Impact of Roux-En-Y Gastric Bypass Surgery on Neurohormonal and Gastrointestinal Physiology: Insights for Future Weight Loss Efforts
Received: 31-Oct-2017 / Accepted Date: 08-Nov-2017 / Published Date: 13-Nov-2017 DOI: 10.4172/2165-7904.1000356
Abstract
Roux-en-Y Gastric Bypass (RYGB), is an effective weight loss intervention for patients failing conventional nonsurgical methods. Despite its popularity, only a subset of patients undergo RYGB due to its cumbersome nature. Furthermore, patients may experience nutritional deficiencies or weight regain after RYGB. This review will delineate the less known impact of RYGB on neurohormonal and gastrointestinal physiology involved in weight loss. Understanding these alterations will contribute to the development of future novel investigations targeting viable weight loss strategies.
Keywords: Obesity; Weight loss; Gastrointestinal physiology; Feeding; Neurohormonal physiology; Roux-en-Y; Gastric bypass
Introduction
RYGB is one of the most common bariatric approaches and leads to marked improvements in inflammatory status, insulin resistance, and several metabolically active hormones including leptin and adiponectin [1-7]. These improvements are associated with lower morbidity and mortality even in severely obese patients [8-17].
Therefore, the number of patients undergoing Roux-en-Y gastric bypass (RYGB) surgery has increased by almost tenfold in the past 2 decades, with approximately 101,645 operations performed in 2011 alone [18,19]. However, although RYGB is effective for the vast majority of patients, a small proportion of RYGB patients develop serious nutritional complications, debilitating gastrointestinal (GI) symptoms, and/or fail to reach their weight loss goals [20-25].
Thus, RYGB use decreased in recent years with the increasing application of sleeve gastrectomy as an effective and less cumbersome bariatric approach [26,27]. Furthermore, newer less invasive weight loss interventions, such as intra-gastric balloons for instance, are gaining popularity and may induce weight loss through delayed gastric emptying and humoral changes [28].
As a result, it is important to review the influence of RYGB on neurohormonal and gastrointestinal physiology in order to understand their role in RYGB induced weight loss and ultimately guide future less invasive weight management innovations that can mimic RYGB effect effectively.
Methods
We performed a literature search in PubMed and Medline, using the terms bariatric surgery, gastric bypass, obesity surgery, and Roux-en-Y. These were searched as Medical Subject Headings “MeSH” terms and also as text words.
These individual MeSH term search results and text word search results were all combined using the Boolean operator “OR”. The combined search was limited to English language using the language filter. Human and animal studies were included.
This search result was then coupled with secondary search terms in relation to our focus topics using the Boolean operator “AND”. The title and abstracts of articles that resulted from this secondary search were screened for relevance in relation to the focus topic. If found relevant, their references were further reviewed to identify additional published studies not indexed in PubMed.
The Roux-en-Y gastric bypass procedure
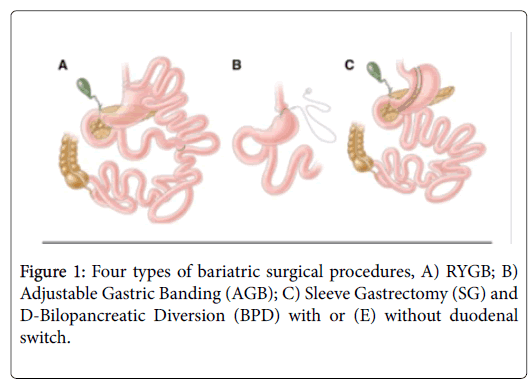
Four bariatric surgical procedures are commonly performed in the United States and worldwide: 1) RYGB; 2) Adjustable Gastric Banding (AGB); 3) Sleeve Gastrectomy (VSG), and 4) Bilopancreatic Diversion (BPD) with or without duodenal switch (Figure 1).
RYGB was introduced in 1967 as a treatment for obesity [29]. The RYGB procedure involves creation of a proximal stomach pouch approximately 30 ml in size. The intestinal jejunum is then transected ~30 cm below the Ligament of Treitz to form the Roux limb.
The distal jejunal segment then forms a gastroenterostomy, while the proximal segment is connected to the small bowel ~100 cm below the jejunal division. Despite a degree of recent standardization, construction of gastric pouches, gastrojejunostomies or the Roux-en-Y limbs all exhibit patient-specific variability [30].
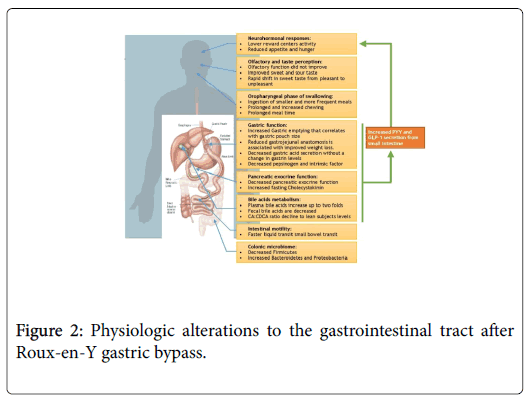
Neurohormonal and Gastrointestinal Physiology Alterations after Roux-en-Y gastric bypass (Figure 2)
Feeding behavior and neuro-hormonal changes after RYGB
Functional Magnetic Resonance Imaging (MRI) and positron emission topography (PET) imaging studies have highlighted some of the key neural responses that occur following food intake in lean, obese, and post RYGB patients. Compared to lean controls, pictorial representations of food to obese subjects more intensely activate regions of the frontal, dorsomedial prefrontal, precentral and parahippocampal cortices.
These regions stimulate attention to (and memory of) food as well as enhance food seeking behavior that is associated with weight gain [31,32]. Conversely, dorsolateral prefrontal and insular brain centers responsible for appetite control are suppressed in obesity, leading to attenuated impulse control [31]. In addition, feeding in obese patients fails to stimulate a ‘high reward’ response in mesolimbic pathways and culminates in overeating [31,33]. Thus obese patients have a more exaggerated memory of food-associated rewards and augmented food seeking behavior. These neuronal changes lead to reduced appetite control and reward during food ingestion that contributes to excessive caloric intake.
After RYGB surgery, the activity of the mesolimbic reward pathway and other reward centers is reduced in response to food pictures, especially visual representations of high caloric density food. This is associated with a decreased desire to eat [34,35]. Furthermore, RYGB surgery patients have indistinguishable hunger reactions and brain activity to food presentation from lean controls [36]. This indicates a degree of RYGB-induced normalization of obesity-associated food neural impulses.
Peptide YY (PYY) and Glucagon-like peptide-1 (GLP-1), produced by the L cells in the GI tract, may play a critical role in this integrated neural response. They have been shown to act on the central nervous system to modulate appetite and feeding behavior [37-39]. A strong body of evidence indicates that PYY and GLP-1 are suppressed in obese patients and increase after RYGB surgery [40-44]. This increase occurs in a dose dependent manner in relation to dietary calorie content and is likely due to RYGB anatomical changes, as opposed to the subsequent weight loss, as the increase occurs prior to significant weight loss [45-47]. Although less well studied, motilin, a peptide released from the upper intestine, stimulates phase III migrating motor complex and hunger. Morbidly obese patients have higher motilin levels compared to lean controls, and evidence suggests that these levels normalize after gastric bypass surgery [48]. Ghrelin, an orexigenic or appetite-stimulating hormone [49-51], is decreased shortly after RYGB in some studies. However, ghrelin returns to preoperative levels a few months after surgery despite continued weight loss [52], arguing against its role as a significant contributor to longterm changes in appetite after RYGB surgery.
The summation of these findings suggests 1) that neural responses to food are altered in obesity and appear to be recovered after RYGB surgery, and 2) that this phenomenon may be related to the neurohormonal effects of gastrointestinal incretin hormones. These findings are critically important for future research in obesity pharmacotherapy and emphasize the need further investigation in the future.
Impact of RYGB on olfactory and taste perception and relation to weight loss
Taste and smell are important modulators of feeding behavior and appetite [53,54]. Taste sensation is decreased in obese compared to lean controls, which may partly explain the inhibited reward during food ingestion [55-57]. After RYGB, the acuity for sweet and sour tastes is increased to levels that resemble lean subjects [57,58]. There is also a rapid shift in sweet taste from pleasant to unpleasant after surgery [59], likely due to altered post-surgical neural responses. These changes potentially lead to improved reward response to food or high caloric food aversion after surgery [34,60-62]. Olfactory sense and discrimination is decreased in obese patients, possibly as result of chronic high fat intake associated with obesity [57,63,64]. However, although olfactory function seems to improve after Sleeve Gastrectomy, RYGB does not lead to similar normalization, irrespective of BMI [57,65,66]. Although incompletely understood, this intriguing finding may be due to more pronounced olfactory dysfunction in RYGB surgery candidates at baseline and/or to unidentified procedure-specific factors. This data suggest that taste may play a role in obesity and weight loss after RYGB.
The oropharyngeal phase of swallowing and weight loss after RYGB
The oropharyngeal phase is stereotyped in humans and starts with solid food transportation to the back of the mouth after ingestion [67]. Then food is processed through mastication cycles in order to soften it and form a bolus suitable for subsequent swallowing [67,68]. A review of studies evaluating mastication in obese compared to lean patients leads to inconsistent findings. In some reports, obese patients assembled food faster, with less chewing time (CT) and chewing cycles (CC) [69,70]. However, two additional studies showed either increased or unchanged CC and CT in obese patients [71,72]. This variation could be due to sample size and study design. After RYGB surgery, patients ingest smaller and more frequent meals [73,74] and solid food mastication CC and CTs are increased [75]. Meal ingestion time is also prolonged [76]. The prolonged eating rate and chewing seen in RYGB can contribute to its positive impact on satiety [77]. It’s noteworthy that improved mastication was thought to contribute to efficacy of other obesity treatments such as aspiration therapy [77,78].
Gastric function after RYGB and impact on weight loss
Significant digestion of macronutrients occurs in the stomach. With accelerated emptying, gastric retention is decreased after RYGB [79-85]. In a recent study, using Scintigraphy and 3DCT, accelerated gastric emptying was found to correlate with small volume of gastric pouch and lower risk of weight regain after RYGB [86]. This accelerated gastric emptying potenitally enhances the postprandial insulin response seen after RYGB, and may be partially responsible for the augmented release of GLP-1 and PYY [84,85]. Reduction of gastrojejunal anastomosis size was also associated with better weight loss maintenance after RYGB, however underlying mechanisms are not completely understood [87]. After bariatric surgery, baseline and peak gastric acid secretion from the excluded stomach remnant is reduced, with no change in fasting and post-prandial gastrin levels [88-92]. The change gastric acid secretion may affect PYY levels after RYGB surgery and subsequent weight loss [93]. Postprandial intrinsic factor and pepsinogen secretion is also substantially decreased [91,94]. In summary, the increased gastric emptying and decreased secretion are important factors in post-RYGB weight loss and may account for the augmented release of GLP-1 and PYY after RYGB.
Pancreatic exocrine function after RYGB and impact on malabsorption and satiety
Fecal elastase-1 is reduced 8 months post-RYGB as opposed to obese controls [95]. In parallel, patients who undergo RYGB exhibit lower trypsin, chymotrypsin and amylase soon after surgery [96-98]. The decreased pancreatic exocrine function could be due to surgical alteration and can be responsible for the activation of PYY and the mild fatty acids malabsorption seen after RYGB [98]. This led to techniques promoting malabsorption such as the EndoBarrier (GI Dynamics, Lexington, MA, USA) and the incision less magnetic anastomosis system (IMAS) [99,100]. Cholecystokinin (CCK) is secreted from I cells in the duodenum and jejunum in the presence of duodenal lipids. It affects gallbladder contraction and pancreatic enzymes secretion. Furthermore, CCK was shown to induce satiety by interacting with the vagus nerve sensory fibers and subsequently brain satiety centers [101]. Fasting CCK levels are increased after RYGB surgery [102]. CCK postprandial levels are either not changed or increased, with a more rapid rise after meal ingestion, in RYGB patients [103,104]. These CCK changes are likely due to the anatomical alteration and rapid gastric transit leading to altered postprandial levels and maybe responsible for the increased satiety after RYGB.
Bile acids and weight loss after RYGB
Plasma bile acids (BA) were suggested to affect genes implicated in inflammation, obesity, and glucose metabolism [105]. Serum BA increases gradually in rats, starting at week 14 post RYGB [106]. A twofold increase was also shown in human studies after RYGB, independent of weight loss [107,108]. This increase in serum BA involves both fasting and peak postprandial levels [102]. Alternatively, although fecal BA levels peak in the first days post RYGB in rats, they normalize afterwards with a trend towards lower fecal BAs compared to sham controls [106]. This was also seen in an ileal transposition rat model which alters the small bowel anatomy in a similar fashion to RYGB [109]. These findings were replicated in a small human study by Odstrcil et al in which fecal BA absorption did not increase when measured at 5 and 14 month post RYGB [110]. Following RYGB surgery, the plasma Cholic acid: Chenodeoxycholic acid (CA: CDCA) ratio in obese rats declines to levels observed in lean animals. Decreased ratio of CA to CDCA derived BAs is also seen in stool, suggesting a change in the bile acid pool post-RYGB surgery [106]. The ratio is not significantly different between RYGB and nonobese, weight- and age-matched human controls [102]. Markers of hepatic BA biosynthesis and uptake do not increase after RYGB surgery, suggesting that there is no increased hepatic production or decreased absorption of BAs to explain the rise in serum levels. In contrast, gene expression analysis indicates more BA reabsorption in the biliopancreatic limb of the small intestine, highlighting the importance of enterohepatic recycling to the increased serum BA levels [106,109]. Thus, in conclusion, serums BA are increased after RYGB with improved CA: CDCA ratio.
Intestinal motility after RYGB
There is a paucity of data on intestinal motility after RYGB. Solid food transit in the small bowel seems to be slower after RYGB while colonic motility was similar up to 72 hours [84,111]. Another study used a lactose breath test and showed accelerated orofecal transit which could be due to faster gastric emptying and/or small bowel bacterial overgrowth [81]. As opposed to solids, liquids were shown to empty faster into the cecum. The faster liquid transit may contribute to the early rise in PYY and GLP-1 after RYGB and potentially improve satiety [85]. In summary, after RYGB, liquids small intestinal transit seems to be faster while the opposite holds for solids. This discrepancy between solids and liquids transit may contribute to improved satiety and satiation after RYGB.
Impact of RYGB on the microbiome and relation to weight loss
An expanding literature supports a role for the gut microbiome in obesity and after RYGB weight loss. When obese-lean discordant twin pairs were compared, gut microbiome of the obese group was less diverse [112]. Further, transfer of gut microbiome from lean and obese subjects can induce metabolic phenotype in germ-free mice [113]. Most of the microbiome-focused studies in RYGB were of small sample size with variable results as summarized in a recent review [114]. Monitoring bacterial genera post-RYGB showed less Firmicutes, such as Lactobacillus, as well as Actinobacteria such as Bifidobacterium decreased after RYGB. Conversely, Bacteroides and Alistipes, of the phylum Bacteroidetes, significantly increased after RYGB [115-117]. Similar gut microbial changes were seen after non-surgical weight loss [112,118]. Unlike in non-surgical weight loss, there was an increased abundance of Proteobacteria, specifically Escherichia coli, after RYGB [115-117,119-123]. Enterobacteriaceae and Pasteurellaceae were also associated with weight loss after RYGB [122,123]. Finally, compared to obese microbiota, RYGB gut microbiota transplant into germ-free mice led to weight loss and improved glucose tolerance [117,124,125]. These findings suggest that RYGB, compared to non-surgical weight loss, produces a specific shift in the gut microbiota which may induce weight loss.
Conclusion
RYGB induced surgical changes lead to significant alterations in neural and gastrointestinal physiology as well as the microbiome. These changes are likely to be collectively responsible for the observed weight loss post RYGB and may guide innovative and viable future weight loss interventions targeting these mechanisms in the future.
Author Contributions
Dr. Hussan was involved in conception, design, review of data, and drafting and critical revision of the manuscript. The above author had full access to all of the data in the study and takes responsibility for the integrity of the data. Drs. Ugbarugba, Krishna, Conwell, Bradley, Clinton and Needleman contributed to the design, interpretation of data, writing of the manuscript, and final review of the manuscript. All gave final approval of the submitted manuscript and take responsibility for the integrity of the work.
Supportive Foundations
No financial support was utilized in the creation of this project.
Conflict-of-interest Statement
The authors do not have any relevant conflicts of interest (including relevant financial interests, activities, relationships, and/or affiliations).
Data Sharing Statement
No additional data are available.
References
- Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, et al. (2014) Long-term follow-up after bariatric surgery: A systematic review. Jama 312: 934-942.
- Yamaura K, Okamoto H, Akiyoshi K, Irita K, Taniyama T, et al. (2001) Effect of low-dose milrinone on gastric intramucosal pH and systemic inflammation after hypothermic cardiopulmonary bypass. J CardiothoracVascAnesth 15: 197-203.
- Vendrell J, Broch M, Vilarrasa N, Molina A, Gómez JM, et al. (2004) Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity. Obes Res 12: 962-971.
- Laimer M, Ebenbichler CF, Kaser S, Sandhofer A, Weiss H, et al. (2002) Markers of chronic inflammation and obesity: A prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J ObesRelatMetabDisord 26: 659-662.
- Trakhtenbroit MA, Leichman JG, Algahim MF, Miller CC 3rd, Moody FG, et al. (2009) Body weight, insulin resistance, and serum adipokine levels 2 years after 2 types of bariatric surgery. Am J Med 122: 435-442.
- Brethauer SA, Heneghan HM, Eldar S, Gatmaitan P, Huang H, et al. (2011) Early effects of gastric bypass on endothelial function, inflammation, and cardiovascular risk in obese patients. SurgEndosc 25: 2650-2659.
- Miller GD, Nicklas BJ, Fernandez A (2011) Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. SurgObesRelat Dis 7: 618-624.
- Â Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, et al. (2009) Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 17: 796-802.
- Christou NV, Lieberman M, Sampalis F, SampalisJs (2008) Bariatric surgery reduces cancer risk in morbidly obese patients. SurgObesRelat Dis 4: 691-695.
- Christou NV, Sampalis JS, Liberman M, Look D, Auger S, et al. (2004) Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 240: 416-423.
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, et al. (2007) Long-term mortality after gastric bypass surgery. N Engl J Med 357: 753-761.
- McCawley GM, Ferriss JS, Geffel D, Northup CJ, Modesitt SC (2009) Cancer in obese women: Potential protective impact of bariatric surgery. J Am CollSurg 208: 1093-1098.
- Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, et al. (2009) Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): A prospective, controlled intervention trial. Lancet Oncol 10: 653-662.
- Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE, et al.(2013) Effect of bariatric surgery on oncologic outcomes: A systematic review and meta-analysis. SurgEndosc 27: 4449-4456.
- Ashrafian H, Ahmed K, Rowland SP, Patel VM, Gooderham NJ, et al. (2011) Metabolic surgery and cancer: Protective effects of bariatric procedures. Cancer 117:1788-1799.
- Weiner R, El-Sayes I, Manger T, Weiner S, Lippert H, et al. (2014) Antidiabetic efficacy of obesity surgery in Germany: A quality assurance nationwide survey. SurgObesRelat Dis 10: 322-327.
- Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, et al. (2012) Bariatric surgery and long-term cardiovascular events. Jama 307: 56-65.
- Nguyen NT, Root J, Zainabadi K, Sabio A, Chalifoux S, et al. (2005) Accelerated growth of bariatric surgery with the introduction of minimally invasive surgery. Arch Surg 140: 1198-1202.
- Buchwald H, Oien DM (2013) Metabolic/bariatric surgery worldwide 2011. ObesSurg 23: 427-436.
- Martins Tde C, Duarte TC, Mosca ER, PinheiroCde F, Marcola MA, et al. (2015) Severe protein malnutrition in a morbidly obese patient after bariatric surgery. Nutrition 31: 535-538.
- Korpershoek HW, Witteman EM, Meinardi JR, Vollaard EJ (2010) Severe vitamin D deficiency and hypocalcaemia after bariatric surgery. Ned TijdschrGeneeskd 154:Â A827.
- Ramos-Levi AM, Pérez-Ferre N, Sanchez-Pernaute A, Torres GarcÃa AJ, Rubio Herrera MA (2013) Severe vitamin A deficiency after malabsortive bariatric surgery. NutrHosp 28: 1337-1340.
- Boutin D, Cante V, Levillain P, Piguel X, Guillet G et al. (2015) Adult kwashiorkor: A rare complication of bariatric surgery. Ann DermatolVenereol 142: 99-103.
- Stroh C, Meyer F, Manger T (2014) Beriberi a severe complication after metabolic surgery - Review of the literature. Obes Facts 7: 246-252.
- Singh S, Suresh S, McClave SA, Cave M (2014) Treating Every Needle in the Haystack: Hyperammonemic Encephalopathy and Severe Malnutrition After Bariatric Surgery-A Case report and review of the literature. JPEN J Parenter Enteral Nutr 39: 977-985.
- Kizy S, Jahansouz C, Downey MC, Hevelone N, Ikramuddin S, et al. (2017) National Trends in Bariatric Surgery 2012-2015: Demographics, Procedure Selection, Readmissions, and Cost. ObesSurg 27: 2933-2939.
- Mion F, Napoleon B, Roman S, Malvoisin E, Trepo F, et al. (2005) Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. ObesSurg 15: 510-516.
- Mason EE, Ito C (1967) Gastric bypass in obesity. SurgClin North Am 47: 1345-1351.
- Schauer PR, Ikramuddin S (2001) Laparoscopic surgery for morbid obesity. SurgClin North Am 81: 1145-1179.
- Brooks SJ, Cedernaes J, Schioth HB (2013) Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: A meta-analysis of fMRI studies. PLoS One 8: e60393.
- Yokum S, Ng J, Stice E (2011) Attentional bias to food images associated with elevated weight and future weight gain: An fMRI study. Obesity (Silver Spring) 19: 1775-1783.
- Burger KS, Stice E (2011) Variability in reward responsivity and obesity: Evidence from brain imaging studies. Curr Drug Abuse Rev 4: 182-189.
- Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM et al. (2011) Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg 253: 502-507.
- Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, et al. (2014) Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 63: 891-902.
- Frank S, Wilms B, Veit R, Ernst B, Thurnheer M, et al. (2014) Altered brain activity in severely obese women may recover after Roux-en Y gastric bypass surgery. Int J Obes (Lond) 38: 341-348.
- Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, et al. (2007) PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 450: 106-109.
- De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, et al. (2011) The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 14: 700-706.
- Manning S, Batterham RL (2014) The role of gut hormone peptide YY in energy and glucose homeostasis: Twelve years on. Annu Rev Physiol 76: 585-608.
- Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, et al. (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J ClinEndocrinolMetab 90: 359-365.
- Chan JL, Mun EC, Stoyneva V, Mantzoros CS, Goldfine AB (2006) Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring), 2006. 14: 194-198.
- Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, et al. (2006) Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 14: 1553-1561.
- Morinigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, et al. (2008) Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg 247: 270-275.
- Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, et al. (2014) Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. ObesSurg 24: 241-252.
- le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, et al. (2007) Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780-785.
- Yan W, Polidori D, Yieh L, Di J, Wu X, et al. (2014) Effects of meal size on the release of GLP-1 and PYY after Roux-en-Y gastric bypass surgery in obese subjects with or without type 2 diabetes. ObesSurg 24: 1969-1974.
- Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, et al. (2008) Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J ClinEndocrinolMetab 93: 2479-285.
- Deloose E, Janssen P, Lannoo M, Van der Schueren B, Depoortere I, et al. (2015) Higher plasma motilin levels in obese patients decrease after Roux-en-Y gastric bypass surgery and regulate hunger. Gut 65: 1110-1118.
- Lai KC, Cheng CH, Leung PS (2007) The ghrelin system in acinar cells: localization, expression, and regulation in the exocrine pancreas. Pancreas 35: 1-8.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656-660.
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, et al. (2001) Ghrelin enhances appetite and increases food intake in humans. J ClinEndocrinolMetab 86: 5992.
- Falken Y, Hellstrom PM, Holst JJ, Naslund E, et al. (2011) Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J ClinEndocrinolMetab 96: 2227-2235.
- Smeets PA, Charbonnier L, van Meer F, van der Laan LN, Spetter MS, et al. (2012) Food-induced brain responses and eating behaviour. ProcNutrSoc 71: 511-520.
- Yeomans MR (2006) Olfactory influences on appetite and satiety in humans. PhysiolBehav 89: 10-14.
- Overberg J, Hummel T, Krude H, Wiegand S (2012) Differences in taste sensitivity between obese and non-obese children and adolescents. Arch Dis Child 97: 1048-1052.
- Goldstein GL, Daun H and Tepper BJ (2005) Adiposity in middle-aged women is associated with genetic taste blindness to 6-n-propylthiouracil. Obes Res 13: 1017-1023.
- Holinski F, Menenakos C, Haber G, Olze H, Ordemann J, et al. (2015) Olfactory and Gustatory Function After Bariatric Surgery. Obes Surg.
- Miras AD and Roux CW (2010) Bariatric surgery and taste: novel mechanisms of weight loss. CurrOpinGastroenterol. 26: 140-145.
- Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, et al. (2014) Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 22: 13-20.
- Tichansky DS, Boughter JD, and Madan AK (2006) Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. SurgObesRelat Dis 2: 440-444.
- Thanos PK, Michaelides M, Subrize M, Miller ML, Bellezza R, et al. (2015) Roux-en-Y gastric bypass alters brain activity in regions that underlie reward and taste perception. PLoSO ne 10: e0125570.
- Olbers T, Bjorkman S, Lindroos AK, Maleckas A, Lonnet L, et al. (2006) Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: A randomized clinical trial. Ann Surg 244: 715-22.
- Thiebaud N, Fadool JC, Gale AM, Gale DS, Fadool DA, et al. (2014) Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J Neurosci 34: 6970-6984.
- Thompson DA, Moskowitz HR and Campbell RG (1977) Taste and olfaction in human obesity. PhysiolBehav 19: 335-337.
- Richardson BE, Vanderwoude EA, Sudan R, Leopold DA, Thompson JS (2012) Gastric bypass does not influence olfactory function in obese patients. ObesSurg 22: 283-286.
- Jurowich CF, Seyfried F, Miras AD, Bueter M, Deckelmann J, et al. (2014) Does bariatric surgery change olfactory perception? Results of the early postoperative course. Int J Colorectal Dis 29: 253-260.
- Hiiemae KM and JB Palmer (1999) Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia 14: 31-42.
- Van der BA (2011) Assessment of mastication with implications for oral rehabilitation: a review. J Oral Rehabil 38: 754-780.
- Wagner M and Hewitt MI (1975) Oral satiety in the obese and no obese. J Am Diet Assoc 67: 344-346.
- Riener R, Schindler K, Ludvik B (2006) Psychosocial variables, eating behavior, depression, and binge eating in morbidly obese subjects. Eat Behav 7: 309-314.
- Spiegel TA (2000) Rate of intake, bites, and chews-the interpretation of lean-obese differences. NeurosciBiobehav Rev 24: 229-237.
- Veyrune JL, Miller CC. Czernichow S, Ciangura CA, Nicolas E, et al. (2008) Impact of morbid obesity on chewing ability. ObesSurg 18: 1467-1472.
- Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, et al. (2012) Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond) 36: 348-55.
- Zheng, H, Shin AC, Lenard NR, Townsend RL, Patterson LM, et al. (2009) Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am JÂ PhysiolRegulIntegr Comp Physiol 297: 1273-1282.
- Godlewski AE, Chaussain CC, Czernichow S, Basdevant A, Hennequin M, et al. (2011) Effect of dental status on changes in mastication in patients with obesity following bariatric surgery. PLoS One 6: 22324.
- Laferrere B (2012) Gut feelings about diabetes. EndocrinolNutr 59: 254-260.
- Ferriday D, Bosworth ML, Lai S, Godinot N, Martin N, et al. (2015) Effects of eating rate on satiety: A role for episodic memory? Physiology & Behavior 152: 389-396.
- Noren E and Forssell H (2016) Aspiration therapy for obesity; a safe and effective treatment. BMC obesity 3: 56.
- Naslund I and KW Beckman (1987) Gastric emptying rate after gastric bypass and gastroplasty. Scand J Gastroenterol 22: 193-201.
- Horowitz M, Cook DJ, Collins PJ, Harding PE, Hooper MJ, et al. (1982) Measurement of gastric emptying after gastric bypass surgery using radionuclides. Br J Surg 69: 655-657.
- Morinigo R, Marin JL, Delgado S, Casamitjana R, Vidal J, et al. (2006) Glucagon-like peptide-1, peptide YY, hunger and satiety after gastric bypass surgery in morbidly obese subjects. J ClinEndocrinolMetab 91: 1735-1740.
- Seyfried F, Lannoo M, Gsell W, Tremoleda JL, Bueter M, et al. (2012) Roux-en-Y gastric bypass in mice-surgical technique and characterisation. ObesSurg 22: 1117-1125.
- Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, et al. (2014) Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J PhysiolEndocrinolMetab 306: 424-432.
- Dirksen C, Damgaard M, Bojsen-Moller KN, Jorgensen NB, Kielgast U, et al. (2013) Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. NeurogastroenterolMotil 25: 346-e255.
- Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, et al. (2014) Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring) 22: 2003-2009.
- Riccioppo D, Santo MA, Rocha M, Buchpiguel CA, Diniz MA, et al. (2017) Small-Volume, Fast-Emptying Gastric Pouch Leads to Better Long-Term Weight Loss and Food Tolerance After Roux-en-Y Gastric Bypass. Obes Surg.
- Jirapinyo P, Dayyeh BK and Thompson CC (2016) Gastrojejunal anastomotic reduction for weight regain in roux-en-y gastric bypass patients: Physiological, behavioral, and anatomical effects of endoscopic suturing and sclerotherapy. SurgObesRelat Dis 12: 1810-1816.
- Mason EE, Munns JR, Kealey GP, Wangler R, Clarke WR, et al. (1976) Effect of gastric bypass on gastric secretion. Am J Surg 131: 162-168.
- Nomiyama S, Doughertyet SH, Vogel SB, Eisenbergal MM (1980) The effect of small bowel bypass and subsequent resection on gastric acid secretion and serum gastrin. J Surg Res 28: 103-109.
- Behrns KE, Smith CD and Sarr MG (1994) Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig Dis Sci 39: 315-320.
- Sillakivi T, Suumann J, Kirsimägi U, Peetsalu A, et al. (2013) Plasma levels of gastric biomarkers in patients after bariatric surgery: biomarkers after bariatric surgery. Hepatogastroenterology 60: 2129-2132.
- Smith CD, Herkes SB, Behrns KE, Fairbanks VF, Kelly KA, et al. (1993) Gastric acid secretion and vitamin B12 absorption after vertical Roux-en-Y gastric bypass for morbid obesity. Ann Surg 218: 91-96.
- Gomez G, Padilla L, Udupi V, Tarasova N, Sundler F, et al. (1996) Regulation of peptide YY homeostasis by gastric acid and gastrin. Endocrinology 137: 1365-1369.
- Marcuard, S.P., et al., Absence of luminal intrinsic factor after gastric bypass surgery for morbid obesity. Dig Dis Sci, 1989. 34(8): p. 1238-42.
- Carswell KA, Vincent RP, Belgaumkar AP, Sherwood RA, Amiel SA, et al. (2014) The effect of bariatric surgery on intestinal absorption and transit time. ObesSurg 24: 796-805.
- Emas S, Billings A and Grossman MI (1968) Effects of gastrin and pentagastrin on gastric and pancreatic secretion in dogs. Scand J Gastroenterol 3: 234-240.
- OKeefe SJD, Rakitt T, Ou J, El Hajj II, Blaney E, et al. (2017) Pancreatic and Intestinal Function Post Roux-en-Y Gastric Bypass Surgery for Obesity. ClinTranslGastroenterol 8: e112.
- Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, et al. (2010) A multicenter, randomized efficacy study of the endobarrier gastrointestinal liner for presurgical weight loss prior to bariatric surgery. Ann Surg 251: 236-243.
- Machytka E, Buzga M, Zonca P, Lautz DB, Ryou M, et al. (2017) Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. GastrointestEndosc 86: 904-912.
- Strader AD and SC (2005) Woods, gastrointestinal hormones and food intake. Gastroenterology 128: 175-191.
- De Giorgi S, Campos V, Egli L, Toepel U, Carrel G, et al. (2014) Long-term effects of Roux-en-Y gastric bypass on postprandial plasma lipid and bile acids kinetics in female non diabetic subjects: A cross-sectional pilot study. ClinNutr.
- Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, et al. (2004) The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 240: 236-242.
- Kellum JM, Kuemmerle JF, O'Dorisio TM, Rayford P, Martin D, et al. (1990) Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 211: 763-770.
- Cariou B, Fruchart JC, Gonzalez FJ, Kuipers F, Staels B, et al. (2006) The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J BiolChem 281: 11039-11049.
- Bhutta HY, Rajpal N, White W, Freudenberg JM, Liu Y, et al. (2015) Effect of Roux-en-Y Gastric Bypass Surgery on Bile Acid Metabolism in Normal and Obese Diabetic Rats. PLoS One 10: e0122273.
- Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, et al. (2013) Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J ClinEndocrinolMetab 98: 708-712.
- Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Kakela P, et al. (2012) Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. ObesSurg 22: 1473-1480.
- Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, et al. (2010) Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J PhysiolGastrointest Liver Physiol 299: 652-660.
- Odstrcil EA, Martinez JG, Santa Ana CA, Xue B, Schneider RE, et al. (2010) The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J ClinNutr 92: 704-713.
- Pellegrini CA, Deveney CW, Patti MG, Lewin M, Way LW (1986) Intestinal transit of food after total gastrectomy and Roux-Y esophagojejunostomy. Am J Surg 151: 117-125.
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, et al. (2009) A core gut microbiome in obese and lean twins. Nature 457: 480-484.
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, et al. (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341: 1241214.
- Anhe FF, Varin TV, Schertzer JD, Marette A (2017) The gut microbiota as a mediator of metabolic benefits after bariatric surgery. Can J Diabetes.
- Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, et al. (2013) Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J ClinNutr 98: 16-24.
- Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, et al. (2010) Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59(12): 3049-3057.
- Tremaroli V, Karlsson F, Werling M, Stahlman M, Kovatcheva-Datchary P, et al. (2015) roux-en-y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metabolism 22: 228-238.
- Ley, R.E., et al., Microbial ecology: human gut microbes associated with obesity. Nature, 2006. 444(7122): p. 1022-3.
- Guo Y (2017) Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: Increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab Res Rev 33.
- Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, et al. (2016) Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Medicine 8: 67.
- Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, et al. (2011) Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 60: 1214-1223.
- Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, et al. (2013) Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 13: 514-522.
- Shao Y, Ding R, Xu B, Hua R, Shen Q, et al. (2017) alterations of gut microbiota after roux-en-y gastric bypass and sleeve gastrectomy in sprague-dawley rats. ObesSurg 27: 295-302.
- Liou AP (2013) Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. SciTransl Med 5: 178ra41.
- Arora T, Seyfried F, Docherty NG, Tremaroli V, le Roux CW, et al. (2017) Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J 11: 2035-2046.
Citation: Hussan H, Ugbarugba E, Krishna SG, Conwell DL, Bradley D, et al. (2017) Impact of Roux-En-Y Gastric Bypass Surgery on Neurohormonal and Gastrointestinal Physiology: Insights for Future Weight Loss Efforts. J Obes Weight Loss Ther 7: 356. DOI: 10.4172/2165-7904.1000356
Copyright: © 2017 Hisham H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6890
- [From(publication date): 0-2017 - Aug 03, 2025]
- Breakdown by view type
- HTML page views: 5874
- PDF downloads: 1016