2 | 0 | 2 |
| EMA | 2 | 1 | 1 | 2 |
| p53 | 0 | 0 | 0 | 0 |
| ki67 | 3 | 3 | 2 | 3 |
Table 5: Immunophenotype of the MPNST with angiosarcomatous differentiation in nude mice (case 5); both original tumor and passages in nude mice are shown and grouped as: Initial (from first inoculation to passage 4), Middle (from passage 5 to 9) and late (last 5 passages). Intensity is expressed from 0 to 3 (0,1: negative and 2,3: positive).
No metastases were found in any of the animal autopsies; although case 5 produced a Kasabach-Merritt-like syndrome in the animals, with purpura and hematomas in the skin, splenomegaly and intraperitoneal hemorrhages, and sudden death between 2 and 5 days after the appearance of the symptoms.
Ultrastructure
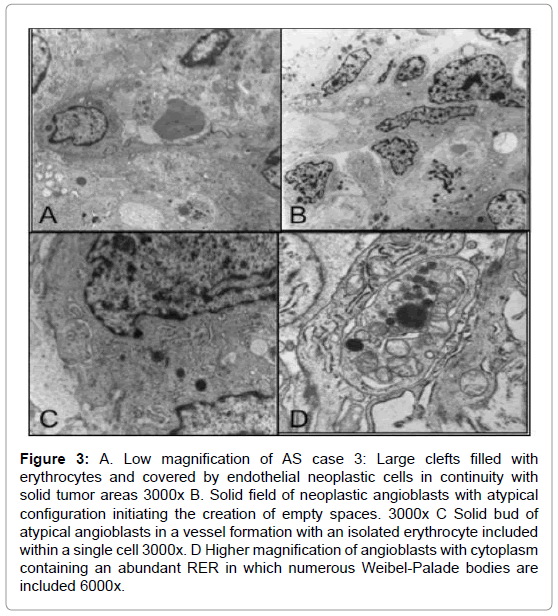
All tumors were similarly composed of atypical endothelial cells arranged in sheets or compact bands with a lobular pattern due to reticular septa and irregular clefts, forming lumens in early vascular stages and with occasional erythrocytes present. The neoformed endothelial cells produced solid buds or abnormal vascular beds with large architectural irregularities and solid masses, but displaying small empty spaces into which superficial microvilli or short digitations were projected. These cells sometimes presented a highly attenuated cytoplasm mixed with other more prominent watery clear areas. The cytoplasm contained isolated mitochondria and several Weibel-Palade bodies in various stages of maturation with closely-packed small microvesicular bodies inside. Occasionally cell mitoses were present.
Basal laminae were either absent or reduplicated. Pericyte-like cells were present, although irregularly distributed. The absence of pericytes or smooth muscle-like cells led to blood vessel dilatation, endothelial cell hyperplasia and microaneurysms (Figure 3).
Figure 3: A. Low magnification of AS case 3: Large clefts filled with erythrocytes and covered by endothelial neoplastic cells in continuity with solid tumor areas 3000x B. Solid field of neoplastic angioblasts with atypical configuration initiating the creation of empty spaces. 3000x C Solid bud of atypical angioblasts in a vessel formation with an isolated erythrocyte included within a single cell 3000x. D Higher magnification of angioblasts with cytoplasm containing an abundant RER in which numerous Weibel-Palade bodies are included 6000x.
Cytogenetics
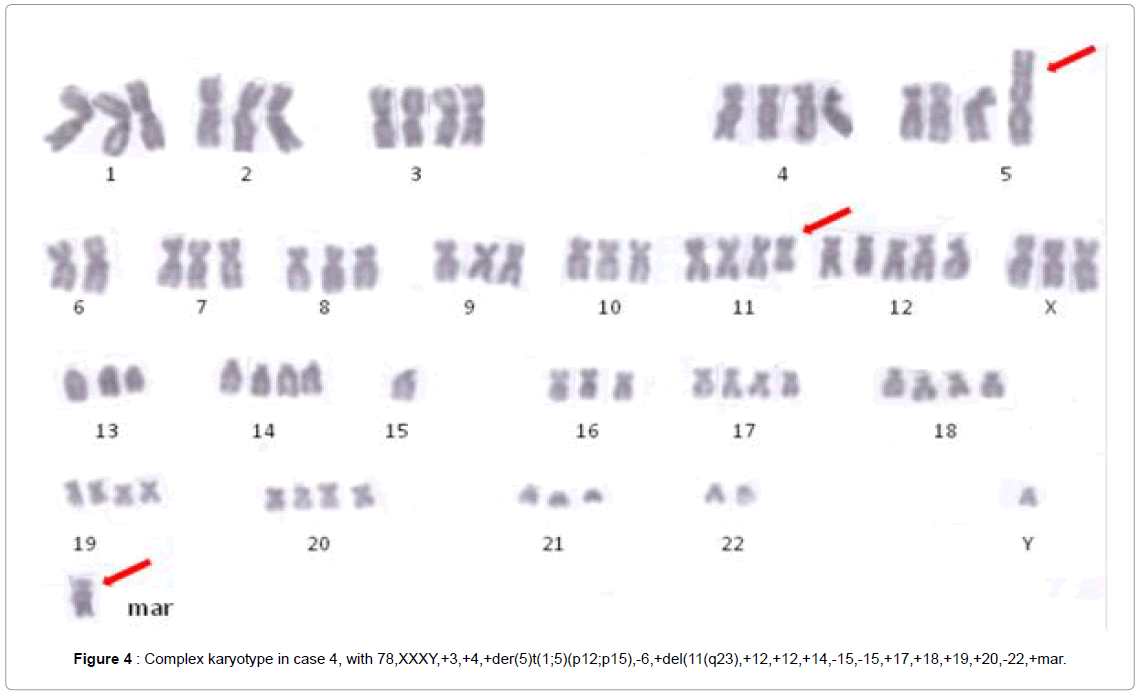
Case 5 revealed a complex karyotype with ring chromosomes, the rest of the cases presented abnormal karyotypes, with hypertriploidy (chromosomes 78-83) in case 3, and (chromosomes 76-80) in case 4. The chromosome analysis revealed the following clonal karyotype:
78,XXXY,+3,+4,+der(5) t ( 1 ; 5 ) (p12;p15) , -6,+del ( 11) (q23),+12,+12,+14,-15,-15,+17,+18,+19,+20,-22,+mar in case 3, and 78, XX,add(X)(q11), +dic (X;17)(p11;q25), del(1)(p21),-2,del(2) (p13),-3,-4,+del(5)(p15),+der(6)t(1;6)(p22;q22),-7,-7,add(8)(q24),- 9,-10,del(10)(p11),-11,der(11)t(1;11)(q25;q24),+12,+7-9mar,+2 microchromosomes in case 4 (Figure 4).
Molecular analysis
None of the cases presented mutations in either p53, or in KDR (KDR15, 16 and 24), or had the WWTR1-CAMTA fusion gene.
Discussion
Human Angiosarcoma (AS) is a highly malignant infrequent soft tissue neoplasm that may be difficult to classify histologically [1,6,8]. This study adds to the literature four deep soft tissues AS, with both typical and atypical morphology, plus a secondary angiosarcoma arising in an MPNST, which we had the opportunity to follow in vivo.
For solid, pleomorphic and epithelioid areas within the tumor, a broad panel of antibodies is necessary to achieve an adequate diagnosis since the traditional markers (CD31, FVIII and CD34) are only focally expressed or even negative [8]. In our cases, ERG, FLI1, V-CAD and D2-40 were the most consistent antibodies for vascular differentiation, being consistently positive in undifferentiated areas; in agreement with the results of Rao et al. Our study adds V-CAD and D2-40 as helpful markers for tumors with a vascular immature nature, or with partial lymphatic nature in the neoformed vessels [8,9].
Common markers, CD31, CD34 and FVIII, used for vascular staining in routine diagnosis were present in those cells arranged in vascular channels while negative in solid and immature areas in our series. This finding has not so far been reported in the literature and should be taken into consideration in daily practice
CAV-1 and endoglin were the least expressed antibodies: endoglin being negative in 100% of the cases and positive only in nontumoral vessels present in the slide as a positive control. This finding has not been published previously in AS and requires further investigation as it can provide a useful tool for distinguishing normal from abnormal neoformed vessels.
Epithelial markers were diffusely positive in epithelioid AS (in case 4), as well as in isolated cells in those with conventional morphology (also case 5 after transformation) as already described in the literature [16,17]; something that needs to be taken into consideration, especially to achieve an appropriate differential diagnosis. Testing the WWTR1- CAMTA fusion gene status may be helpful in distinguishing epithelioid AS from Epithelioid Hemangioendothelioma (EHE), as it has been described as a hallmark in the latter [15]. In our cases, this fusion gene was not present in any of the cases. This finding confirms that we are dealing with an AS displaying epithelioid differentiation and not with an epithelioid HE. None of our cases were located in breast, or presented the KDR (also known as VEGFR2 gene) mutation, described by Antonescu [18] as being associated with this location. However, our cases did show intense positivity for VEGFR2 by IHC. This is another feature to take into account when studying the kinase receptor status. A recent publication by an international group [19], sequenced the whole genome of three AS; identifying recurrent mutations in two genes related to angiogenesis, PTPRB and PLCG1, not present in the EHE, Kaposi sarcomas or hemangiomas included in their series. They also investigated other genes related to angiogenesis (not including KDR), with no mutual exclusivity among them and a higher portion of mutation in those cases with Myc-amplification. All these findings in our opinion deserve further confirmation with larger series. p53 immunoexpression did not correlate with the mutational status in our cases, as demonstrated in other series [20].
Information available on cytogenetics in AS in the literature is limited [21-24], yet very complex chromosomal abnormalities have been demonstrated [23]; and while numeric changes as the sole karyotypic aberration have been described in two cases [21,22], no recurrent structural abnormalities have so far been identified. It appears that deletions of 4p and 7p, and abnormality involving 22q [24] are the most common changes described in the literature. Our cases presented a hypertriploid range with many numerical abnormalities and, interestingly, three chromosomes (1, 5 and 11) implicated in common with other unknown marker chromosomes. This heterogeneity could be implicated in tumor progression; nevertheless, too few cases were analyzed to conclude which abnormality may be implicated in tumor initiation.
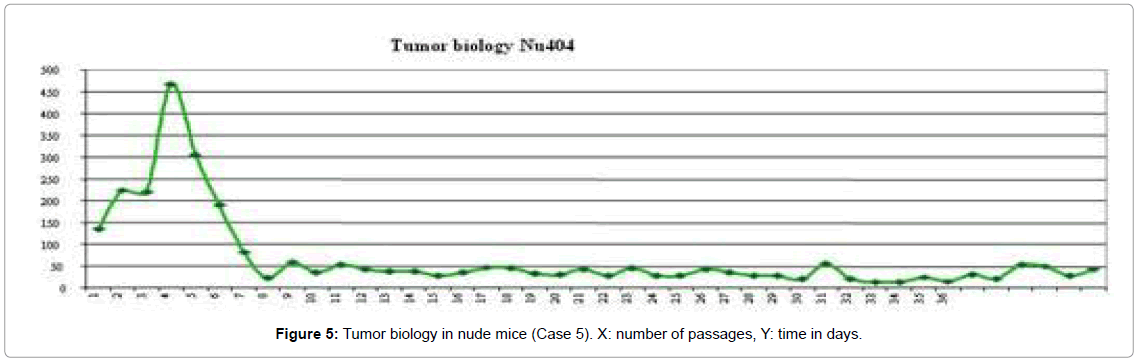
Nude mice provide an excellent in vivo platform to study the biology of rare malignancies; especially those with a metaplastic morphology as in our case with MPNST. In fact, this is the first time that a progressive transformation from an MPNST into an angiosarcoma has been described in vivo. In this context, few cases have been published describing the association of AS with a neural tumor [25-29] where the angiosarcomatous component appeared years later. In our case we observed a progressive transformation of an MPNST with fusiform patterns in the original neoplasm and the early passages, gaining an increasingly vascular differentiation with channels covered by atypical endothelial-like cells, finally becoming an AS in the late passages. This phenomenon was associated with a more aggressive behavior, with the tumor growing more rapidly in the nude mice (Figure 5). The present xenograft experience also generated a Kasabach-Merritt-like syndrome in the animals that suffered splenomegaly, sporadic haemorrhagia and extramedullary hematopoiesis a finding that has not been described in AS so far.
In conclusion, in addition to the more usual antibodies employed to detect AS (CD31 and CD34), the present analysis demonstrated that FLI-1 and ERG are the most sensitive and specific antibodies, whereas V-Cad and D2-40 are useful for determining the more immature areas, being positive in the undifferentiated tumor fields.
Nude mice xenograft technology provides a useful tool for investigating the diagnosis, biology and phenotype of these uncommon neoplasms, being of particular value in detecting cases such as the MPNST displaying a secondary angiosarcomatous metaplasia.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank Laura Martinez for her technical assistance, and David Harrison for his help with the revision of the paper. This study was performed with the financial support of EuroBoNeT (contract: 018814) and the Instituto Valenciano de Oncología, Valencia, Spain (IVO).
References
- Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ (2010)Angiosarcoma. Lancet Oncol 11:983-991
- Akutsu H, Tsuboi K, Sakamoto N, Nose T, Honma S, et al. (2004) Cerebral metastasis from angiosarcoma of the aortic wall: case report. SurgNeurol 61:68-71.
- Hwang SL, Howng SL, Sun ZM, Kwan AL (1996) Brain metastasis from pericardial angiosarcoma. J Formos Med Assoc 95:484-486.
- Liu DS, Smith H, Lee MM, Djeric M (2013) Small intestinal angiosarcoma masquerading as an appendiceal abscess. Ann R CollSurgEngl 95:1.
- Al Beteddini OS, Brenez D, Firket C, Algaba R, Tabech A (2013) Colonic angiosarcoma: A case report and review of literature. Int J Surg Case Rep 4:208-211.
- Fletcher C (2013) WHO Classification of tumours of soft tissue and bone. (4thedn) Lyon.
- Weiss S. and Goldblum J (2008)Enzinger and Weiss´ Soft tissue tumours(5thedn).
- Rao P, Lahat G, Arnold C Gavino AC, LahatS,et al. (2013)Angiosarcoma: a tissue microarray study with diagnostic implications. Am J Dermatopathol 35:432-437.
- Folpe AL, Chand EM, Goldblum JR, Weiss SW (2001) Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J SurgPathol 25:1061-1066.
- Mayordomo E, Machado I, Giner F (2010) A tissue microarray study of osteosarcoma: histopathologic and immunohistochemical validation of xenotransplanted tumors as preclinical models. ApplImmunohistochem Mol Morphol 18:453-461.
- Machado I, Giner F, Mayordomo E, Carda C, Navarro S, et al. (2008) Tissue microarrays analysis in chondrosarcomas: light microscopy, immunohistochemistry and xenograft study. DiagnPathol3 Suppl 1:S25.
- Kuijjer ML, Namløs HM, Hauben EI (2011) mRNA expression profiles of primary high-grade central osteosarcoma are preserved in cell lines and xenografts. BMC Med Genomics.4:66.
- Mosakhani N, Guled M, Leen G (2012) An integrated analysis of miRNA and gene copy numbers in xenografts of Ewing's sarcoma..JExpClin Cancer Res 31:24.
- Kresse SH, Meza-Zepeda LA, Machado I, Llombart-Bosch A, Myklebost O (2012) Preclinical xenograft models of human sarcoma show nonrandom loss of aberrations. Cancer 118:558-70
- Â Errani C, Zhang L, Sung YS (2011) A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioidhemangioendothelioma of different anatomic sites. Genes ChromosomesCancer 50:644-653.
- Errani C, Zhang L, Sung YS (2011) A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioidhemangioendothelioma of different anatomic sites. Genes ChromosomesCancer 50:644-653.
- Hart J, Mandavilli S (2011)Epithelioidangiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med135:268-272.
- Gray MH, Rosenberg AE, Dickersin GR, Bhan AK (1990) Cytokeratin expression in epithelioid vascular neoplasms. Hum Pathol 21:212-127.
- Antonescu CR, Yoshida A, Guo T (2009)KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res69:7175-7179.
- Â Behjati S (2014) Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet.46(4):376-9.
- Italiano A, Chen CL, Thomas R (2012) Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer 118:5878-5887.
- Kindblom LG, Stenman G, Angervall L (1991) Morphological and cytogenetic studies of angiosarcoma in Stewart-Treves syndrome. Histopathol419:439-445.
- Wong KF, So CC, Wong N, Siu LL, Kwong YL, et al. (2001)Sinonasalangiosarcoma with marrow involvement at presentation mimicking malignant lymphoma: cytogenetic analysis using multiple techniques. Cancer Genet Cytogenet 129:64-68.
- Schuborg C, Mertens F, Rydholm A (1998)Cytogenetic analysis of four angiosarcomas from deep and superficial soft tissue. Cancer Genet Cytogenet100:52-56.
- Gil-Benso R, López-Ginés C, Soriano P, Almenar S, Vazquez C, et al. (1994) Cytogenetic study of angiosarcoma of the breast. Genes Chromosomes Cancer10:210-212.
- Ogawa T, Kato T, Ikeda A (2014) Case of malignant transformation of vagus nerve schwannoma to angiosarcoma. Head Neck36:E17-20.
- Mentzel T, Katenkamp D (1999) Intraneuralangiosarcoma and angiosarcoma arising in benign and malignant peripheral nerve sheath tumours: clinicopathological and immunohistochemical analysis of four cases. Histopathology 35:114-120.
- Li C, Chen Y, Zhang H, Zheng X, Wang J (2012)Epithelioidangiosarcoma arising in schwannoma: report of three Chinese cases with review of the literature. PatholInt62:500-505.
- Rückert RI, Fleige B, Rogalla P, Woodruff JM (2000)Schwannoma with angiosarcoma. Report of a case and comparison with other types of nerve tumors with angiosarcoma. Cancer 89:1577-1585.
Citation: Mayordomo-Aranda E, Machado I, Navarro L, Gil-Benso R, Peydro A, et al. (2015) Human Angiosarcoma: A Histological and Biological Phenotyping Using Xenografts in Nude Mice: Analysis of Five Cases. J Clin Exp Pathol 5:213. DOI: 10.4172/2161-0681.1000213
Copyright: © 2015 Mayordomo-Aranda E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15674
- [From(publication date): 4-2015 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 11044
- PDF downloads: 4630