Investigating the Effect of Phenylbutyrate and Valproate Supplementation on Atherosclerotic Plaque Regression in a High Fat Diet Fed LDLR-/- Mouse Model
Received: 13-Mar-2019 / Accepted Date: 27-Mar-2019 / Published Date: 02-Apr-2019
Abstract
Objective: Evidence suggests that Endoplasmic Reticulum (ER) stress plays a causative role in the development of atherosclerosis. Our previous studies have shown that ER stress signals through glycogen synthase kinase (GSK)-3αβ to activate pro-atherogenic pathways. The purpose of this study is to determine if small molecule inhibitors of ER stress-GSK3αβ signaling can promote the regression of existing atherosclerotic lesions.
Methods: Four-week-old female low density lipoprotein receptor deficient (LDLR-/-) mice were fed a high fat diet for 16 weeks to establish atherosclerotic lesions. A subset of mice was sacrificed at this time to set the baseline for atherosclerotic progression. The remaining mice were switched to stand chow diet (control) or a standard chow diet supplemented with phenylbutyrate, a chemical chaperone that reduces ER stress, or valproate, a branch chain fatty acid that selectively inhibits GSK3αβ. These mice were harvested at 30 weeks of age and atherosclerotic lesions were quantified and characterized.
Results: Dietary supplementation with phenylbutyrate and valproate had no effect on body weight but did significantly reduce plasma cholesterol levels. Atherosclerotic lesion areas at the aortic sinus and total atherosclerotic volumes were significantly larger in the control group compared to the baseline group, indicating that the lesions continued to grow despite the switch from a high fat diet to chow diet. The supplemented mice had significantly smaller lesions than the control group, but not the baseline group. Both phenylbutyrate and valproate supplementation reduced lesional macrophage/foam cell content and necrotic core area, and increased smooth muscle cell content and collagen content, relative to control and baseline groups. These changes are indicative of more stable atherosclerotic lesions.
Conclusion: Small molecule inhibitors of ER stress-GSK3αβ signaling do attenuate the growth of existing atherosclerotic lesions and appear to increase lesion stability. It remains unclear whether these interventions can actually promote atherosclerotic regression.
Keywords: Atherosclerosis; Mouse model; Plaque regression; Dietary supplementation; ER stress
Introduction
Atherosclerosis is an inflammatory disease that involves the accumulation of lipid engorged macrophages in the walls of medium and large arteries [1]. Initially, atherosclerotic lesions grow asymptomatically, however more advanced lesions can significantly impede blood flow through the artery and cause unstable angina, stoke or myocardial infarction. These cardiovascular diseases are the leading cause of death in the world [2].
A great deal of research has been focused upon understanding the underlying molecular and cellular mechanisms of atherogenesis. It is now well established that atherosclerosis initiates at sites of endothelial injury, usually in regions of turbulent blood flow, including vascular bifurcations and inner curvatures [3]. Leukocytes and Low Density Lipoprotein (LDL) particles infiltrate the intima through gaps in the damaged endothelium. Intimal monocytes differentiate into macrophages which endocytose oxided-LDL and efferocytose apoptotic bodies thus becoming lipid laden foam cells. Macrophage/ foam cells secrete cytokines that perpetuate the inflammatory response and promote vascular smooth muscle cell (VSMC) migration into the intima. VSMCs secrete collagen which contributes to the development of a fibrous cap over the growing lesion. If foam cell apoptosis exceeds macrophage efferocytosis, an acellular necrotic region of cellular debris (predominately cholesterol/lipids) accumulates within the lesion. This necrotic core destabilizes the lesion making it prone to rupture. If the lesion ruptures and the circulating blood contacts the lipid-rich contents of the lesion, a thrombus will rapidly form which may occlude blood flow through the vessel. Occlusion of a major artery will cause myocardial infarction or stroke [1,4].
Many interventions have been identified that can slow or block the progression of atherosclerosis, in pre-clinical model systems [5]. Previous studies from our lab and others have shown that cardiovascular risk factors can disrupt protein processing in the endoplasmic reticulum (ER) of endothelial cells [6], VSMCs [7] and macrophages [8]. ER stress can signal through glycogen synthase kinase (GSK)3αβ to activate pro-atherogenic processes including inflammation [9], lipid accumulation [8] and apoptosis [10]. Furthermore, inhibiting ER stress-GSK3αβ signaling can attenuate the development and progression of atherosclerosis in LDL receptor knockout (LDLR-/-) mice or apolipoprotein E knockout (ApoE-/-) mice [11,12].
However, blocking the development of atherosclerotic lesions is not currently a viable treatment option because patients typically present with existing disease. Clinically relevant interventions must be effective at stabilizing or regressing established lesions. Although it is clear that atherosclerotic lesions can regress in humans and in animal models [13,14] much less is known about the underlying mechanisms of regression. Murine regression models require interventions that drastically reduce circulating lipid levels that are attained through dietary changes [15], genetic manipulation [16], or transplant [17].
In this study we investigate the potential of small molecule interventions that block ER stress-GSK3αβ signaling on atherosclerotic lesion regression using a high fat diet (HFD) fed LDLR-/- mouse model. These mice are supplemented with the chemical chaperone, 4- phenylbutyric acid, to attenuate ER stress or with the short chained fatty acid, valproate to inhibit GSK3αβ. Lesion size and volume are determined and the lesions are characterized for VSMC and macrophage content.
Materials and Methods
Mouse models of atherosclerotic regression
All experimental procedures were pre-approved by the McMaster University Animal Research Ethics Board. LDLR-/- mice were used as the regression model (n=8/treatment group). These mice develop atherosclerosis lesions when fed a high fat diet (HFD) but not when fed a standard chow diet [18]. Mice were maintained on a 12-hour light/dark cycle and had unlimited access to food and water. Four week old, female LDLR-/- mice were fed a high fat diet (HFD) containing 21% fat and 0.2% cholesterol, with 42% calories from fat (Harlan Teklad TD97363) or a standard control diet containing 18% protein and 5% fat, with 18% calories from fat (TD92078). The HFD was supplemented with sodium valproate (625 mg/kg HFD) or the chemical chaperone phenylbutyrate (3.8 g/L in the drinking water). The valproate and phenylbutyrate concentrations are based on experience from previous studies [19,12].
Mice were fasted for 6 h prior to sacrifice. Blood was collected via cardiac puncture and liver and perigonadal fat pad were removed. The vasculature was flushed with 1X PBS buffer and perfusion fixed with 10% neutral buffer formalin. Hearts and aortas were collected and fixed in formalin.
Characterization of atherosclerotic lesions
Hearts and aortas were embedded in paraffin and 5 μm sections of the aortic sinus were collected onto slides, as previously described [20]. Sections were stained with Masson’s trichrome for atherosclerotic lesion quantification. Images of the stained sections were collected using Olympus BX41 microscope connected to a DP71 Olympus camera. Lesion area staining was quantified using Image J 1.51v software.
For immunofluorescent staining, sections were deparaffinized and antigen retrieval performed by using antigen unmasking solution (Vector Laboratories, H-3300). Sections were blocked in 10% goat serum and then immunostained overnight with primary antibodies against the macrophage marker CD107b/Mac3 1:50 (BD Transductions, 550292), vascular smooth muscle cell (VSMC) marker α-actin 1:200 (Santa Cruz, sc-32251), ER Stress marker KDEL 1:100 (ENZOL, ADI-SPA-827) or pro-apoptotic UPR marker C/EBPhomologous protein (CHOP)(Santa Cruz sc-575). Sections were then incubated with secondary antibodies Alexa Fluor 488 goat anti-mouse IgG 1:250 (Thermofisher Scientific, A11001), Alexa Fluor 488 goat anti-rabbit IgG 1:250 (Thermofisher Scientific A11008) or Alexa Fluor 488 goat anti-rat IgG 1:250 (Thermofisher Scientific, A11006) for 2 h, and then stained with the DAPI (1:5000) (Invitrogen, D1306). Slides were mounted with Fluoromount Aqueous Mounting Medium (Sigma, F4680) and stored at 4°C in the dark. Images of the stained sections were collected using an Olympus BX41 microscope connected to a DP71 Olympus camera. Image J 1.51v software was used to quantify immunofluorescent staining.
Determination of plasma lipids
Total plasma cholesterol and triglyceride levels were determined by using the Infinity Cholesterol Kit (TR13421) and Infinity Triglyceride Kit (TR22421), respectively. Assays were performed according to manufacturer’s instructions.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism 7 software. Data were analyzed by one or two-way ANOVA, followed by the Bonferroni multiple comparison test between all groups. All error bars on graphs represent the standard error of the mean (SEM). For all experiments, a p value lower than 0.05 was considered statistically significant. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Results
Effect of phenylbutyrate or valproate supplementation on atherosclerosis
It has been established that supplementation with the chemical chaperone phenylbutyrate, or the short branch chain fatty acid valproate, can attenuate the progression of atherosclerosis in ApoE-/- and HFD-fed LDLR-/- mice [12,19,21,22]. To determine if these treatments could promote the regression of existing plaques, we have previously measured the effects of a 4 week treatment with phenylbutyrate or valproate on HFD-fed LDLR-/- mice with existing atherosclerotic lesions [12]. Results of this experiment showed changes in the morphology of the lesion that are consistent with plaque stabilization; however, there was no reduction in lesion area or volume. The purpose of these experiments was to determine if a longer (10 week) treatment with phenylbutyrate or valproate can promote atherosclerotic lesion regression.
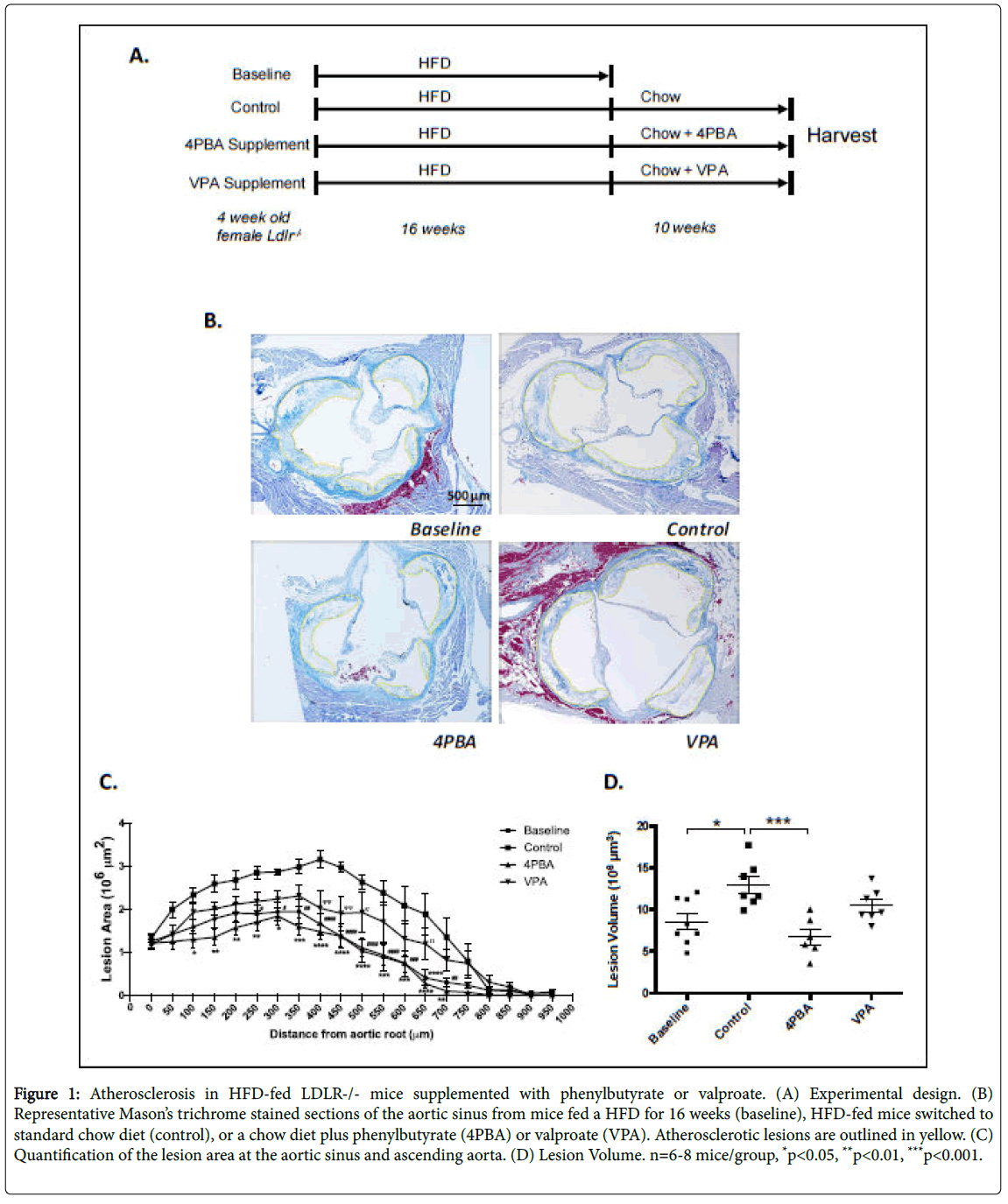
Four week old male and female LDLR-/- mice were fed a HFD for 16 weeks (Figure 1A). At 20 weeks of age, the baseline group was sacrificed. The control group was switched to a standard chow diet and other groups of mice were switched to the standard diet and treated with phenylbutyrate (3.8 g/L in the drinking water), or control diet containing valproate (625 mg/kg). Control, phenylbutyrate-treated and valproate-treated groups were sacrificed after 10 additional weeks (30 weeks of age).
Figure 1: Atherosclerosis in HFD-fed LDLR-/- mice supplemented with phenylbutyrate or valproate. (A) Experimental design. (B) Representative Mason’s trichrome stained sections of the aortic sinus from mice fed a HFD for 16 weeks (baseline), HFD-fed mice switched to standard chow diet (control), or a chow diet plus phenylbutyrate (4PBA) or valproate (VPA). Atherosclerotic lesions are outlined in yellow. (C) Quantification of the lesion area at the aortic sinus and ascending aorta. (D) Lesion Volume. n=6-8 mice/group, *p<0.05, **p<0.01, ***p<0.001.
To investigate the effects of phenylbutyrate and valproate on atherosclerosis, the aortic sinus was removed and processed, as previously described [20]. Cross-sections of the aortic root were stained with Masson’s trichrome stain (Figure 1B) and the atherosclerotic lesion areas (Figure 1C) and volumes were quantified (Figure 1D) [20].
Effect of supplementation on metabolic parameters
There were no significant differences in fasting blood glucose level, body weight, liver weight, adipose weight between experimental groups (Table 1). Hepatic triglyceride and cholesterol levels were lower in the treatment groups compared to baseline (Figure 2A and 2B). Control, phenylbutyrate- and valproate-treated mice had significantly lower total plasma triglyceride and cholesterol levels compared to baseline (Figure 2C and 2D). This difference is likely due to the switch from HFD to standard diet. Both phenylbutyrate- and valproatetreated mice had significantly lower total cholesterol levels compared to control.
| Baseline | Control | Phenylbutyrate | Valproate | |
|---|---|---|---|---|
| Blood Glucose (mM) | 10.56 ± 0.48 | 11.50 ± 0.40 | 10.49 ± 0.06 | 8.87 ± 0.42 |
| Adipose weight (g) | 0.47 ± 0.07 | 0.42 ± 0.05 | 0.34 ± 0.08 | 0.49 ± 0.06 |
| Liver weight (g) | 1.38 ± 0.06 | 1.21 ± 0.13 | 1.11 ± 0.06 | 1.18 ± 0.04 |
| Body weight (g) | 26.78 ± 0.42 | 26.70 ± 0.65 | 25.53 ± 0.94 | 27.20 ± 0.88 |
Table 1: Metabolic parameters of 30 week old mice. Data represent the mean ± SEM; n=7-10 mice/group.
Characterization of atherosclerotic lesions
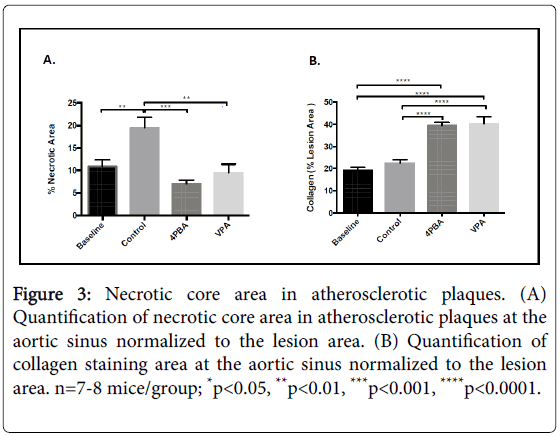
Mason’s trichrome staining was used to assess necrotic core areas and collagen content within the atherosclerotic lesions. There were significantly larger acellular necrotic regions the atherosclerotic lesions from control mice compared to baseline and supplemented mice (Figure 3A and 3B). Collagen content, a marker for more stable atherosclerotic plaques, was significantly elevated in the phenylbutyrate and the valproate treated mice compared to both the baseline and the controls (Figure 3C and 3D).
Figure 3: Necrotic core area in atherosclerotic plaques. (A) Quantification of necrotic core area in atherosclerotic plaques at the aortic sinus normalized to the lesion area. (B) Quantification of collagen staining area at the aortic sinus normalized to the lesion area. n=7-8 mice/group; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
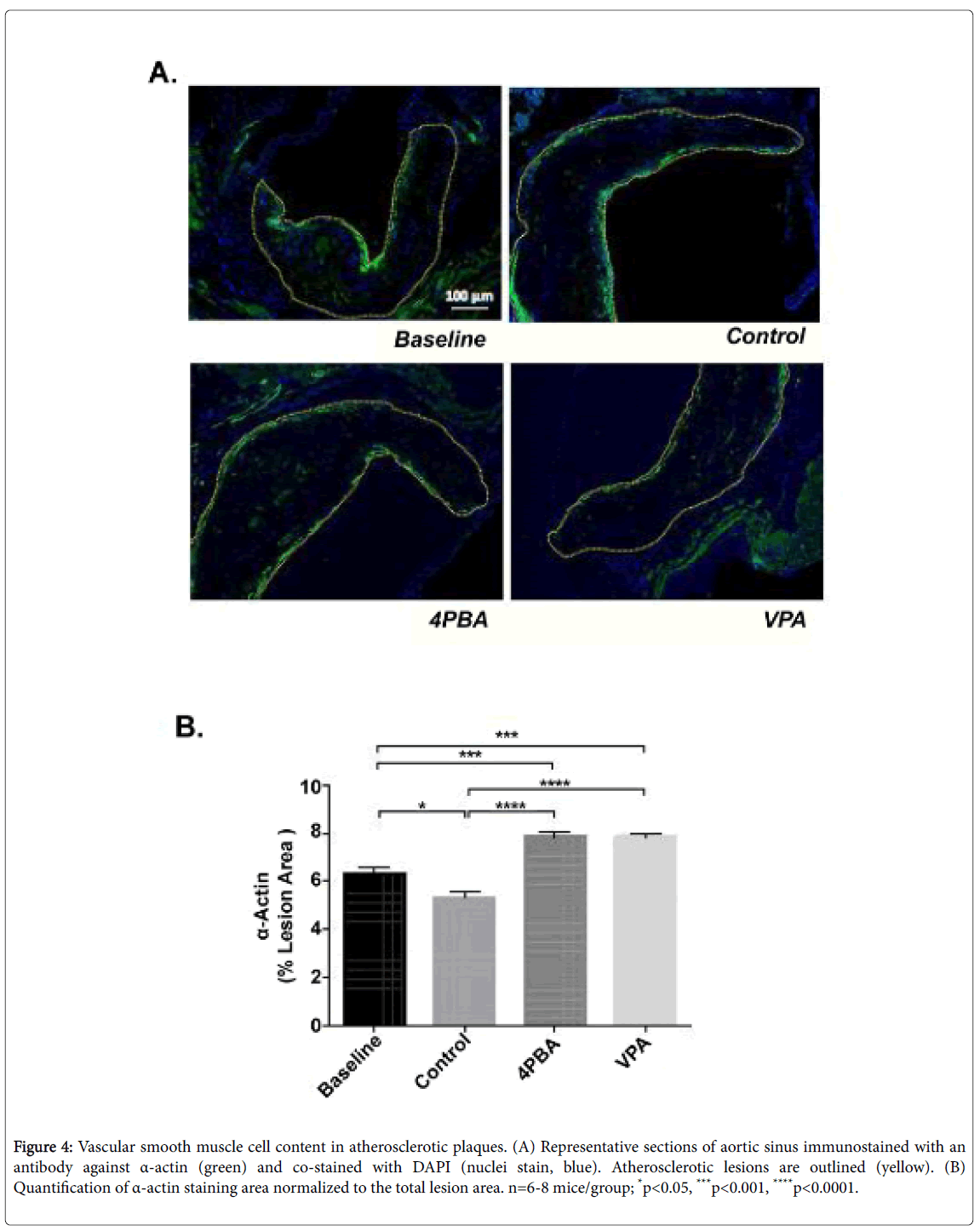
Lesional vascular smooth muscle cell and macrophage/foam cell content was quantified in each of the experimental groups using immunofluorescent staining with antibodies against smooth muscle α actin and CD107b (Mac-3), respectively. Both phenylbutyrate and valproate treatment resulted in significantly increased lesional VSMC content relative to baseline and control groups (Figure 4). This is consistent with the observed increase in plaque collagen (Figure 3C).
Figure 4: Vascular smooth muscle cell content in atherosclerotic plaques. (A) Representative sections of aortic sinus immunostained with an antibody against α-actin (green) and co-stained with DAPI (nuclei stain, blue). Atherosclerotic lesions are outlined (yellow). (B) Quantification of α-actin staining area normalized to the total lesion area. n=6-8 mice/group; *p<0.05, ***p<0.001, ****p<0.0001.
Control, phenylbutyrate and valproate groups had significantly decreased lesional macrophage content compared to baseline, indicative of an effect that can be attributed to the switch to a chow diet (Figure 5). Phenylbutyrate treatment also significantly decreased lesional macrophage content relative to control mice.
Figure 5: Macrophage content in atherosclerotic plaques. (A) Representative sections of aortic sinus immunostained with an antibody against CD107b/Mac3 (green) and co-stained with DAPI (nuclei stain, blue). Atherosclerotic lesions are outlined (yellow). (B) Quantification of CD107b/Mac3 staining area normalized to the total lesion area. n=6-8 mice/group; **p<0.01, ***p<0.001, ****p<0.0001.
Effects of supplementation on lesion ER stress levels
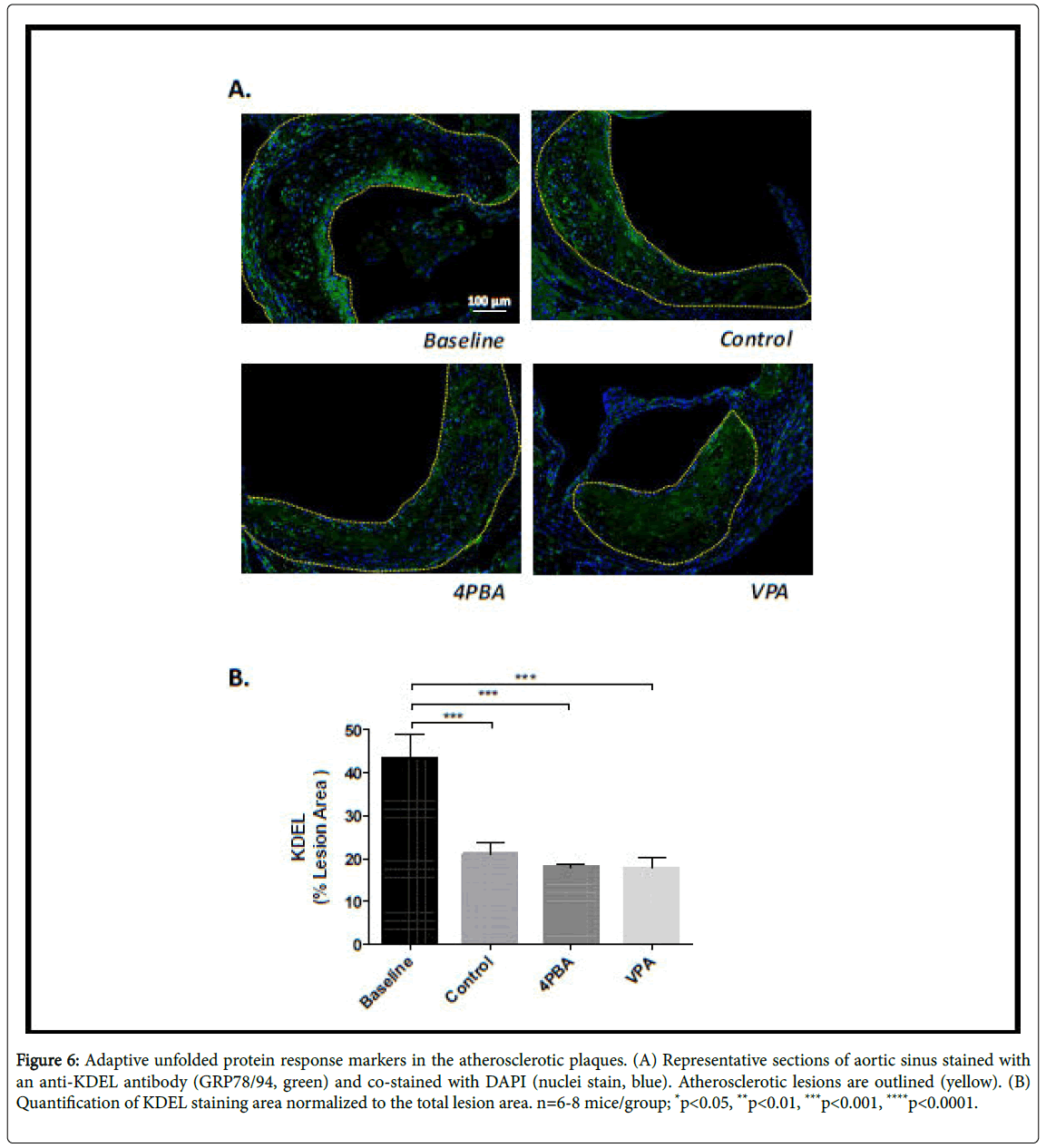
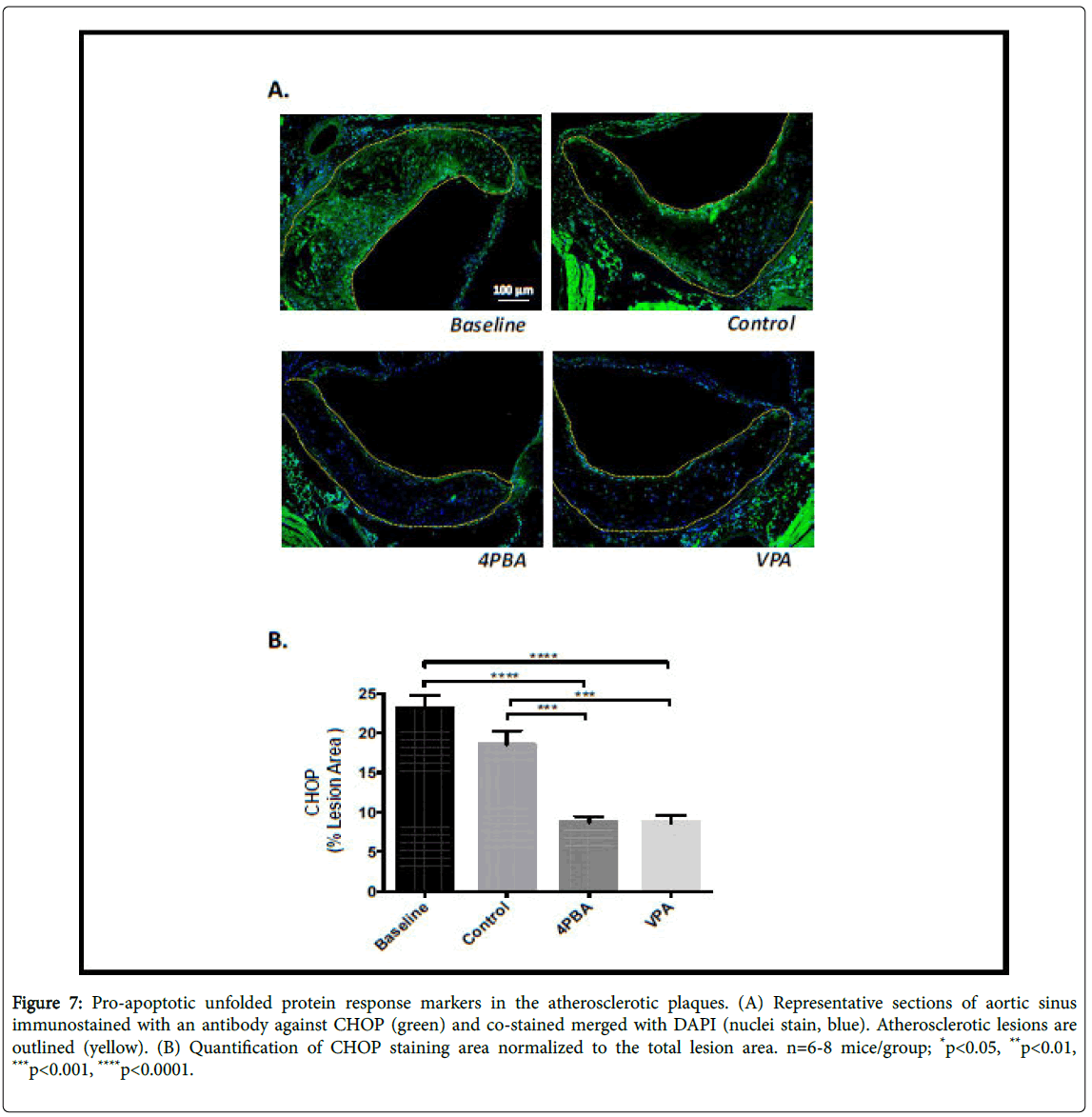
Lesional ER stress was determined indirectly by performing immunofluorescence staining in each of the experimental groups using antibodies against KDEL – a marker of ER resident chaperone proteins (GRP78/94) that are induced by ER stress, and an antibody against the C/EBP-homologous protein (CHOP), a pro-apoptotic transcription factor. There was no significant difference in KDEL levels in the control, phenylbutyrate or valproate treatment groups (Figure 6). The baseline group had significantly elevated KDEL levels compared to the other groups. This likely indicates that HFD-feeding promotes ER stress in LDLR-/- mice, an observation consistent with previous reports [12]. CHOP staining was significantly lower in the phenylbutyrate and valproate treated groups, relative to baseline and control (Figure 7). This suggests that these treatments suppress the pro-apoptotic UPR. This is consistent with the smaller necrotic cores observed in the supplemented mice.
Figure 6: Adaptive unfolded protein response markers in the atherosclerotic plaques. (A) Representative sections of aortic sinus stained with an anti-KDEL antibody (GRP78/94, green) and co-stained with DAPI (nuclei stain, blue). Atherosclerotic lesions are outlined (yellow). (B) Quantification of KDEL staining area normalized to the total lesion area. n=6-8 mice/group; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Figure 7: Pro-apoptotic unfolded protein response markers in the atherosclerotic plaques. (A) Representative sections of aortic sinus immunostained with an antibody against CHOP (green) and co-stained merged with DAPI (nuclei stain, blue). Atherosclerotic lesions are outlined (yellow). (B) Quantification of CHOP staining area normalized to the total lesion area. n=6-8 mice/group; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Discussion and Conclusion
We have previously shown that the ER stress-GSK3αβ signaling pathway promotes the development of atherosclerosis in LDLR-/- and ApoE-/- mice [22-24]. In this study we set out to investigate the potential ability of small molecules, phenylbutyrate and valproate, to induce the regression of existing atherosclerotic plaques in a HFD-fed LDLR-/- mouse model. Phenylbutyrate is a chemical chaperone that reduces levels of ER stress [25] and valproate is a branch chain fatty acid that is a selective inhibitor of GSK3αβ [26]. To investigate the effects on atherosclerotic regression, a “dietary switch” strategy in LDLR-/- mice was utilized. LDLR-/- mice only develop atherosclerosis when fed a HFD and switching to a standard chow diet has been reported to arrest plaque development [27,28]. In this study the plaques were established in all mice by 16 weeks of HFD-feeding at which time the baseline group were sacrificed. All other mice were switched to a standard diet (control group), or a standard diet plus phenylbutyrate (3.8 g/L in the drinking water), or a standard diet containing valproate (625 mg/kg) for ten additional weeks (Figure 1A).
The switch to standard diet resulted in a significant drop in plasma lipid levels and hepatic cholesterol levels, but not hepatic triglycerides. Sixteen weeks of HFD-feeding induced advanced atherosclerosis at the aortic sinus and ascending aorta. Unexpectedly, the control group had significantly larger lesions than the baseline group. This means that, despite the shift to the standard diet and the normalization of plasma lipid levels, the lesions continued to grow in the LDLR-/- control mice. The growth of the lesions after the shift to chow diet makes it difficult to distinguish regression from arrested progression, unless the final lesions are smaller than those in the baseline group. The unanticipated continuation of atherogenesis in the control mice after the switch to standard chow is likely due to a localized, unresolved inflammatory cycle in the microenvironment of the plaque [4]. This appears to result in significantly more apoptosis and the development of a large acellular necrotic core within the lesion. The enlarged necrotic core accounts for all of the plaque growth during the 10 week standard diet feeding period. In fact, both macrophage and smooth muscle cell content was significantly lower in control mice compared to baseline.
Both phenylbutyrate and valproate treatments reduced atherosclerotic lesion area, compared to control, but not baseline (Figure 1D). The phenylbutyrate treatment reduced lesion area and volume significantly in comparison to control. These results indicate that the treatment with phenylbutyrate for 10 weeks can impede the development of existing atherosclerosis in LDLR-/- mice. It is not clear if it can promote atherosclerotic regression. The increase in lesional VSMC, decrease in lesional macrophage, increase in lesional collagen in both phenylbutyrate and valproate treated mice suggests that phenylbutyrate and valproate treatment promote lesional stability. Our results are consistent with a previous study which showed that inducing ER stress in ApoE-/- mice caused a reduction in lesional VSMCs and collagen content, and an increase in necrotic core destabilization [29]. Furthermore, the increase in lesional VSMC and collagen found in valproate-treated mice are consistent with reports that have suggested a role of GSK3α/β in regulating VSMC migration and survival, and plaque stability [30,31].
Together, these data add further evidence of the importance of the ER stress-GSK3αβ in atherosclerosis. Additional research is required to specifically define the potential role of this pathway in atherosclerotic regression. These studies will be facilitated by the creation of new regression models, the identification of more potent and more specific GSK3αβ inhibitors and/or the development of inducible genetic GSK3 knockout mouse lines.
Acknowledgement
The authors would like to thank Rhea Jangra for technical assistance with immunostaining. This research was supported by operating grants from the Canadian Institutes of Health Research (MOP62910 and MOP133612).
References
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2015) Executive summary: heart disease and stroke statistics—2015 update. A report from the American Heart Association. Circulation 131: 434-441.
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, et al. (1983) Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 53: 502-514.
- Kasikara C, Doran AC, Cai B, Tabas I (2018) The role of non-resolving inflammation in atherosclerosis. J Clin Invest 128: 2713-2723.
- Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, et al. (2007) Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol 27: 1706-1721.
- Wang YI, Bettaieb A, Sun C, DeVerse JS, Radecke CE, et al. (2013) Triglyceride-rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)-1 expression via differential regulation of endoplasmic reticulum stress. PLoS One 8: e78322.
- Camargo LL, Harvey AP, Rios FJ, Tsiropoulou S, Da Silva RNO, et al. (2018) Vascular Nox (NADPH Oxidase) compartmentalization, protein hyperoxidation, and endoplasmic reticulum stress response in hypertension. Hypertension 72: 235-246.
- McAlpine CS, Werstuck GH (2014) PKR-like endoplasmic reticulum kinase (PERK) signals through glycogen synthase kinase (GSK)-3α/β to regulate endoplasmic reticulum (ER) stress-induced foam cell formation. J Lipid Res 55: 2320-2233.
- Kim S, Joe Y, Kim HJ, Kim YS, Jeong SO, et al. (2015) Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J Immunol 194: 4498-4506.
- Song L, De Sarno P, Jope RS (2002) Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem 277: 44701-44708.
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, et al. (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 15: 1383-1391.
- Huang A, Young TL, Dang VT, Shi YY, McAlpine CS, et al. (2017) 4-Phenylbutyrate and valproate treatment attenuate the progression of atherosclerosis and stabilize existing plaques. Atherosclerosis 266: 103-112.
- Peled M, Fisher EA (2014) Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front Immunol 5: 579.
- Feig JE (2014) Regression of atherosclerosis: insights from animal and clinical studies. Ann Glob Health 80: 13-23.
- Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, et al. (2011) Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest 121: 2921-2931.
- Lieu HD, Withycombe SK, Walker Q, Rong JX, Walzem RL, et al. (2003) Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation 107: 1315-1321.
- Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, et al. (2006) Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci USA 103: 3781-3786.
- Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF (1998) Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice, J. Biol. Chem 273: 30427-30434.
- Bowes AJ, Khan MI, Shi YY, Robertson L, Werstuck GH (2009) Valproate attenuates accelerated atherosclerosis in hyperglycemic apoE-deficient mice: evidence in support of a role for endoplasmic reticulum stress and glycogen synthase kinase-3 in lesion development and hepatic steatosis. Am J Pathol 174: 330-342.
- Venegas-Pino DE, Banko N, Khan MI, Shi, Y, Werstuck GH (2013) Quantitative analysis and characterization of atherosclerotic lesions in the murine aortic sinus. J Vis Exp 82: 50933.
- Weng S, Sprague JE, Oh J, Riek AE, Chin K, et al. (2013) Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS One 8: e54625.
- McAlpine C, Bowes AJ, Khan MI, Shi YY, Werstuck GH (2012) Endoplasmic reticulum stress and glycogen synthase kinase-3β activation in apolipoprotein E-deficient mouse models of accelerated atherosclerosis. Arterioscler Thromb Vasc Biol 32: 82-91.
- McAlpine C, Huang A, Emdin A, Banko N, Beriault D, et al. (2015) Deletion of myeloid GSK3α attenuates atherosclerosis and promotes an M2 macrophage phenotype. Arterioscler Thromb Vasc Biol 35: 1113-1122.
- Huang A, Patel S, McAlpine CS, Werstuck GH (2018) The role of endoplasmic reticulum stress - glycogen synthase kinase-3 signaling in atherogenesis. Int J Mol Sci 19: 6.
- Özcan U, Yilmaz E, Özcan L, Furuhashi M, Vaillancourt E, et al. (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137-1140.
- Werstuck GH, Kim AJ, Brenstrum T, Ohnmacht SA, Panna E, et al. (2004) Examining the correlations between GSK-3 inhibitory properties and anticonvulsant efficacy of valproate and valproate-derived compounds. Bioorg Med Chem Lett 14: 5465-5467.
- Nakaya H, Summers BD, Nicholson AC, Gotto AM Jr, Hajjar DP, et al. (2009) Atherosclerosis in LDLR-knockout mice is inhibited, but not reversed, by the PPAR gamma ligand pioglitazone. Am J Pathol 174: 2007-2014.
- Willecke F, Yuan C, Oka K, Chan L, Hu Y, et al. (2015) Effects of high fat feeding and diabetes on regression of atherosclerosis induced by low-density lipoprotein receptor gene therapy in LDL receptor-deficient mice. PLoS One 10: e0128996.
- Herck JL, Meyer GRY, Martinet W, Bult H, Vrints CJ, et al. (2010) Proteasome inhibitor bortezomib promotes a rupture-prone plaque phenotype in ApoE deficient mice. Basic Res Cardiol 105: 39-50.
- Allard D, Figg N, Bennett MR, Littlewood TD (2008) Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem 283: 19739-19747.
- Fernandez-Hernando C, Jozsef L, Jenkins D, Di Lorenzo A, Sessa WC (2009) Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol 2: 2033-3040.
Citation: Patel S, De Jong M, Huang A, Shi Y, Werstuck GH (2019) Investigating the Effect of Phenylbutyrate and Valproate Supplementation on Atherosclerotic Plaque Regression in a High Fat Diet Fed LDLR-/- Mouse Model. Atheroscler Open Access 4: 123.
Copyright: © 2019 Patel S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4069
- [From(publication date): 0-2019 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 3141
- PDF downloads: 928