Research Article Open Access
Influence of Renal ABC and SLC Transporter Polymorphisms on Cisplatin-induced Nephrotoxicity in Patients with Esophageal Cancer
Kazuma Fujita1, Takenori Niioka1*, Satoru Motoyama2 and Masatomo Miura1
1Department of Pharmacy, Akita University Hospital, Akita, Japan
2Department of Surgery, Akita University School of Medicine, Akita, Japan
- Corresponding Author:
- Takenori Niioka
Department of Pharmacy
Akita University Hospital, 1-1-1 Hondo
Akita 010-8543, Japan
Tel: +81-18-884-6462
Fax: +81-18-836-2628
E-mail: t-niioka@hos.akita-u.ac.jp
Received date: June 18, 2016; Accepted date: July 12, 2016; Published date: July 19, 2016
Citation: Fujita K, Niioka T, Motoyama S, Miura M (2016) Influence of Renal ABC and SLC Transporter Polymorphisms on Cisplatin-induced Nephrotoxicity in Patients with Esophageal Cancer. Clin Pharmacol Biopharm 5:158. doi: 10.4172/2167-065X.1000158
Copyright: © 2016 2016 Fujita K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Background: The individual and collective contributions of genetic polymorphisms in drug transporter genes in human renal proximal tubules to cisplatin-induced nephrotoxicity are still unclear. Methods: In this study, we investigated the effects of polymorphisms in SLC22A2 (808G>T), SLC31A1 (rs10981694A>C, rs12686377G>T, rs7851395A>G), SLC47A1 (rs2289669G>A), ABCB1 (1236C>A, 2677G>T/A, 3435C>T), ABCC2 (-24C>T), and ABCG2 (421C>A) on cisplatin-induced nephrotoxicity in 131 patients with esophageal cancer receiving 5-fluorouracil and cisplatin (FP) chemotherapy. The change rate of the estimated glomerular filtration rate (eGFR) was calculated according to the following formula: eGFR before FP chemotherapy-lowest eGFR during first cycle after FP chemotherapy)/eGFR before FP chemotherapy. Results: In univariate analysis, there was a significant correlation between patient age and the change rate of the eGFR by cisplatin (P = 0.021). However, there were no significant differences in the change rate of the eGFR by cisplatin between SLC22A2, SLC47A1, SLC31A1, ABCB1, ABCC2, and ABCG2 polymorphisms. Multivariate analysis revealed that only age was an independent variable predicting a higher risk of cisplatin-induced acute renal dysfunction. Conclusion: In FP chemotherapy for esophageal cancer patients, cisplatin-induced nephrotoxicity was not affected by polymorphisms in the uptake transporters OCT2 and Ctr1 or efflux transporters MATE1, P-glycoprotein, MRP2, and BCRP. Since degradation of renal function due to aging reduces cisplatin clearance, the cisplatin dosage should be carefully chosen in elderly patients.
Keywords
Cisplatin; Nephrotoxicity; CTR1; OCT2; MATE1
Introduction
Cisplatin (cis-diamminedichloroplatinum), a potent platinumbased alkylating anticancer drug, is widely used as therapy for many types of cancer such as lung, breast, bladder, esophageal, head and neck cancers [1-4]. However, the use of cisplatin is limited by severe side effects such as nephrotoxicity, cardiotoxicity, gonadotoxicity, hepatotoxicity, and central and peripheral neurotoxicity [5-7]. Cisplatin is an extremely hydrophilic compound that has difficulty crossing plasma membranes [8]. Cisplatin is excreted primarily by the kidneys, i.e., it is filtered in the glomerulus and is secreted by the human kidney [9]. Consequently, movement of cisplatin through plasma membranes is mediated by transport proteins.
Renal tubular epithelial cells contain brush-border (apical) membranes and basolateral membranes. Cisplatin is a substrate of uptake transporters, organic cation transporter (OCT) 2 (encoded by the SLC22A2 gene) and copper transporter (Ctr) 1 (encoded by the SLC31A1 gene), expressed in the basolateral membrane of renal proximal tubules [10-16]. On the other hand, cisplatin is transported by the H+/organic cation antiporter multidrug and toxin extrusion transporter (MATE) 1 (encoded by the SLC47A1 gene) expressed in the renal apical membrane [15-17]. In addition, although cisplatin is not a substrate of ATP-binding cassette (ABC) transporters, such as multidrug resistance-associated protein (MRP) 2 (encoded by the ABCC2 gene), breast cancer resistance protein (BCRP) (encoded by the ABCG2 gene) and P-glycoprotein (encoded by the ABCB1 gene), its metabolites, including cisplatin-thiol and unidentified substances induced by cisplatin, may cause cisplatin-induced nephrotoxicity [18-23]. The activities of these drug transporters in human renal proximal tubules are considered a determinant of cisplatin-induced nephrotoxicity.
In our previous study, we found that cisplatin-induced nephrotoxicity in 95 esophageal cancer patients receiving a 5-fluorouracil (5-FU) and cisplatin (FP) regimen was unaffected by the SLC22A2 808G>T polymorphism [24]. This result is different from those of previous studies [25,26]. However, the ABCC2-24T variant shows 18.7% reduced activity compared with the -24C/C genotype [27], and clinical evidence has shown that common ABCC2 polymorphisms may affect the disposition or efficacy of drugs that are known ABCC2 substrates [28]. In addition, cisplatin induces BCRP and P-glycoprotein in the kidneys [22,29]. Hoffmeyer et al. reported that the expression levels of P-glycoprotein induced by rifampin are lowest in subjects with the ABCB1 3435T/T genotype [30]. Therefore, patients with the ABCB1 3435T/T genotype may exhibit higher nephrotoxicity. However, the individual and collective contributions of genetic polymorphisms in the above transporters to cisplatin-induced nephrotoxicity are still unclear.
In the present study, we re-examined the influence of the SLC22A2 808G>T polymorphism using an increased sample size of 131 patients with esophageal cancer receiving FP chemotherapy. In addition, we investigated the effects of polymorphisms in SLC31A1 (rs10981694A>C, rs12686377G>T, rs7851395A>G), SLC47A1 (rs2289669G>A), ABCB1 (1236C>A, 2677G>T/A, 3435C>T), ABCC2 (-24C>T), and ABCG2 (421C>A) on cisplatin-induced nephrotoxicity.
Materials and Methods
Protocol
This study was approved by the Ethics Committee of Akita University Graduate School of Medicine. Each participant provided informed consent in compliance with the code of ethics of the World Medical Association (Declaration of Helsinki) before this study and signed a human subject institutional review board consent form. Eighty-four esophageal cancer patients receiving FP chemotherapy in this study had participated in our previous studies [24].
The disease was classified according to the UICC International Union against Cancer tumor-node-metastasis (TNM) Classification of Malignant Tumors (sixth edition). Between 2004 and 2014, 131 patients with normal renal function before FP chemotherapy who agreed to genetic analysis of polymorphisms were included in the study. Patients with renal dysfunction who required reduced doses of cisplatin lower than those indicated in the protocol during the first cycle were not included in this study (n = 12). We treated patients with stage II - IV disease with esophagectomy with extended lymphadenectomy followed by adjuvant chemotherapy consisting of protracted infusion of 5-FU (800 mg/m2/day) on days one to five and cisplatin (80 mg/ m2/day) on day one. This protocol was repeated every four weeks. After 2009, we treated the stage II and III patients with neoadjuvant chemotherapy or neoadjuvant chemoradiotherapy (40 Gy) consisting of the same protocol followed by surgery. Stage IV patients received definitive chemoradiotherapy or chemotherapy alone. In these patients, chemotherapy consisted of protracted infusion of 5-FU (400 mg/m2/ day) on days one to five, eight to 12, and cisplatin (40 mg/m2/day) on days one and eight. This protocol was repeated every four weeks. After 2009, cisplatin was administered in 500 mL of normal saline solution over 2 h in combination with 5-FU and 2500-3000 mL of hydration. In addition, after 2013, all patients received 8 mEq of magnesium sulfate to prevent nephrotoxicity. However, we were unable to confirm information regarding hydration and the use of magnesium sulfate for treatments given during 2012 owing to incomplete medical records. The toxicity grade was determined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 criteria.
Genotyping
Single nucleotide polymorphisms that influence transporter activity were chosen for analysis. DNA was extracted from peripheral blood samples using a QIAamp Blood Kit (Qiagen, Hilden, Germany) and was stored at -80°C until analyzed. Genotyping procedures identifying the G and T alleles of SLC22A2 (808G>T, rs316019) were performed using a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method described by Wang et al. [31] Genotyping procedures identifying the C and T alleles in exon 12 (1236 C>T, rs1128503), the G and T/A alleles in exon 21 (2677 G>T/A, rs2032582), and the C and T alleles in exon 26 (3435 C>T, rs1045642) of the ABCB1 gene were performed using PCR-RFLP methods described by Wu et al. [32], Tanaka et al. [33] and Cascorbi et al. [34], respectively. The ABCG2 421C>A (rs2231142) and ABCC2 -24C>T (rs717620) polymorphisms were genotyped by the PCR-RFLP methods of Kobayashi et al. [35] and Rau et al. [36], respectively. The SLC47A1 rs2289669G>A and SLC31A1 rs7851395A>G polymorphisms were genotyped using a fully automated single nucleotide polymorphism (SNP) detection system (prototype i-densy, ARKRAY Inc., Kyoto, Japan) and by the PCR-RFLP method of He et al. [37]. The SLC31A1 rs10981694A>C and SLC31A1 rs12686377G>T polymorphisms were genotyped by the PCR-RFLP method of Wu et al. [38] All frequencies for the different loci analyzed were at Hardy-Weinberg equilibrium.
Statistical analyses
The Shapiro-Wilk test was used to assess distribution. The characteristics of esophageal cancer patients were expressed as the mean ± standard deviation (range). The estimated glomerular filtration rate (eGFR) was calculated for each esophageal cancer patient according to the following formula: eGFR = 194* serum creatinine (Scr) (mg/dL) -1·094* age -0·287* body surface area (m2)/1.73 (*0·739 for female). The change rates in laboratory test values were calculated for each patient with esophageal cancer according to the following formula: Change rate = (value before FP chemotherapy-lowest value during first cycle after FP chemotherapy)/value before FP chemotherapy. The change rate of the eGFR between genotype groups for each transporter was expressed as the median (quartile 1-quartile 3). The Kruskal-Wallis test or Mann-Whitney U test was used to determine the difference in continuous values between groups. The Spearman’s rank correlation coefficient test was used to assess correlations in continuous values between groups, and all results were expressed as a correlation coefficient (r). Stepwise multiple linear regression analysis was performed to determine the effect of all factors in a univariate analysis. For each patient, the transporter genotypes were replaced with dummy variables (in 2 groups, 1 and 0; in 3 groups, 1 and 0, 0 and 0, and 0 and 1, respectively). For post-hoc power analysis, the power (1 – β) was calculated about the results of comparisons of the change rates of eGFR between genotypes for each drug transporter. A 2-sided P-value less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS 20.0 for Windows (SPSS IBM Japan Inc., Tokyo, Japan). Power was calculated using G*Power version 3.1 software.
Results
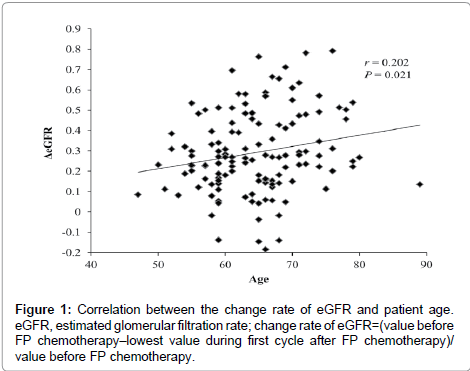
The baseline patient characteristics are listed in Table 1. The mean (± SD) age of patients was 64.5 ± 7.2 years, and the mean body weight (± SD) was 53.1 ± 9.9 kg. There were no patients with serious renal or hepatic dysfunction before FP chemotherapy. In univariate analysis, there was a significant correlation between patient age and the change rate of the eGFR by cisplatin (P = 0.021, Table 2). However, there were no significant differences between substrata of patients by gender, dose of cisplatin in cycle, disease stage, or the change rate of the eGFR by cisplatin (Table 2). In addition, there were no significant differences in the change rate of the eGFR by cisplatin between SLC22A2, SLC47A1, SLC31A1, ABCB1, ABCC2, and ABCG2 polymorphisms (Table 2). However, all power levels were less than 50% in comparisons with change rates of eGFR between genotype groups for each drug transporter (SLC47A1, SLC31A1 and ABCC2). Multivariate analysis showed that only age was an independent variable predicting a higher risk of cisplatin-induced acute renal dysfunction. The correlation between the change rate in the eGFR and age is shown in Figure 1.
| Gender (male: female) | 114:17 | |
| Age (year) | 64.5 ±7.2 | (47-89) |
| Body weight (kg) | 53.1 ±99 | (32-88) |
| Body surface area (m2) | 1.6 ± 0.2 | (1.2-2.1) |
| Laboratory test values | ||
| Estimated Glomerular Filtration Rate (mL/min/1.73m2) | 82.1 ± 16.5 | (53-136.1) |
| Serum creatinine (mg/dL) | 0.66 ± 0.13 | (0.38-0.92) |
| Blood urea nitrogen (mg/dL) | 13.4 ± 4.4 | (4.1-28.3) |
| Aspartate transaminase (IU/L) | 20.9 ± 7.4 | (8-46) |
| Alanine transaminase (IU/L) | 20.6 ± 13.1 | (6-73) |
| Total bilirubin (mg/dL) | 0.5 ± 0.3 | (0.1-1.4) |
| White blood cell (×103/µL) | 6.4 ± 2..2 | (2.4-12.8) |
| Red blood cell (×104/µL) | 395.5 ± 49.8 | (=240-544) |
| Platelet (×104/µL) | 256 ± 84 | (91-548) |
| Na (mEq/L) | 139 ± 3.1 | (132-145) |
| K (mEq/L) | 4.3 ± 0.4 | (3-5.5) |
| CL (mEq/L) | 102.4 ± 3 | (95-109) |
| Chemotherapeutic regimen | ||
| Dose of cisplatin/1 cycle (mg) | 124.3 ± 12.4 | (90:170) |
| 80 mg/m2 (Day1):40 mg/m2 (Day 1 and Day 8) | 76:55 | - |
| Radiation (with: without) | 74:57 | - |
| Stage (I : II : III : IVa : IVb) | 4 : 24 : 50 : 46 : 7 | |
| SLC22A2 808G>T | G/G : G/T : T/T = 101 : 29 : 1 | |
| SLC47A1 rs2289669G>A | G/G : G/A : A/A = 33 : 71 : 27 | |
| SLC31A1 rs10981694A>C | A/A : A/C : C/C = 100 : 29 : 2 | |
| SLC31A1rs12686377G>T | G/G:G/T:T/T=43:68:20 | |
| SLC31A1rs7851395A>G | A/A:A/G:G/G=28:58:45 | |
| ABCB11236C>T | C/C:C/T:T/T=14:53:64 | |
| ABCB12677G>T/A | G/G:G/T+G/A:T/T+T/T+A/A=27:59:45 | |
| ABCB13435C>T | C/C:C/T:T/T=35:63:33 | |
| ABCC2-24C>T | C/C:C/T:T/T=68:54:9 | |
| ABCG2421C>A | C/C:C/A:A/A=66:52:13 | |
Data presented as number or mean ± standard deviation (range)
Table 1: Clinical characteristics of 131 patients with esophageal cancer before chemotherapy.
| Correlation coefficient (r) | P-value | ||
| Age | 0.202 | 0.021 | |
| Body weight | -0.086 | 0.331 | |
| Body surface area | -0.057 | 0.519 | |
| Dose of cisplatin/1 cycle (mg) | -0.06 | 0.497 | |
| Median | (25th-75th percentile) | P-value | |
| Gender | - | - | 0.789 |
| Male | 0.268 | (0.155-0.434) | - |
| Female | 0.308 | (0.121-0.386) | - |
| Radiation | - | - | 0.293 |
| With | 0.255 | (0.136-0.457) | |
| Without | 0.278 | (0.202-0.396 | |
| Chemotherapeutic regimen/1 cycle | - | - | 0.53 |
| 80 mg/m2 (Day 1) | 0.274 | (0.154-0.471) | - |
| 40 mg/m2 (Day 1,8) | 0.248 | (0.157-0.404) | - |
| Stage | |||
| I | 0.239 | (0.188-0.380) | 0.46 |
| II | 0.268 | (0.109-0.393) | - |
| III | 0.279 | (0.163-0.501) | - |
| IVa | 0.267 | (0.139-0.396) | - |
| IVb | 0.23 | (0.205-0.256) | - |
|
SNPs |
|||
| SLC22A2 808G>T | - | - | 0.833 |
| G/G | 0.268 | (0.154-0.438) | - |
| G/T+T/T | 0.273 | (0.162-0.386) | - |
| SLC47A1 rs2289669G>A | - | - | 0.626 |
| G/G | 0.279 | (0.202-0.433) | - |
| G/A | 0.268 | (0.158-0.411) | - |
| A/A | 0.239 | (0.138-0.465) | - |
| SLC31A1 rs10981694A>C | - | - | 0.082 |
| A/A | 0.276 | (0.165-0.465) | - |
| A/C+C/C | 0.202 | (0.128-0.309) | - |
| SLC31A1 rs12686377G>T | - | - | 0.701 |
| G/G | 0.268 | (0.141-0.442) | - |
| G/T | 0.273 | (0.185-0.420) | - |
| T/T | 0.252 | (0.117-0.474) | - |
| SLC31A1 rs7851395A>G | - | - | 0.117 |
| A/A | 0.201 | (0.118-0.328) | |
| A/G | 0.271 | (0.188-0.438) | - |
| G/G | 0.282 | (0.181-0.480) | - |
| ABCB1 1236C>T | - | - | 0.223 |
| C/C | 0.269 | (0.142-0.433) | - |
| C/T | 0.283 | (0.201-0.485) | - |
| T/T | 0.234 | (0.147-0.395) | |
| ABCB1 2677G>T/A | - | - | 0.244 |
| G/G | 0.27 | (0.229-0.513) | - |
| G/T+G/A | 0.248 | (0.139-0.374) | - |
| T/T+T/A+A/A | 0.255 | (0.154-0.433) | - |
| ABCB1 3435C>T | - | - | 0.654 |
| C/C | 0.27 | (0.165-0.487) | - |
| C/T | 0.276 | (0.181-0.410) | - |
| T/T | 0.239 | (0.136-0.412) | - |
| ABCC2 -24C>T | - | - | 0.144 |
| C/C | 0.244 | (0.151-0.370) | - |
| C/T+T/T | 0.298 | (0.165-0.484) | - |
| ABCG2 421C>A | - | - | 0.613 |
| C/C | 0.271 | (0.183-0.438) | - |
| C/A+A/A | 0.264 | (0.150-0.428) | - |
eGFR: Estimated Glomerular Filtration Rate
Table 2: Correlation and comparison of change rate of eGFR between patient backgrounds.
Discussion
In the present study, in FP chemotherapy for esophageal cancer patients, there were no significant differences in the change rate of the eGFR by cisplatin administration between SLC22A2 808G>T or SLC31A1 (rs10981694A>C, rs12686377G>T and rs7851395A>G) genotypes. The contribution ratio of cisplatin taken up by the transporters OCT2 and Ctr1 is not clear. In patients with reduced OCT2 activity, cisplatin may primarily be transported by Ctr1. Therefore, definitive renal transporter polymorphisms promoting cisplatin-induced nephrotoxicity may not be found. On the other hand, there were no significant differences in the change rate of the eGFR between patients with different MATE1 genotypes. Iwata et al. reported that the SLC47A1 rs2289669G>A polymorphism is not associated with cisplatin-induced nephrotoxicity [25]. Although the SLC47A1 rs2289669G>A polymorphism has been reported to be associated with the glucose-lowering effect of metformin [37,39], similar to the result obtained by Iwata et al., our present study also showed that cisplatin-induced nephrotoxicity was not affected by the SLC47A1 rs2289669G>A polymorphism.
In single and multiple regression analyses, cisplatin-induced nephrotoxicity was affected by patient age. Since degradation of renal function due to aging reduces cisplatin clearance, the cisplatin dosage should be carefully chosen in elderly patients. However, the effects of age on cisplatin-induced nephrotoxicity were limited in our study. Although only two patients who were 80 years of age or older were enrolled in this study, their change rates of eGFR were not high (Figure 1). However, since elderly patients tend to have impaired physiology functions, cisplatin should be administered with caution in these patients.
In this study, cisplatin-induced nephrotoxicity was unaffected by ABCC2 polymorphisms. Sprowl et al. have also reported that cisplatin-induced nephrotoxicity is not affected by the ABCC2 -24C>T polymorphism [40]. These data are consistent with our present study. Additionally, Wen et al. suggested that cisplatin is conjugated with tripeptide glutathione (GSH) by glutathione S-transferases in renal tubular epithelial cells, and cisplatin-GSH but not cisplatin, is subsequently effluxed by MRP2 to the tubule lumen [19]. Cisplatin-GSH is then metabolized by γ-glutamyltranspeptidase and aminodipeptidase to cisplatin-cysteine (CYS) on the cell surface [41,42], and the cisplatin- CYS conjugate is then reabsorbed. Cisplatin-CYS is metabolized by cysteine-S-conjugate β-lyase to form cisplatin-thiol, which leads to nephrotoxicity [19,43]. According to the discussion of Wen et al., the concentration of cisplatin-GSH in renal tubular epithelial cells would be elevated by the ABCC2 -24T mutation [19]. The formation of cisplatin-GSH reduces the amount of platinum bound to DNA and protects dividing cells from cisplatin nephrotoxicity [44]. Therefore, the formation of cisplatin-GSH and its transport out of the cell seem to be the first steps in detoxification to prevent nephrotoxicity. That is, the elevation of cisplatin-GSH in cells resulting from the ABCC2 mutation does not seem to influence cisplatin-induced nephrotoxicity.
There were some limitations to this study. First, this study was carried out with a small sample size of 131 patients with esophageal cancer receiving FP chemotherapy. Therefore, further studies using a larger sample size are necessary. Second, although hydration and the administration of magnesium sulfate are useful for prevention of cisplatin-induced nephrotoxicity [45], it was not possible to compare these parameters from our study with those in previous studies due to lack of sufficient information. The influence of renal drug transporter polymorphisms on cisplatin-induced nephrotoxicity may differ according to hydration and/or the administration of magnesium sulfate. Third, we have not investigated the expression of inflammatory markers in this study. The majority of studies under review have reported that these markers are associated with the pathogenesis of cisplatininduced nephrotoxicity [46]. In addition, Shinke et al. have recently reported that urinary kidney injury molecule-1 and/or monocyte chemotactic protein-1 may represent biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients [47]. Hence, these results should be interpreted within the context of the study limitations. However, in FP chemotherapy for patients with esophageal cancer, it became clear that a polymorphism in each drug transporter did not directly affect cisplatin-induced nephrotoxicity alone. A combination of multiple drug transporters, including MATE1, P-glycoprotein, BCRP, and MRP2, reacts with the efflux of cisplatin and its metabolites, resulting in complex transport mechanisms. In addition, other single nucleotide polymorphisms, in addition to the transporter polymorphisms observed in the present study, may influence cisplatin-induced nephrotoxicity. Therefore, further studies are necessary to unravel the pathophysiological mechanisms of cisplatin-induced nephrotoxicity.
In FP chemotherapy for esophageal cancer patients, there were no significant differences in the change rate of the eGFR by cisplatin between uptake transporters SLC22A2 808G>T or SLC31A1 (rs10981694A>C, rs12686377G>T and rs7851395A>G) genotypes, and between efflux transporter SLC47A1 rs2289669G>A genotypes. In the present study, cisplatin-induced nephrotoxicity was affected by patient age. Cisplatin dosage should be chosen carefully in elderly patients.
Conflict of Interest
All of the authors state they have no conflicts of interest.
References
- Desoize B, Madoulet C (2002) Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev OncolHematol 42: 317-325.
- Flore AM, Busselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanism of activity, drug resistance and induced side effects. Cancers 3: 1351-1371.
- Chen D, Milacic V, Frezza M, Dou QP (2009) Metal complexes, their cellular targets and potential for cancer therapy. Curr Pharm Des 15: 777-791.
- Amptoulach S, Tsavaris N (2011) Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract 843019: 1-5.
- Rabik CA, Dolan ME (2007) Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33: 9-23.
- Yazici A, Sari ES, Yay A, Aksit H, Kilic A, et al. (2014) The protective effect of selenium in cisplatin related retinotoxicity. CutanOculToxicol 33: 327-332.
- Erken HA, Koç ER, Yazici H, Yay A, Önder GÖ, et al. (2014) Selenium partially prevents cisplatin-induced neurotoxicity: a preliminary study. Neurotoxicology 42: 71-75.
- Houjou T, Nakano K, Ike O, Wada H, Hitomi S, et al. (1996) Oral sustained-release cisplatin capsule. J Pharm Pharmacol 48: 474-478.
- Jacobs C, Coleman CN, Rich L, Hirst K, Weiner MW (1984) Inhibition of cis-diamminedichloroplatinum secretion by the human kidney with probenecid. Cancer Res 44: 3632-3635.
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, et al. (2002) Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am SocNephrol 13: 866-874.
- Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K (2006) Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family). J PharmacolExpTher 319: 879-886.
- Yonezawa A, Inui K (2011) Organic cation transporter OCT/SLC22A and H(+)/organic cationantiporter MATE/SLC47A are key molecules for nephrotoxicity of platinum agents. BiochemPharmacol 81: 563-568.
- Harrach S, Ciarimboli G (2015) Role of transporters in the distribution of platinum-based drugs. Front Pharmacol 6: 85.
- Kim ES, Tang X, Peterson DR, Kilari D, Chow CW, et al. (2014) Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer 85: 88-93.
- Motohashi H, Inui K (2013) Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J 15: 581-588.
- Ciarimboli G (2014) Membrane transporters as mediators of cisplatin side-effects. Anticancer Res 34: 547-550.
- StaudF, Cerveny L, Ahmadimoghaddam D, Ceckova M (2013) Multidrug and toxin extrusion proteins (MATE/SLC47); role in pharmacokinetics. Int J Biochem Cell Biol 45: 2007-2011.
- Ceckova M, Vackova Z, Radilova H, Libra A, Buncek M, et al. (2008) Effect of ABCG2 on cytotoxicity of platinum drugs: interference of EGFP. ToxicolIn Vitro 22: 1846-1852.
- Wen X, Buckley B, McCandlish E, Goedken MJ, Syed S, et al. (2014) Transgenic expression of the human MRP2 transporter reduces cisplatin accumulation and nephrotoxicity in Mrp2-null mice. Am J Pathol 184: 1299-1308.
- Chan YY, Kalpana S, Chang WC, Chang WC, Chen BK (2013) Expression of aryl hydrocarbon receptor nuclear translocator enhances cisplatin resistance by upregulating MDR1 expression in cancer cells. MolPharmacol 84: 591-602.
- Gibalová L, Sereš M, Rusnák A, Ditte P, Labudová M, et al. (2012) P-glycoprotein depresses cisplatin sensitivity in L1210 cells by inhibiting cisplatin-induced caspase-3 activation. ToxicolIn Vitro 26: 435-444.
- Demeule M, Brossard M, Beliveau R (1999) Cisplatin induces renal expression of P-glycoprorein and canalicularmultispecific organic anion transporter. Am J Physiol 277: 832-840.
- Yang X, Pagé M (1995) P-glycoprotein expression in ovarian cancer cell line following treatment with cisplatin. Oncol Res 7: 619-624.
- Hinai Y, Motoyama S, Niioka T, Miura M (2013) Absence of effect of SLC22A2 genotype on cisplatin-induced nephrotoxicity in esophageal cancer patients receiving cisplatin and 5-fluorouracil: report of results discordant with those of earlier studies. J Clin Pharm Ther 38: 498-503.
- Iwata K, Aizawa K, Kamitsu S, Jingami S, Fukunaga E, et al. (2012) Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. ClinExpNephrol 16: 843-851.
- Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A (2009) Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. ClinPharmacolTher 86: 396-402.
- Haenisch S, Zimmermann U, Dazert E, Wruck CJ, Dazert P, et al. (2007) Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenomics J 7: 56-65.
- Cascorbi I, Haenisch S (2010) Pharmacogenetics of ATP-binding cassette transporters and clinical implications. Methods MolBiol 596: 95-121.
- Zhang Q, Li K, Xu JH, Zhao CG, Gao Q, et al. (2013) Role of ABCG2 expression driven by cisplatin in platinum-containing chemotherapy for gastric cancer. World J Gastroenterol 19: 6630-6636.
- Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, et al. (2002) Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. ProcNatlAcadSci USA 97: 3473-3478.
- Wang ZJ, Yin OQ, Tomlinson B, Chow MS (2008) OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics 18: 637-645.
- Wu L, Xu X, Shen J, Xie H, Yu S, et al. (2007) MDR1 gene polymorphisms and risk of recurrence in patients with hepatocellular carcinoma after liver transplantation. J SurgOncol 96: 62-68.
- Tanaka H, Imamura N, Oguma N, Shintani T, Tanaka K, et al. (2001) Acute myelogenous leukemia with PIG-A gene mutation evolved from aplastic anemia-paroxysmal nocturnal hemoglobinuria syndrome. Int J Hematol 73: 206-212.
- Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, et al. (2001) Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. ClinPharmacolTher 69: 169-174.
- Kobayashi D, Ieiri I, Hirota T, Takane H, Maegawa S, et al. (2005) Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug MetabDispos 33: 94-101.
- Rau T, Erney B, GÃres R, Eschenhagen T, Beck J, et al. (2006) High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. ClinPharmacolTher 80: 468-476.
- He R, Zhang D, Lu W, Zheng T, Wan L, et al. (2015) SLC47A1 gene rs2289669 G>A variants enhance the glucose-lowering effect of metformin via delaying its excretion in Chinese type 2 diabetes patients. Diabetes Res ClinPract 109: 57-63.
- Xu X, Duan L, Zhou B, Ma R, Zhou H, et al. (2012) Genetic polymorphism of copper transporter protein 1 is related to platinum resistance in Chinese non-small cell lung carcinoma patients. ClinExpPharmacolPhysiol 39: 786-792.
- Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, et al. (2009) Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes 58: 745-749.
- Sprowl JA, Gregorc V, Lazzari C, Mathijssen RH, Loos WJ, et al. (2012) Associations between ABCC2 polymorphisms and cisplatin disposition and efficacy. ClinPharmacolTher 91: 1022-1026.
- Hanigan MH, Lykissa ED, Townsend DM, Ou CN, Barrios R, et al. (2001) Gamma-glutamyltranspeptidase-deficient mice are resistant to the nephrotoxic effects of cisplatin. Am J Pathol 159: 1889-1894.
- Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH (2003) Metabolism of Cisplatin to a nephrotoxin in proximal tubule cells. J Am SocNephrol 14: 1-10.
- Zhang L, Hanigan MH (2003) Role of cysteine S-conjugate beta-lyase in the metabolism of cisplatin. J PharmacolExpTher 306: 988-994.
- SadowitzPD, Hubbard BA, Dabrowiak JC, Goodisman J, Tacka KA, et al. (2002) Kinetics of cisplatin binding to cellular DNA and modulations by thiol-blocking agents and thiol drugs. Drug MetabDispos 30: 183-190.
- Bodnar L, Wcislo G, Gasowska-Bodnar A, Synowiec A, Szarlej-Wcislo K, et al. (2008) Renal protection with magnesium subcarbonate and magnesium sulphate in patients with epithelial ovarian cancer after cisplatin and paclitaxel chemotherapy: a randomised phase II study. Eur J Cancer 44: 2608-2614.
- Ozkok A, Edelstein CL (2014) Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int 2014: 967826.
- Shinke H, Masuda S, Togashi Y, Ikemi Y, Ozawa A, et al. (2015) Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer ChemotherPharmacol 76: 989-996.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 11084
- [From(publication date):
August-2016 - Aug 07, 2025] - Breakdown by view type
- HTML page views : 10171
- PDF downloads : 913