Interferon-Gamma +874 (T/A) Polymorphism and Susceptibility to Aplastic Anemia: A Systematic Review and Meta-Analysis
Received: 07-Apr-2017 / Accepted Date: 23-May-2017 / Published Date: 09-Jun-2017 DOI: 10.4172/2471-9919.1000112
Abstract
Background: Many studies have assessed the relation between IFN-γ +874(T/A) polymorphism and risk of aplastic anemia. However, the results of these studies were inconclusive. In the current study, we performed a metaanalysis to evaluate the association between IFN-γ +874(T/A) polymorphism and susceptibility to aplastic anemia.
Methods: All publications were searched precisely to find eligible articles on IFN-γ polymorphism +874(T/A) and aplastic anemia. Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated to evaluate the strength of association in the dominant model, recessive model, allelic model, homozygotes contrast, and heterozygotes contrast.
Results: A total of 4 case-control studies, including 210 cases and 537 healthy controls were elegble for this metta-analysis. Combined analysis of these studies showed no significant association between the IFN-γ polymorphism +874(T/A) and aplastic anemia risk in the overall population (dominant model: OR=1.52, 95% CI=0.57-2.46; recessive model: OR=1.27, 95% CI=0.47-2.08; allelic model: OR=0.98, 95% CI=0.63-1.34; TT vs. AA: OR=3.68, 95% CI=0.21-7.15, and AT vs. AA: OR=1.20, 95% CI=0.43-1.97). No heterogeneity or publication bias was observed in this study. Conclusion: This meta-analysis showed that the IFN-γ +874(T/A) polymorphism was not associated with the risk of aplastic anemia.To confirm our results, further studies are needed.
Keywords: IFN- γ, Polymorphism, Aplastic anemia, Meta-analysis
Abriviations
IFN-γ: Interferon-Gamma; AA: Aplastic Anemia
Introduction
Aplastic anemia (AA) is a rare bone marrow failure syndrome characterized by hypocellularity of bone marrow and peripheral blood cytopenias [1]. Patients with AA frequently manifest with symptoms of purpura or hemorrhage, anemia and less often infection, leading to medical outcomes [2,3]. Most cases of AA are acquired and idiopathic [4]. Furthermore, in most cases, no agitating factor is known [1,4,5]. However, the principal etiology is thought to be immune-mediated damage of progenitor cells and hematopoietic stem cells [6,7]. Epidemiologic studies also suggest an influence of environmental exposures and genetic factors on AA pathogenesis [8]. The recent genome-wide transcriptional analysis of peripheral blood T cells from AA patients described various dysregulated genes in CD4 and CD8 T cells of the patients with AA [9]. In accordance with these findings, impaired functions of T cells has been reported in patients with aplastic anemia [10].
Interferon (IFN)-γ is one of the essential cytokines secreted by activated T cells and has immunomodulatory, antiviral and antiproliferative activities [11,12]. The involvement of IFN-γ in the immunopathogenesis of AA has been well addressed and upregulation of IFN-γ in the bone marrow and peripheral blood of patients with AA is predominant [13,14]. The IFN-γ gene is located on chromosome 12q14.1, spanning approximately six kb and is considered as a conserved region with limited genetic polymorphisms [15]. Various single nucleotide polymorphisms (SNP) in the non-coding region of INF-γ has been reported in different diseases [16]. T to A single nucleotide polymorphism (SNP), which is located at position +874 of the first intron of the IFN-γ gene (IFN-γ +874), can obviously affect the expression of the IFN-γ gene [17].
The SNP +874 A>T of IFN-γ has been evaluated in several association studies with aplastic anemia and bone marrow hypocellularity [18,19]. But, the results were inconsistent and inconclusive, probably due to different study populations and limited sample sizes. Thus, we performed this metaanalysis on all eligible case-control studies to further elucidate the effect of IFN-γ +874 A/T polymorphisms on the risk of AA.
Materials and Methods
The current Meta-analysis carried out based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement including search strategy, inclusion and exclusion criteria, data extraction and statistical analysis [20].
Search strategy
A detailed literature search was carried out using Scopus, PubMed central and Web of Science databases. Our search was performed from database inception to last updated on January 7, 2017. The following keywords were applied for search: (interferon OR IFN OR interferongamma OR interferon-g OR IFN-g OR interferon-γ OR IFN-γ) AND (Aplastic Anemia OR Aplastic OR Anemia OR Pancytopenia) AND (polymorphism OR variant OR mutation OR allele OR genotype). Just English publications were included in searches, and all favorable articles were obtained and their references were evaluated for other relevant studies. Moreover, we applied the “Related Articles” function in PubMed database to search for other possible relevant articles.
Inclusion and exclusion criteria
All candidate articles, at the beginning, were scanned in titles and/ or abstracts by two trained researchers independently. The main criteria for selection of studies to be considered in this meta-analysis were as following: 1) studies that had assessed the association between IFN-g polymorphism +874 (T/A) and risk of aplastic anemia; 2) case-control or nested case-control studies; and 3) reporting genotype frequency for the +874 (T/A) polymorphism in cases and controls. Animal studies, reviews, letters to the editor, summaries, book’s chapters and duplicated articles were all excluded.
Data extraction and quality assessment
The following data were extracted from the qualified article: the first author, year of publication, ethnicity, country of origin, gender, mean or range of age, the number of cases and controls for each genotype, and the sample size of cases and controls. Data were collected independently by two investigators. The Newcastle-Ottawa Scale (NOS) was used to evaluate the methodological quality [21]. This quality assessment tool judges studies based on a star system. Studies awarded 0-3, 4-6 or 7-9 were considered as a low, moderate or high-quality study, respectively.
Statistical analysis
Deviation from Hardy-Weinberg equilibrium (HWE) was calculated by using the χ2 test [22]. The association between IFN-γ polymorphism +874 (T/A) and aplastic anemia was assessed using ORs and their corresponding 95% (CIs). The allelic model (T vs. A), dominant model (TT+TA vs. AA), recessive model (TT vs. TA+AA), homozygote comparison (TT vs. AA) and heterozygote comparison (TA vs. AA) were examined. We used Cochran Q and the I2 statistics to evaluated heterogeneity between included studies (I2=(Q−df)/Q × 100%; I2<25%, no heterogeneity; I2=25-50%, moderate heterogeneity; I2=50-75%, large heterogeneity, I2>75%, extreme heterogeneity) [23]. Heterogeneity was considered to be significant if the Q statistic had p<0.1 or I2>50%. In the presence of significant heterogeneity the random‑effects model was applied, otherwise, the fixed ‑ effect model was performed in the absence of significant heterogeneity (Q statistic p>0.1 or I2<50%). Possible publication bias was assessed by Egger’s and Begg’s tests [24,25]. All statistical analysis for this meta-analysis were performed by STATA (version 14.0; Stata Corporation, College Station, TX) and SPSS (version 23.0; SPSS, Inc. Chicago, IL).
Results
Characteristics of eligible studies
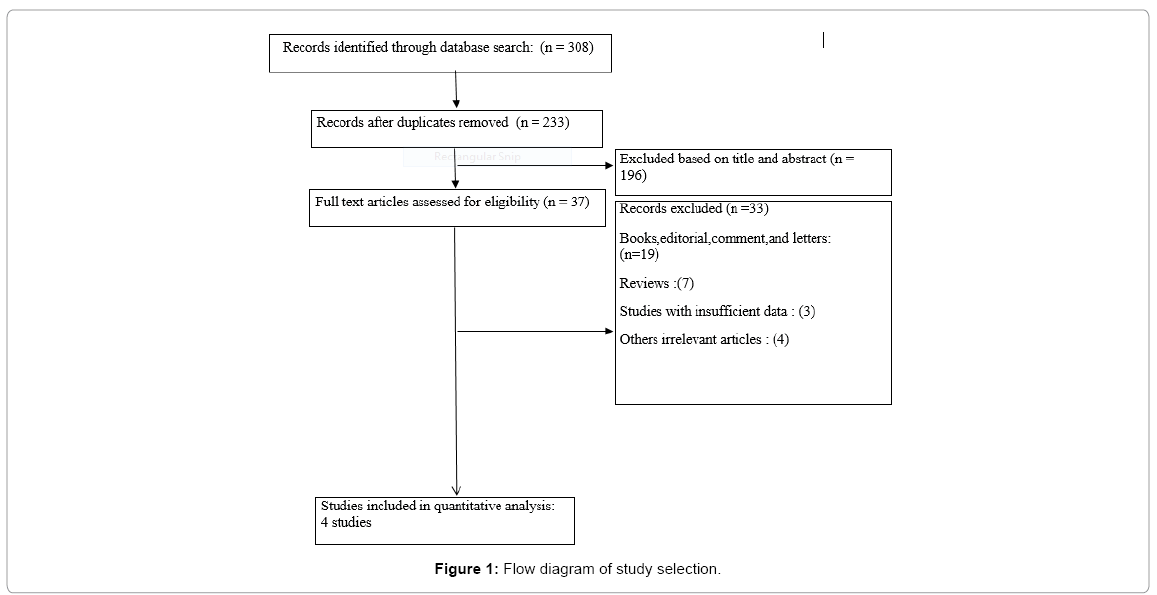
Figure 1 shows the procedure of including/excluding potential studies selection. Based on the inclusion and exclusion criteria, 4 articles (210 cases and 537 healthy subjects) were included in the metaanalysis. The studies were performed in different countries; two studies were from Egypt [26,27], one from Italy [19] and the other one was from South Korea [18]. Based on the criteria of the NOS, all included studies had an overall good methodological quality with a total score ranging from 7 to 9. The general characteristics and the allele and genotype distributions of studies included in this meta-analysis are reported in Tables 1 and 2.
| Study Author | Year | Country | Ethnicity | Sex | Total Cases/Controls | Case Age/Control Age (mean ± SD) | Genotype Method | Quality Score |
|---|---|---|---|---|---|---|---|---|
| Roderick et al. [7] | 2013 | Egypt | Caucasian | M/F | 50/50 | 11.2 ± 7.13/15.1 ± 7.25 | PCR–RFLP | 9 |
| Lee et al . [18] | 2011 | S.korea | Caucasian | M/F | 80/84 | 38/37 | PCR–RFLP | 7 |
| Fermo et al. [19] | 2004 | Italy | Caucasian | M/F | 40/363 | NR/NR | PCR–RFLP | 7 |
| Zayed et al. [26] | 2016 | Egypt | Caucasian | M/F | 40/40 | 11/12 | PCR–RFLP | 8 |
NR: Not Reported; M: Male; F: Female
Table 1: The characteristics of the studies included in the meta-analysis of the aplastic anemia.
| Study Author | AA Cases | Healthy Control | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AT | TT | A | T | AA | AT | TT | A | T | P-HWE | MAF | |||
| Roderick et al. [7] | 16 | 21 | 13 | 53 | 47 | 31 | 13 | 6 | 47 | 25 | 0.03 | 0.34 | ||
| Lee et al . [18] | 61 | 15 | 3 | 137 | 21 | 66 | 17 | 0 | 149 | 17 | 0.29 | 0.10 | ||
| Fermo et al. [19] | 6 | 19 | 15 | 49 | 31 | 116 | 170 | 77 | 402 | 324 | 0.31 | 0.44 | ||
| Zayed et al. [26] | 9 | 17 | 14 | 35 | 45 | 19 | 12 | 9 | 50 | 30 | 0.02 | 0.37 | ||
P-HWE: p-value for Hardy-Weinberg Equilibrium; MAF: Minor Allele Frequency of Control Group; NR: Not Reported; M: Male; F: Female
Table 2: Distribution of genotype and allele among AA patients and controls.
Meta-Analysis of the association between IFN-γ +874 A/T polymorphism and Aplastic anemia risk. The pooled results of metaanalysis in different models are shown in Table 3. when the eligible studies were pooled, there was no significant association between IFN-γ +874 A/T polymorphism and aplastic anemia risk in any genetic model tested (dominant model: OR=1.52, 95% CI=0.57-2.46; recessive model: OR=1.27, 95% CI=0.47-2.08; allelic model: OR=0.98, 95% CI=0.63-1.34; TT vs. AA: OR=3.68, 95% CI=0.21-7.15 and AT vs. AA:OR=1.20, 95% CI=0.43-1.97) (Table 3). Also, the results of heterogeneity tests in different analysis models are presented in b. There was no significant heterogeneity among the studies in the dominant model (I2=0.0, P=0.45), recessive model (I2=0.0, P=0.92) allelic model (I2=0.35, P=0.20), TT vs. AA (I2=0.0, P=0.98) and AT vs. AA (I2=0.0, P=0.63).
| Genetic Models | Sample Size | Test of Association | Test of Heterogeneity | Test of publication bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| Begg’s | Egger’s | ||||||||
| Case /Control | OR | 95% CI | I2 (%) | P | Z | P | t | P | |
| Dominant model | 210/537 | 1.52 | 0.57-2.46 | 0.0 | 0.45 | 0.49 | 0.68 | 0.29 | 1.39 |
| Recessive model | 210/537 | 1.27 | 0.47-2.08 | 0.0 | 0.92 | 0.60 | -0.5 | 0.37 | -1.50 |
| Allelic model | 210/537 | 0.98 | 0.63-1.34 | 0.35 | 0.20 | 0.49 | 0.68 | 0.15 | 2.22 |
| TT vs. AA | 210/537 | 3.68 | 0.21-7.15 | 0.0 | 0.98 | 0.60 | -0.5 | 0.98 | -0.11 |
| AT vs. AA |
210/537 | 1.20 | 0.43-1.97 | 0.0 | 0.63 | 1 .00 | 0.0 | 0.33 | 1.26 |
Table 3: Main results of the pooled ORs in meta-analysis of the IFN-γ 874 A/T polymorphism.
Evolution of publication bias and sensitivity analysis
The sensitivity of this meta-analysis was assessed by removing non- HWE studies. However, the results remained unaltered, indicating the reliability of the results. Publication bias was calculated by using Egger’s and Begg’s tests (Table 3). For the IFN-γ 874 A/T polymorphism, Egger’s test showed no evidence of publication bias for the dominant model (t=1.39, p=0.29), recessive model (t=-1.50, p=0.37), allelic model (t=2.22, p=0.15), TT vs. AA (t=-0.11, p=0.98) and AT vs. AA (t=1.26, p=0.33).
Discussion
The pathogenesis of aplastic anemia includes a change in the hematopoietic microenvironment, destruction and reduction of the hematopoietic stem cells, and altered cellular immunity, which finally culminate to bone marrow failure [28,29]. The immune mechanisms involved in AA consist of cell-mediated killing and cytokines secretion by Th1, which have an inhibitory activity on the hematopoietic progenitors, such as IFN-γ and TNF-α [30,31]. Moreover, it is known that many immunogenic factors may play a critical role in the increase and the decrease of predisposition to immune responses. Functional polymorphisms of cytokines are an interested group of immunogenic factors for the understanding of diseases risk [32-34]. These polymorphisms have been reported to be associated with specific clinical manifestations or susceptibility to some diseases [35]. Recently, several studies have focused on the association between the IFN-γ +874 (T/A) polymorphism and aplastic anemia, although the results of these studies were controversial. Therefore, this meta-analysis was performed to reveal the possible effect of the IFN-γ +874(T/A) polymorphism on susceptibility to AA.
To the best of the author’s knowledge, this meta-analysis is the first quantitative assessment of the association between IFN-γ +874T/A polymorphism and risk of aplastic anemia. The results suggested that there was no significant association between the IFN-γ +874 (T/A) polymorphism and genetic susceptibility to aplastic anemia. Although the present meta-analysis did not find associations of IFN-γ +874 (T/A) polymorphism and the risk of AA, but results should be interpreted with cautions. For evaluation of the investigated polymorphism, there were limited published studies. Due to the relatively small sample size, the results are unable to come to a confirmed conclusion. Therefore, to conclude a reliable result, further studies are needed to assess the association between IFN-γ +874 (T/A) polymorphism and the risk of AA in different ethnicities.
The results of this meta-analysis are in agreement with some crosssectional studies that have examined the risk of AA according to IFN-γ +874 (T/A) polymorphism. In the study that was carried out by Bestach et al. [31], no association was reported between polymorphisms in IFN-γ gene and susceptibility to AA, while, unlike to this study, Gidvani et al. [36] described a relationship between AA and IFN-γ polymorphisms. In consistent with Gidvani et al. [36], other studies [19,37] emphasize on the role of IFN-γ polymorphisms in clinical characteristics of AA. However, these studies did not evaluate the association between IFN-γ +874 (T/A) polymorphism and AA risk.
Limitations
The current study has some limitations. First, the number of studies included in the analysis was limited and, for this reason, we could not perform subgroup analysis to evaluate the possible differences caused by age, sex and race of the participants. Second, this meta-analysis was restricted to English-language publications, therefor may result in the exclusion of some relevant article in other languages. Third, because of lack of sufficient data in the original studies, we could not evaluate the possible interactions between gene-environmental factors and genegene interactions, which this shortage might affect our results.
Conclusion
In conclusion, this gene-based meta-analysis found no significant association between IFN-γ +874 (T/A) polymorphism and genetic susceptibility to aplastic anemia. Because of the scarcity of data, additional large, well-designed studies are needed to assess the association between this polymorphism and risk of aplastic anemia.
Conflicts of Interests
The authors declared no conflicts of interests.
References
- Young NS, Maciejewski J (1997) The pathophysiology of acquired aplastic anemia. N Engl J Med 336: 1365-1372.
- Young NS, Calado RT, Scheinberg P (2006) Current concepts in the pathophysiology and treatment of aplastic anemia. Blood 108: 2509-2519.
- Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H; German Aplastic Anemia Study Group (2003) Antithymocyte globulin with or without cyclosporin A: 11 year follow-up of a random ized trial comparing treatments of aplastic anemia. Blood 101: 1236-1242.
- Davies JK, Guinan EC (2007) An update on the management of severe idiopathic aplastic anaemia in children. Br J Haematol 136: 549-564.
- Nakao S, Gale R (2016) Are mild/moderate acquired idiopathic aplastic anaemia and low-risk myelodysplastic syndrome one or two diseases or both and how should it/they be treated and quest. Leukemia 30: 2127-2130.
- Zeng Y, Katsanis E (2015) The complex pathophysiology of acquired aplastic anaemia. Clin Exp Immunol 180: 361-370.
- Roderick JE, Gonzalez-Perez G, Kuksin CA, Dongre A, Roberts ER et al. (2013) Therapeutic targeting of NOTCH signaling ameliorates immune-mediated bone marrow failure of aplastic anemia. J Exp Med 210: 1311-1329.
- Melinkeri SR (2015) Epidemiology, pathogenesis and diagnosis of aplastic anaemia. J Assoc Physicians India 63: 8-12.
- Zeng W, Kajigaya S, Chen G, Risitano AM, Olga Nunez, et al. (2004) Transcript profile of CD4+ and CD8+ T cells from the bone marrow of acquired aplastic anemia patients. Exp Hematol 32: 806-814.
- Kordasti S, Marsh J, Al-Khan S, Jiang J, Smith A, et al. (2012) Functional characterization of CD4+ T cells in aplastic anemia. Blood 119: 2033-2043.
- Boehm U, Klamp T, Groot M, Howard JC (1997) Cellular responses to interferon-γ. Annu Rev Immunol 15: 749-795.
- Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-γ: An overview of signals, mechanisms and functions. J Leukoc Biol 75: 163-189.
- Nakao S, Yamaguchi M, Shiobara S, Yokoi T, Miyawaki T, et al. (1992) Interferon-gamma gene expression in unstimulated bone marrow mononuclear cells predicts a good response to cyclosporine therapy in aplastic anemia. Blood 79: 2532-2535.
- Chang H, Zeng F, Zhang JY, Mu XY, Meng WT, et al. (2010) Association of the interferon-gamma single nucleotide polymorphism+ 874 (T/A) with response to immunosuppressive therapy in patients with severe aplastic anemia. Blood Cells Mol Dis 45: 313-316.
- Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnott PJ, et al. (1999) In vitro production of IFNâ€Î³ correlates with CA repeat polymorphism in the human IFNâ€Î³ gene. Eur J Immunogenet 26: 1-3.
- Silva G, Santos MP, Mota-Passos I, Boechat AL, Malheiro A, et al. (2012) IFN-γ+ 875 microsatellite polymorphism as a potential protection marker for leprosy patients from Amazonas state, Brazil. Cytokine 60: 493-497.
- Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV (2000) A single nucleotide polymorphism in the first intron of the human IFN-γ gene: Absolute correlation with a polymorphic CA microsatellite marker of high IFN-γ production. Hum Immunol 61: 863-866.
- Lee YG, Kim I, Kim JH, Bae JY, Kwon JH, et al. (2011) Impact of cytokine gene polymorphisms on risk and treatment outcomes of aplastic anemia in Korea. Blood 116: 4432-4432.
- Fermo E, Bianchi P, Barcellini W, Pedotti P, Boschetti C, et al. (2004) Immunoregulatory cytokine polymorphisms in Italian patients affected by paroxysmal nocturnal haemoglobinuria and aplastic anaemia. Eur J Immunogenet 31: 267-269.
- Moher D, Liberati A, Tetzlaff J, Altman DG; The Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: e1000097.
- Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25: 603-605.
- Wigginton JE, Cutler DJ, Abecasis GR (2005) A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet 76: 887-893.
- Huedo-Medina TB, Sánchez-Meca J, MarÃn-MartÃnez F, Botella J et al. (2010) Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol Methods 11: 193.
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634.
- Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088-1101.
- Zayed RA, Abdel-Hamid SM, El-Lithy H (2016) The association of cytokine genes polymorphisms and susceptibility to aplastic anemia in Egyptian patients. Hematology 21: 106-112.
- El Mahgoub IR, Afify RAA, Botros SKA, Fawzy R (2014) Immunoregulatory cytokines gene polymorphisms in Egyptian patients affected with acquired aplastic anemia. Ann Hematol 93: 923-929.
- Camitta BM, Thomas ED, Nathan DG, Gale RP, Kopecky KJ, et al. (1979) A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood 53: 504-514.
- Maciejewski J, Selleri C, Anderson S, Young NS (1995) Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood 85: 3183-3190.
- Giannakoulas NC, Karakantza M, Theodorou GL, Pagoni M, Galanopoulos, et al. (2004) Clinical relevance of balance between type 1 and type 2 immune responses of lymphocyte subpopulations in aplastic anaemia patients. BJH 124: 97-105.
- Bestach Y (2015), Polymorphisms in TNF and IFNG are associated with clinical characteristics of aplastic anemia in Argentinean population. Leuk lymphoma 56: 1793-1798.
- Huang HR, Zhong YQ, Wu JF (2012) The association between IFN-γ and IL-4 genetic polymorphisms and childhood susceptibility to bronchial asthma. Gene 494: 96-101.
- Saunthararajah Y, Nakamura R, Nam JM, Robyn J, Loberiza F, et al. (2002) HLA-DR15 (DR2) is overrepresented in myelodysplastic syndrome and aplastic anemia and predicts a response to immunosuppression in myelodysplastic syndrome. Blood 100: 1570-1574.
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD (1998) How cells respond to interferons. Annu Rev Biochem 67: 227-264.
- Schena FP, Cerullo G, Torres DD, Scolari F, Foramitti M, et al. (2006) Role of interferon-γ gene polymorphisms in susceptibility to IgA nephropathy: A family-based association study. Eur J Hum Genet 14: 488-496.
- Gidvani V, Ramkissoon S, Sloand EM, Young NS (2007) Cytokine gene polymorphisms in acquired bone marrow failure. Am J Hematol 82: 721-724.
- Dufour C, Capasso M, Svahn J, Marrone A, Haupt R (2004) Homozygosis for (12) CA repeats in the first intron of the human IFNâ€Î³ gene is significantly associated with the risk of aplastic anaemia in Caucasian population. BJH 126: 682-685.
Citation: Razi B, Alizadeh S, Imani D, Rezaei R, Omidkhoda A (2017) Interferon- Gamma +874 (T/A) Polymorphism and Susceptibility to Aplastic Anemia: A Systematic Review and Meta-Analysis. Evid Based Med Pract 3: 112. DOI: 10.4172/2471-9919.1000112
Copyright: © 2017 Razi B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12525
- [From(publication date): 0-2017 - Dec 18, 2025]
- Breakdown by view type
- HTML page views: 11242
- PDF downloads: 1283