Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Short Communication Open Access
Macromolecular Design of Platinum Drug Conjugates
| Neli Koseva* | |
| Institute of Polymers, Bulgarian Academy of Sciences, Bulgaria | |
| Corresponding Author : | Neli Koseva Institute of Polymers Bulgarian Academy of Sciences Acad. Georgi Bonchev Str., Bl. 103 A 1113 Sofia, Bulgaria Tel: +35929796630 E-mail: koseva@polymer.bas.bg |
| Received March 27, 2014; Accepted April 30, 2014; Published May 02, 2014 | |
| Citation: Koseva N (2014) Macromolecular Design of Platinum Drug Conjugates. Clin Pharmacol Biopharm S2:006. doi:10.4172/2167-065X.S2-006 | |
| Copyright: © 2014 Koseva N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Cisplatin and its analogues display common drawbacks such as unfavorable toxicological profile, short blood circulation time and non-specific bio-distribution. Besides that, in biological fluids cisplatin is inactivated by nitrogenand sulphur-containing biomolecules. Drug conjugation to a water soluble polymer is a feasible approach to enhance therapeutic efficiency. Examples of carboxylate-containing polymer carriers of cisplatin and the effect of macromolecular architecture on the behavior of conjugates are discussed.
| Since early 1970’s when cisplatin was successfully commercialized and introduced clinically thousands of platinum coordination compounds have been synthesized and evaluated as antineoplastic agents. The result is five cisplatin analogues approved for clinical use and only two of them, carboplatin and oxaliplatin, have obtained widespread application [1]. Unfortunately none of the successors of cisplatin could be considered superior to the prototype in terms of lower toxicity, superior clinical efficacy and bypassing resistance mechanisms. Cisplatin and its analogues display common drawbacks such as unfavorable toxicological profile, limited spectrum of antitumour activity, short blood circulation time and non-specific biodistribution. Besides that, in biological fluids cisplatin reacts irreversibly with a variety of nitrogen- and sulphur-containing biomolecules that reduce its therapeutic concentration [2,3]. |
| An alternative approach to the development of cisplatin-superior platinum drugs is the application of drug delivery systems capable of modulating the bio-disposition of the immobilized platinum drugs and to enhance their tumor uptake. It has been demonstrated that long-circulating polymeric carriers can preferentially and effectively accumulate in solid tumors-a phenomenon known as the “Enhanced Permeability and Retention (EPR) effect” [4,5]. The EPR effect occurs because of the imperfect angiogenesis upon tumor formation, creating leaky blood vessels. A mechanism similar to the infection induced vascular permeability also operates in the tumor tissue [6]. Further, the dysfunctional lymphatic drainage additionally facilitates the EPR effect. Thus, the result is macromolecular drug accumulation inside of the tumor (passive targeting) and decrease of the systemic toxic effects compared to the administration of low molecular-weight drugs which have no tumor selectivity. Besides these general features, the EPR phenomenon is influenced by the heterogeneity of the tumor tissues, surrounding stroma, patient characteristics, etc. which poses the problem of unpredictable drug accumulation [7]. Nevertheless, most polymer anticancer-drug conjugates rely on passive tumor targeting [8]. |
| For treatment of solid malignancies [9] platinum drugs are usually administered intravenously. Therefore conjugation to a water soluble polymer is a rational choice of route for delivery. The concept, proposed by Ringsdorf [10], has been developed and proven for polymer conjugates of anticancer compounds that entered clinical trials [11,12]. The properties of the hydrophilic polymer are directly responsible for defining the circulation half-life, rate of cellular uptake and the rate of drug release thus reducing the toxic side effects. Moreover, the polymer can impart favourable physicochemical properties, e.g. increasing solubility of lipophilic drugs or stability of agents prone to hydrolytic or proteolytic degradation [13,14]. Therefore, the macromolecular carrier should meet a set of requirements: biocompatibility and nonimmunogenicity, repeated administration, appropriate molecular mass characteristics, presence of active sites for drug binding and a suitable linker chemistry affording drug release in a sustained manner. Synthetic polymers are attractive candidates as delivery vehicles because of the great flexibility regarding the type and size of the bioactive molecules/ agents delivered and possibility for chemical attachment of further components with additional properties enhancing the therapeutic efficiency. |
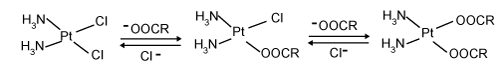
| The therapeutic conjugates are tailor-made structures taking into account the nature of the loaded drug. Specifically, cisplatin possesses two chloride ligands and undergoes ligand exchange reactions even with weak nucleophiles, i.e. carboxylate ions (Figure 1). The resulting species are able to undergo the reverse exchange reaction with chloride ions to regenerate cisplatin at physiological salt concentrations [15]. The property of carboxylate ligand as a good leaving group has been exploited to design cisplatin delivery systems based on carboxylatecontaining polymers. Macromolecules with different composition and architecture have been studied as cisplatin carriers. Their behavior in terms of stability of the nanocolloidal solution, drug payload, rate of drug release, etc. differs considerably though one and the same exchange reaction has been applied for drug conjugation. |
| It was observed that conjugation of cisplatin to a carboxylatecontaining homopolymer resulted in the formation of poorly soluble drug-polymer conjugates. For example, binding of cisplatin to the side chains of poly(L-glutamic acid) through ligand substitution caused precipitation when the molar ratio of cisplatin to L-glutamic units in the polymer exceeds 0.2 [16]. However, when cisplatin was added to poly(ethylene oxide)-b-poly(aspartic acid) (PEG-b- PAsp) or poly(ethylene oxide)-b-poly(glutamic acid) (PEG-b-PGlu) block copolymers solution in distilled water, complex micelles were spontaneously formed [17-19]. Drug loading capacity of about 40% w/w was achieved. The cisplatin-incorporated micelles had a size of 20-30 nm and narrow size distribution. They were extremely stable in distilled water whereas in physiological saline the micelles disintegrated to single chains, accompanied with sustained release of platinum (II) complexes. The micelles formed from poly(ethylene oxide)-bpoly( aspartic acid) underwent faster structural decay (about 30 h) that those from poly(ethylene oxide)-b-poly(glutamic acid) copolymers. The latter were stable for about 50 h and displayed improved selectivity and efficiency in tumor targeting [18]. |
| Bonta et al. [20] used poly(ethylene oxide)-b-poly(methacrylic acid) copolymer in the design of a stable micelles for cisplatin immobilization. Their approach included micelle formation via metal complexation followed by crosslinking of the core of the micelles and removal of the metal ions. Thus prepared micelles were loaded with cisplatin again applying the metal ligand coordination. The size of the loaded micelles was about 150 nm and the drug content was determined to be 22% (w/w). In physiological saline slow release of platinum complexes was measured. More than 20 h were required for 40% of the loaded Pt(II) to be released from the micelles. In vitro studies using human A2780 ovarian carcinoma cells demonstrated that the cross-linked micelles rapidly internalized and delivered cisplatin into cells. |
| Dendrimers are alternative to the micellar systems in drug delivery. Dendrimers are synthetic macromolecules composed of multiple layers with large number of tunable surface functional groups which can be used for drug conjugation [21]. The low level of polydispersity of these macromolecules is a prerequisite for reproducible pharmacokinetic and biodistribution behavior [21]. Carboxylated polyamidoamine (PAMAM) dendrimers were studied as cisplatin carriers [22-24]. A dendrimer G 2.5 was loaded with 26 % (w/w) of cisplatin [23]. Nanoparticles below 30 nm in diameter were visualized by TEM. Bigger particles were also formed due to crosslinking of nanocomplexes with cisplatin as a crosslinker. The drug was slowly release - 40% cisplatin was released over a period of 50 h. Besides, dendrimers possess advantageous properties; their synthesis is tedious stepwise procedure. |
| Microspheres bearing malonate moieties were designed as a cisplatin drug delivery system for the treatment of liver cancer via transarterial chemoembolization [25]. The microspheres were obtained via suspension polymerization of ethyleneglycol dimethacrylate resulting in an excess of vinyl functionalities. The latter were modified through Michael Addition of diethyl malonate. The hydrolysis of the ester groups yielded carboxylate functionalized carrier suitable for cisplatin conjugation. Loaded microspheres (9 wt% of platinum) were found to be highly toxic to liver cancer cells (ATCC, HepG2) while the carrier itself was non-toxic [25]. |
| Other candidates present the core-shell type star polymers with hyperbranched cores and shells of linear polymers with functional groups. The structural stability and multifunctionality of the stars display potential for conjugation or encapsulation of active compounds [26]. A core-shell nanocarrier was designed as a delivery vehicle of cisplatin. The macromolecules composed of a highly branched core and covalently attached linear arms of poly(acrylic acid) [27]. The high density of carboxylate groups in the shell allowed exceptionally high drug payload (45% w/w), stability in aqueous milieu upon storage and sustained release of the agent under physiological conditions. The average size of the loaded particles varied between 17 nm and 23 nm for macromolecules bearing arms of 38 and 58 degree of polymerization, respectively. The system displayed prominent capability for intracellular uptake and exhibited concentration and time- dependent cytotoxicity in a panel of human tumor cell lines [27]. Unfortunately, star coupling via ligand exchange with drug molecules lead to increase of particle size distribution. Therefore, the macromolecular carrier was further developed via modification with PEG [28]. The synthetic strategy was based on an original approach of PEGylation via incorporation of cisplatin as a reversible linker. The formation of PEG shell reduced inter-stars cross-linking, increased the stability of the nano-colloidal solution and allowed higher drug payload to be achieved. The In vitro bioassay in a panel of human tumor cell lines confirmed that the PEGylated conjugates exhibited superior growth inhibitory activity compared to the cisplatin-loaded nonPEGylated carrier. |
| The structural peculiarities of the carrier and the drug release profiles are factors determining the drug disposition in the body. Biodistribution studies carried out with cisplatin-loaded micelles administered intravenously to LLC-bearing mice revealed higher level of platinum in the bloodstream for the more stable micelles. Cisplatin- PEG-b-PGlu system maintained 13% of the injected dose after 24 h [29] compared to 1.5% for the cisplatin-PEG-b-PAsp micelles [30]. The micellar drug systems displayed advantageous tissue distribution in comparison with the free drug. The prolonged blood circulation time favored drug accumulation in tumor. PEG-b-PGlu micelles were found to be more efficient than the PEG-b-PAsp system displaying 20- fold and 4.9-fold higher accumulation, respectively, than free cisplatin. Moreover, the micellar systems did not show rapid accumulation in kidney which is related with nephrotoxicity side effects. Though the incorporated cisplatin exhibited 4.0-fold higher accumulation in the liver compared to the free drug, 24 h after injection the ratio of the liver to tumor accumulation was less than one [29]. Considering the above findings it is expected the stability of the delivery system to correlate with an enhanced accumulation in tumor, i.e. less undesirable damage to the normal tissues. On the other side, favorable accumulation does not quantitatively accounts for the antitumor activity. Cellular internalization of the drug is a critical step to therapeutic effectiveness. The prolonged blood circulation requires nanomedicines with hydrophilic surfaces, i.e. PEGylated conjugates. However, such stealth nanosystems are poorly taken up by cancer cells [31]. The reversible PEG modification can provide improved control over the pharmacological behavior of the entity without hampering the interaction between the nanomedicine and cell surfaces [32]. |
| In conclusion, the examples of cisplatin-polymer conjugates demonstrate that the design of the macromolecular carrier can be used to control key characteristics of the delivery systems though one and the same chemistry of conjugation is used. The structural characteristics of the carrier might facilitate drug delivery to tumors affording selectivity and passing over many barriers before the molecular targets are reached. |
| References |
|
|
References
- Desoize B, Madoulet C (2002) Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev OncolHematol 42: 317-325.
- Gullo JJ, Litterst CL, Maguire PJ, Sikic BI, Hoth DF, et al. (1980) Pharmacokinetics and protein binding of cis-dichlorodiammine platinum (II) administered as a one hour or as a twenty hour infusion. Cancer ChemotherPharmacol 5: 21-26.
- Vermorken JB, van der Vijgh WJ, Klein I, Hart AA, Gall HE, et al. (1984) Pharmacokinetics of free and total platinum species after short-term infusion of cisplatin. Cancer Treat Rep 68: 505-513.
- Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46: 6387-6392.
- Maeda H, Fang J, Inutsuka T, Kitamoto Y (2003) Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. IntImmunopharmacol 3: 319-328.
- Maeda H (2013) The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Sci 104: 779-789.
- Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY (2013) Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev 65: 1866-1879.
- Duncan R, Vicent MJ (2013) Polymer therapeutics-prospects for 21st century: the end of the beginning. Adv Drug Deliv Rev 65: 60-70.
- Sherman SE, Lippard SJ (1987) Structural aspects of platinum anticancer drug interactions with DNA. Chem Rev 87: 1153- 1181.
- Ringsdorf H (1975) Structure and properties of pharmacologically active polymers. JPolym Sci: Polymer Symposia 51: 135-153.
- Brigger I1, Dubernet C, Couvreur P (2002) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 54: 631-651.
- Duncan R (2003) The dawning era of polymer therapeutics. Nat Rev Drug Discov 2: 347-360.
- Riggio C, Pagni E, Raffa V, Cuschieri A (2011) Nano-oncology: clinical application for cancer therapy and future perspectives. J Nanomaterials2011:1-10.
- Pencheva I, Bogomilova A, Koseva N, Obreshkova D, Troev K (2008) HPLC study on the stability of bendamustine hydrochloride immobilized onto polyphosphoesters. J Pharm Biomed Anal 48: 1143-1150.
- Howe-Grant ME, Lippard SJ (1980) Aqueous platinum(II) chemistry; binding to biological molecules. Metal ions in biological systems, Mercel Dekker, New York 63-125.
- Schechter B, Neumann A, Wilchek M, Arnon RJ (1989) Soluble polymers as carriers of cisplatinum. J Controlled Release 10: 75-87.
- Yokoyama M, Okano T, Sakurai Y, Suwa S, Kataoka K (1996) Introduction of cisplatin into polymeric micelle. J Control Release 39: 351–356.
- Nishiyama N, Yokoyama M, Aoyagi T, Okano T, Sakurai Y, et al. (1999) Preparation and characterization of self-assembled polymer-metal complex micelle from cis-dichlorodiammineplatinum(II) and poly(ethylene glycol)-poly(alpha,beta-aspartic acid) block copolymer in an aqueous medium. Langmuir 15: 377-383.
- Nishiyama N, Kataoka K (2001) Preparation and characterization of size-controlled polymeric micelle containing cis-dichlorodiammineplatinum(II) in the core. J Control Release 74: 83-94.
- Bontha S, Kabanov AV, Bronich TK (2006) Polymer micelles with cross-linked ionic cores for delivery of anticancer drugs. J Control Release 114: 163-174.
- Gillies ER, Fréchet JM (2005) Dendrimers and dendritic polymers in drug delivery. Drug Discov Today 10: 35-43.
- Malik N, Evagorou EG, Duncan R (1999) Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs 10: 767-776.
- Ngoc QT, Cuu KN, Thi P (2013) Dendrimer-based nanocarriers demonstrating a high efficiency for loading and releasing anticancer drugs against cancer cells in vitro and in vivo.Adv Nat Sci: NanosciNanotechnol 4: 045013.
- Yellepeddi VK, Vangara KK, Palakurthi S (2013) Poly(amido)amine (PAMAM) dendrimer–cisplatin complexes for chemotherapy of cisplatin-resistant ovarian cancer cells. J Nanopart Res 15:1897-1905.
- Gu W, Gaborieau M, Huynh VTh, De Souza PL, Stenzel MH (2011) Functionalization of microspheres with malonates using Michael Addition as a pathway to create a drug delivery system for platinum drugs for the treatment of liver cancer. Polymer 52: 5993-6002.
- Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4: 307-320.
- Kowalczuk A, Stoyanova E, Mitova V, Shestakova P, Momekov G, et al. (2011) Star-shaped nano-conjugates of cisplatin with high drug payload. Int J Pharm 404: 220-230.
- Stoyanova E, Mitova V, Shestakova P, Kowalczuk A, Momekov G, et al. (2013) Reversibly PEGylatednanocarrier for cisplatin delivery. J InorgBiochem 120: 54-62.
- Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, et al. (2003) Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res 63: 8977-8983.
- Nishiyama N, Kato Y, Sugiyama Y, Kataoka K (2001) Cisplatin-loaded polymer-metal complex micelle with time-modulated decaying property as a novel drug delivery system. Pharm Res 18: 1035-1041.
- Mishra S, Webster P, Davis ME (2004) PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol 83: 97-111.
- Filpula D, Zhao H (2008) Releasable PEGylation of proteins with customized linkers. Adv Drug Deliv Rev 60: 29-49.
Figures at a glance
 |
| Figure 1 |
Post your comment
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 13917
- [From(publication date):
specialissue-2014 - Aug 23, 2025] - Breakdown by view type
- HTML page views : 9335
- PDF downloads : 4582
