Omental Transplantation against NMO and MS: A Method to Decrease or Prevent Relapses
Received: 04-Jan-2018 / Accepted Date: 23-Jan-2018 / Published Date: 30-Jan-2018
Abstract
To date, we do not know the etiological agents of Neuromyelitis Optica (NMO) and Multiple Sclerosis (MS) that trigger the production of specific antibodies (autoimmune reaction) in the bloodstream, against aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) antigens located in the astrocytes and oligodendrocytes, respectively. Agents that also cause immunological abnormalities, which damage the intima of the arteries and the blood brain barrier (BBB). Thus, AQP4 and MOG antibodies, as well as cytokines and activated leukocytes cross the injured BBB to cause inflammation, demyelination and degeneration. The clinical course in both diseases is generally characterized by periods of relapses and remissions. For these reasons, based on previous neurosurgical experiences, I believe that an omental transplantation on the optic chiasma and anteromedial temporal lobes can decrease or prevent relapses. Because the omentum produces revascularization and provides stem cells to the hypothalamus and surrounding zones.
Keywords: NMO; MS; Hyperactivity of the Hypothalamic-pituitary-adrenal (HPA) axis; Chronic stress; AQP4 antigen; MOG antigen
Introduction
To date, the etiological agents that trigger the production of antibodies in the bloodstream against the water channel aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG), are not known. AQP4 antigen is located in the membranes of astrocytes of the central nervous system (CNS) [1-5], while the MOG antigen is located in the membranes of oligodendrocytes [6-8]. The damage to the AQP4 antigen is cause of neuromyelitis optica (NMO) and injury to the MOG antigen seems to be related with Multiple Sclerosis (MS). NMO is a disease which almost selectively affects the optic nerves and spinal cord [3,9-11], while in MS there are a more diffuse distribution within the CNS, both cortical and subcortical (white matter) [12]. Moreover, relapses in both diseases have a direct relationship with the chronic stress and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis [13-17].
In the absence of knowledge of the etiological agents producing specific antibodies for both diseases, medical treatment is aimed at minimizing and avoiding relapses of both autoimmune, inflammatory and demyelinating diseases of the CNS, using high doses of methylprednisolone and immunosuppressive drugs, among other medications [9,11,18]. For the above-mentioned reasons and based on previous neurosurgical experiences [11,19-21], I believe that an omental transplantation at the chiasmatic cistern and anteromedial temporal lobe(dentate gyrus, hippocampus, entorhinal cortex and amygdala) may normalize the function of the HPA axis and so, decrease stress. In other words, normalize blood levels of cortisol and activate the immune system.
Clinical Data of NMO and MS
NMO is an autoimmune and demyelinating disease preferentially targeting the optic nerves and spinal cord [3,10,11,18], usually with a relapsing course [9,14,22]. Initially considered a variant of MS, NMO is now clearly recognized to be a separate disorder with distinct clinical, radiographic, pathologic, and serologic features [12,18]. Generally, NMO is started with ocular pain to the movement of one or both eyes, followed or parallel to the visual impairment uni or bilaterally that advance in hours to days until blindness [21-23]. On other cases, the patients began with legs cramps and months later, weakness in both legs [21,24]. Computed tomography (CT) is not useful for the diagnosis of NMO [22].
MS can occur at any age, but most of them develop the first symptoms between 20 to 40 years [25-27]. Women are about twice as likely as men are to develop MS. There are four clinical forms of MS [26,28]:1) relapsing remitting MS (RRMS), 2) secondary progressive MS (SPMS), 3) primary progressive MS (PPMS), and 4) progressive relapsing MS (PRMS). In the early stages, about 87% of patients present with RRMS, characterized by acute attacks (relapses) followed by partial or full recovery (remission) [6,28,29]. That is, most people with MS have a relapsing-remitting disease course. Patients can manifest with a heterogeneous group of symptoms including depression, diplopia, weakness in one or more limbs, dis-coordination, tremor, sensory loss or distortions, or changes in bowel and bladder function [6,16,28,30]. Minimal depression in early stages of RRMS, is the most frequent psychiatric disorder [15,16], suggesting a lesion in the prefrontal lobes. By contrast, in late stages, 50 % of the patients are incapable to walk about 15 years after the beginning [6,30]. The primary form of MS (PPMS) is the most difficult to diagnose. Over 95% of pediatric MS patients have an initial RRMS course, whereas PPMS is exceptional in children and should prompt detailed consideration of alternative diagnoses [25,26]. Therefore, the appearance of the symptoms and signs is directly related to the affected area in the CNS. That is, MS lesions known as plaques can form in brain and spinal cord white matter in any location and experience anatomopathological stages of functional recovery (remission).
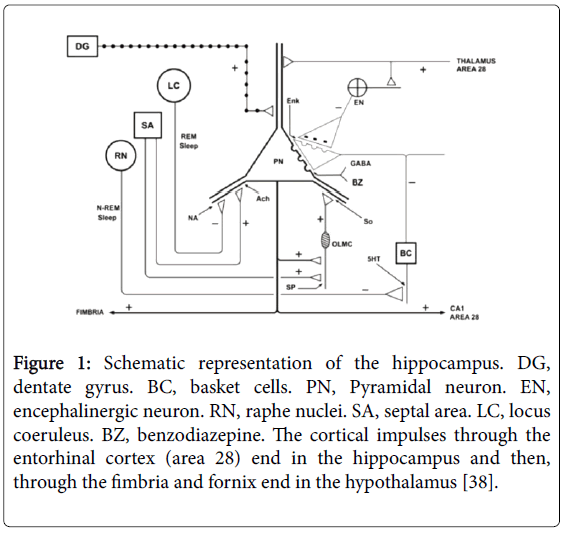
Moreover, several authors [13-15,17,31-33] report that in MS there is hyperactivity of the HPA axis in stages of relapses. Suggesting ischemia and hypoxia [34-36] in prefrontal lobes, anteromedial temporal lobes (Figure 1) and/or hypothalamus at these stages [19,20,37,38]. Because there is a direct relationship between hypothalamic lesions and atrophy of the hippocampus with hyperactivity of the HPA axis [16,19,39-43]. For example, in 173 patients with defined MS, all of them presented plasma levels of ACTH and cortisol significantly higher than in controls [13]. Therefore, chronic stress is directly related with periods of relapses in both diseases, due to the blood increase of cortisol and catecholamines [13,15,20,31,41]. In response to stress, corticotropin-releasing factor (CRF) (released by the hypothalamus) initiates a cascade of events that culminate in the release of cortisol from the adrenal cortex, and so it reduce the production of natural killer cells (leukocytes and macrophages), and increased blood markers of inflammation as reactive C proteins, interleukins, and tumor necrosis factors. In the whole blood of MS patients, the interleukins (IL-1, IL-2 and IL-4) and Interferon gamma (IFN-gamma) levels are increased [14,15,40,44,45]. In conclusion, ischemic lesions in the limbic system and hypothalamus may cause chronic stress [13,15,19,37,38] and then, deterioration of the immune system, as well as hypotrophy of the thymus and lymph nodes [15,17,31,33].
Figure 1: Schematic representation of the hippocampus. DG, dentate gyrus. BC, basket cells. PN, Pyramidal neuron. EN, encephalinergic neuron. RN, raphe nuclei. SA, septal area. LC, locus coeruleus. BZ, benzodiazepine. The cortical impulses through the entorhinal cortex (area 28) end in the hippocampus and then, through the fimbria and fornix end in the hypothalamus [38].
The diagnosis of MS is essentially clinical based on the analysis of symptoms and signs, alongside evident of dissemination of CNS lesions in space and time. The diagnosis of MS in children is somewhat more difficult than in adults. But not so, if we fear that in children there may also be diencephalic ischemia [34,35]. MRI images is often sufficient to confirm the diagnosis when characteristic lesions accompay a typical clinical syndrome [26,27]. A MRI scan will show different things based on the type of MS involved. So much so that some authors argue that the use of MRI is revolutionizing the investigation, diagnosis, and even the treatment of MS.
Normal and Injured Blood-Brain Barrier
Normally the blood-brain barrier (BBB) is formed by 1) endothelial cells, 2) basement membrane, 3) astrocytes end-feet and 4) pericytes [46-49]. Endothelial cells are connected by tight junction around the capillaries that do not exist in general circulation. That is, in the general circulation there is no BBB. This normal BBB allows the passage of water, some gases (oxygen and carbon dioxide), and lipid-soluble molecules by passive diffusion, as well as the selective transport of molecules such as glucose and amino acids that are crucial to neural function. In both diseases, NMO and MS, the BBB is affected. Some hypothalamic and monoaminergic nuclei of the brainstem do not have BBB [48,50,51].
In NMO the astrocytes are damaged, whereas in MS is the oligodendrocytes-myelin complex. The target antigen of autoimmunity in NMO is the water channel aquaporin-4 (AQP4), while in MS seems to be related with myelin oligodendrocyte glycoprotein (MOG). However, to date the etiological agents for each disease are not known. Many researchers believe that external and internal agents of origin or a combination of them(within the bloodstream) are the cause of damage to the intima of the arteries and the BBB [52-55]. But we know that the harmful agent produce non-specific antibodies (AQP4-IgG) for AQP4 in the NMO, and antibodies against the MOG antigen, within the bloodstream. These antibodies selectively affect the CNS myelin, but not PNS myelin [52]. In other words, these agents affect the astrocytes and oligodendrocytes, but not the Schwann´s cells; still circulating in the bloodstream.
Etiological agents [35,53,56-59] in the bloodstream injure the intima of the arteries in general, which cause the release of inflammatory cytokines such as tumor necrosis factor (TNF)-alpha, Ikappa-B Kinase beta (IKK-beta), nuclear factor kappa-B(NF-kB), interferon(IFN)- gamma, interleukins and matrix metalloproteinases (MMPs). Thus, on the one hand, they cause different degrees of atherosclerosis [34,35,56,60-62], and on the other hand, these same agents can also damage the BBB [46,49,61], especially the TNF-alpha and MMPs which facilitate transendothelial migration of the active leukocytes and cause a localized inflammatory process [24,55]. In the whole blood of patients with MS the IFN-gamma, interleukin (IL)-1, IL-2, IL-4 and IL-6, they are all increased [14,44,63].
Astrocytes and Neuromyelitis Optica
Astrocytes are the most numerous glial type of the CNS, in which 4 cell types are distinguished: 1) protoplasmatic astrocytes, located in the gray matter; 2) fibrous astrocyte, located in the white matter (axons) and covering the nodes of Ranvier; 3) radial astrocytes, located in a perpendicular direction to the ependymal cells of the ventricles, and 4) modified astrocytes, such as Bergmann glial cells in the cerebellum and Müller cells in the retina [23,64-66]. Astrocytes, especially protoplasmatic and fibrous types, both nourish neurons and oligodendrocytes, as well as synthesize and recapture neurotransmitters [55,65].
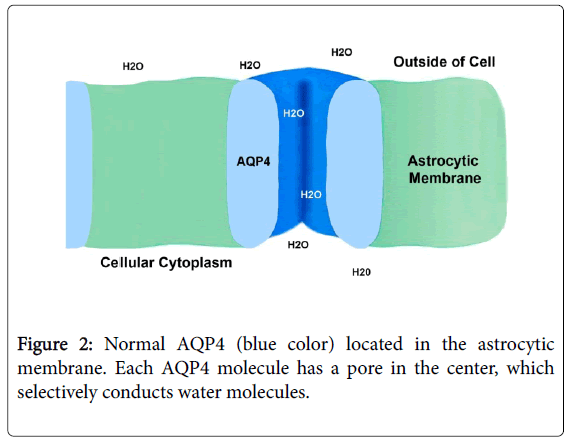
NM0 (also known as Devic´s disease) is a rare autoimmune disorder, distinct from MS, causing inflammatory lesions in the optic nerves and spinal cord [9,24,67,68]. However, it is not known with certainty, which is the etiological agent that circulates through the bloodstream and causes the release of antibodies against the AQP4. Aquaporins (AQPs) that are present in the membranes of the biological cells [1,2,69]. There are 13 known mammalian AQPs (AQP-0 to AQP-12) in the biological cells for the transport of water forward and backward through the cellular membranes [1,2,70]. Of 13 different AQPs, aquaporin-4 is the predominant type found in brain and spinal cord [68,71,72], which plays the critical role in regulating the water transportation, and participating in the CNS physiological processes. In CNS, AQP4 is prefentially expressed in the retina, optic nerve, hypothalamus, cerebellum, perivascular and ependymal cells, anteromedial temporal lobe and the spinal cord, and low levels are observed in the cerebral cortex [1,4,10,24,73]. In the astrocytes of the CNS, AQP4 are distributed throughout the cell membrane, especially in the astrocyte end-feet on the BBB [1,2,55]. Besides, AQP4 are also located in the ear, skeletal muscle, stomach parietal cells, and kidney collecting ducts [4,10]. In this way, AQP4 prevents us from dying of dehydration by reabsorption of the water in the kidneys. On the other hand, the AQP1 present in choroidal plexus, contributes to production of cerebrospinal fluid [1]. By contrast, many AQP4 inhibitors have been discovered, including acetazolamide, sumatriptan and rizatriptan, among others [72,73].
Similar to the insulin receptor located in the cell membrane of the cells of the body [60]; AQP4 are typically composed of subunit proteins, which form pores in the membranes of cells [2] and selectively conduct water molecules through the membrane, while preventig the passage of ions (such as sodium and potassium) and other small molecules [1-3]. The structural analysis of the AQP4 molecules has revealed the presence of a pore in the center of each molecule (Figure 2). Therefore, AQP4 is widely expressed in the astrocyte cell plasma membrane, and primarily localized in specific regions such as astrocyte foot processes [5,47,48,55]. Most authors [3-5,24,52,55,67] believe that AQP4-IgG antibodies against AQP4, at the level of the astrocyte end-feet, is the primary cause of NM0, however they do not point out the harmful effect of these antibodies, in others AQP4 located in other organs of the body as for example in the kidneys.
Autopsy findings have shown evidence that abrupt astrocyte destruction precedes demyelination in NMO [46,62,74]. That is, the astrocyte is damaged first, followed by the oligodendrocyte and finally the axons (demyelination and neuronal loss). AQP4 antibody (or AQP4-Ab) is positive in a high percentage (about 73%) of NMO patients and it is directed against this water channel richly expressed on foot processes of astrocytes [3,9,12,22,46,55]. That is, AQP4 antibodies are not specific for AQP4 antigens. In conclusion, there is evidence that AQP4-IgG antibodies produced by leukocytes (T and B lymphocytes and plasma cells) in the blood stream, they cross the injured BBB to act on the AQP4 antigen distributed throughout the astrocyte membrane, especially on the astrocyte end-feet.
Medical treatment of NMO is directed to the management of acute attacks and prevent future exacerbations [9,11,18,52]. Six medications are used: azathioprine, rituximab, prednisone, methotrexate, mycophenolate mofetil and mitoxantrone. Moreover, recently I reported to a 45-year-old woman who suffered advanced NMO [21]. She received omental tissue on the prechiasmatic space, optic chiasma and anteromedial surface of the left temporal lobe. One day after surgery, she experienced neurological improvement in recent memory, motor on her limbs and days later, visual improvement. Results similar to previous experiences with omental transplantation on the optic chiasma and surrounding areas [19,20,50,75]. Neurological improvement observed in this patient indicates that the disease is associated with ischemia, hypoxia and inflammation in the optic chiasma and surrounding structures, especially the pyramidal pathways at the level of the internal capsules. Because the omentum causes revascularization (neoformation of blood vessels) in underlying and adajacent zones to the omental tissue, and through these neovessels, the affected nervous tissue receives an increase in blood flow, oxygen, neurotransmitters, neurotrophic factors, adipocytokines and omental stem cells [20,50,76].
Oligodendrocytes and Multiple Sclerosis
The subventricular zone (SVZ) of the lateral ventricles, especially from the sphenoidal horn, is the origin of the neurons and glial cells [36,80]. Oligodendrocytes precursor cells, which appear in the late embryonic and postnatal periods, mature into oligodendrocytes (from the bipolar form, proliferation, migration and differentiation) [77,78] and form myelin in the postnatal brain [54,66,79,80]. In the cerebellum, the majority of oligodendrocytes are originated from ventral rhombencephalon and to a lesser extent from the cerebellar SVZ [81,82]; meanwhile in the spinal cord, the oligodendrocytes are originated from the ventral regions of the embryonic cord several weeks before myelination [81,83]. Therefore, infants born prematurely are at high risk to develop white matter injury due to exposure to ischemia and hypoxia insults [36,78]. Such perinatal insults negatively impact the maturation of oligodendrocytes, thereby causing deficits in myelination [75,78].
In adults, three types of oligodendrocytes have been described in the CNS [77,78,80,81,84]: 1) interfascicular oligodendrocytes (i-OLs), 2) satellite oligodendrocytes (s-OLs) and, 3) perivascular oligodendrocytes(p-OLs). The first, the i-OLs are responsible for the myelination of axons of the CNS and are located in rows parallel to the nerve fibers. An oligodendrocyte have many extensions that are wrapped around axons of several neurons, so that a single oligodendrocyte can extend its processes to 50 axons, wrapping approximately 1 um of myelin sheath around each axon; while a Schwann cells in the PNS can wrap only one axon [81,85]. The second, mostly s-LOs are closely opposed to the soma of neocortical layer 5 pyramidal neurons [86] and are the most metabolically active of the neuroglia [87]. In smaller quantity, other s-LOs are located in the periventricular gray matter were clearly seen to be remyelinating axons and other cells seem to play ejector function on the muscles of the cavernous tissue of the male sex organ. That is, these cells are related with remyelinating and causing the exit of sperm [81,86,87]. The third, the p-OLs are associated with the capillary complex of the cerebrum and little is known about their function [84,87].
Myelination occurs primarily in the postnatal period [79,85,88,89]. Each interfascicular oligodendrocyte send several processes that cause rolling in successive turns around the axon, but separated by the node of Ranvier (or myelin-sheath gaps). Besides, several studies have demonstrated the presence of perinodal astrocyte processes at nodes of Ranvier in the CNS, suggesting that astrocyte participate in the formation of mature central nodes [64]. So that, each axon is surrounded by spiral sheets of double membranes, from a few up to 100 or more depending on the diameter of the fiber [85,88,89].
Myelin is a lipid-rich membrane and in situ, the water represents about 40%. The dry mass of both CNS and PNS myelin is characterized by a high proportion of lipids (about 70 to 85%); of which the main lipids present in the myelin sheath are cerebrosides, cholesterol, lecithin’s, phospholipids and, to a lesser extent, sphingomyelins, and consequently, a low proportion of proteins (15 to 30 %) [85,88]. The main proteins of the CNS myelin include: proteolipidic protein, basic proteins and glycoproteins These myelin proteins of the CNS are different from those of the PNS, in variety and proportion. The myelin in the human CNS have more proteins than in monkey [89,90]. Myelin oligodendrocyte glycoprotein (MOG), is a myelin protein solely expressed at the outermost surface of myelin sheaths and oligodendrocyte membranes [54]. This makes MOG as a target auto antigen of MOG antibodies, and cellular and humoral response in demyelinating diseases [8,54,65]. MOG antibodies are also detected in other demyelinating diseases.
Currently the objective of MS treatment is aimed at preventing disability and reducing the course of the disease. Interferon beta (IFN-beta) was first approved by the FDA for MS treatment [28,30]. Other medications are used for the same purpose, such as glatiramer acetate, monoclonal antibodies, natalizumab, rituximab and methotrexate among others [28]. In my opinión, I think clonazepam can also be used to decrease chronic stress by its action on GABA-ergic receptor in the hippocampus [38]. From the surgical point of view, they have been used rhizolysis for neuralgia and rhizotomy for spasticity and deep brain stimulation, among other neurosurgical techniques in the treatment of symptoms of MS [91]. However, based on neurosurgical experiences with omental transplantation on the optic chiasma, anterior perforated space and anteromedial temporal lobes [19-21,50,75], I think that the same surgical technique can decrease chronic stress and thus prevent relapses. Because in this way we revascularize the hypothalamus, temporal lobes and surrounding structures and thus, we normalize the function of the HPA axis.
Etiological Agents of NMO and MS
The etiological agents that cause the production of antibodies (autoimmune reaction) against of AQP4 and MOG in the bloodstream are not known. But based on the onset of the clinical data and associated with chronic stress, I believe that both diseases (NMO and MS) are initiated in the limbic system and hypothalamus caused by ischemia [21,34,75], which causes hyperactivity of the HPA axis, among other axes [20,32,33,37,38]. Thus, during chronic stress, continuous exposure to cortisol induces desensitization of glucocorticoid receptors in lymphocytes, monocytes and macrophages and on the other hand, high levels of catecholamines cause the synthesis of proinflammatory proteins.
Whatever the etiological agent, what we know is that the AQP4- antibodies and MOG-antibodies, both cross the injured BBB to affect the astrocytes and oligodendrocytes, respectively. The majority of investigators believe that exogenous and/or endogenous harmful agents of origin are the etiological agents that they damage to the intima of the arteries, BBB and to the blood cells (leukocytes and plasma cells) [35,56,58,59,62,63].
In addition to the inflammatory cytokines secreted by adipose tissue [60,76], the intima and blood cells also release a series of antibodies and cytokines such as IFN-gamma, MMPs, TNF-alpha, NF-kB, IKK-beta, and Interleukins, among others [35,45,55,61,63]. It seems that MMP-9, IFN-gamma, TNF-alpha and IL-6 are the main cytokines causing damage to the BBB [53,58,63] and thus, the antibodies against the AQP4 or MOG cross the injured BBB and then, the activated leukocytes(neutrophils, monocytes and eosinophils) migrate to the perivascular space, where they cause different degrees of temporary inflammation. In my opinion, the onset and clinical course (periods of relapses and remissions) of both diseases is related to vascular anomalies of the circle of Willis [75,92,93], atherosclerosis [21,34,94] and stress [32,39,41,48]. In this way, vascular anomalies and atherosclerosis can cause varying degrees of ischemia and hypoxia ,and cause hyperexcitability of the HPA axis. Thus we would explain the temporal and changing location of the damage within the CNS, due to circulating antibodies in the bloodstream. So the damage to AQP4 molecule causes destruction of the astrocytes, followed by demyelination, and the injury to MOG molecules would cause damage to the oligodendrocyte-myelin complex. Therefore, pathologically in the affected zone of NMO or MS there are loss of astrocytes and oligodendrocytes, inflammation, demyelination, neurodegeneration and atrophy.
Conclusion
To date, the specific etiological agents that cause NMO or MS are not unknown. But we do know that these harmful agents cause the production of antibodies (autoimmune reaction) in the bloodstream, which (AQP4 and MOG antibodies) in company of inflammatory cytokines cross the injured BBB to affect the astrocytes and oligodendrocytes; followed by perivascular inflammatory reaction due to activated leukocytes. Thus the AQP4 antibodies act on the AQP4 antigens of astrocytes and cause NMO, while the MOG antibodies will act on MOG antigens to cause MS. However, the antibodies are not totally specific for NMO and MS. As a result of this, there is a perivascular pathological change characterized by loss of astrocytes and oligodendrocytes, demyelination, inflammation, neurodegeneration and atrophy.
As the etiological agents are not known, the treatment is aimed at reducing the symptoms and signs through drug therapy and some surgical procedures. Relapses in both diseases are directly related to chronic stress and, however, there is almost no treatment to decrease hyperexcitability of the HPA axis. For these reasons, I think that an omental transplantation on the optic chiasm and anteromedial temporal lobes can normalize the function of the HPA axis and thus reduce chronic stress as well as actívate the immune system. Because the omentum causes revascularization, provides omental stem cells, and favors the proliferation of neural stem cells from the SVZ of the sphenoid horn.
References
- Agre P (2006) The aquaporin water channels. Proc Am Thorac Soc 3: 5-13.
- Takata K, Matsuzaki T, Tajika Y (2004) Aquaporins: Water channels proteins of the cell membrane. Prog Histochem Cytochem 39: 1-83.
- Kinoshita M, Nakatsuji Y (2012) Where do AQP4 antibodies fit in the pathogenesis of NMO? Mult Scler Internat, Article ID 862169
- Song YC, Li WJ, Li LZ (2015) Regulatory effect of miRNA320 a on expression of aquaporin-4 in brain tissue of epileptic rats. Asian Pac J Trop Med 8: 807-812.
- Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14: 265-277.
- Hohifeld R, Dommair K, Meinl E, Wekerle H (2016) The research for the target antigens of multiple sclerosis. Part 2: cD8+T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol; 15: 317-331.
- Weber MS, Hemmer B, Cepok S (2011) The role of antibodies in multiple sclerosis. Biochem Biophy Acta 12: 239-245.
- Sato D, Callegaro D, Lana-Peixoto MA, Fujihara K (2012) Treatment of mielitis optica: An evidence based review. Arq Neuro-Psiquiat 70: 59-66.
- Zeka B, Lassmann H, Bradp M (2017) Müller cells and retinal axons can be primary targets in experimental neuromyelitis optica spectrum disorder. Clin Exp Neuroimmunol 8: 3-7.
- Kimbrough DS, Fujihara K, Jacob A, Lana-Peixoto MA, Levy M, et al. (2012)Treatment of neuromyelitis óptica: Review and recommendations. Mult Scler Relat Disord 1: 180-187.
- Kawachi I, Lassmann H (2016) Neurodegenerative in multiple sclerosis and neuromyelitis optica. J Neurol Neurosurg Psychiat 88: 137-145.
- Ysrraelit MC, Gaitan MI, López AS, Correale J (2009) Actividad del eje hipotálamo-hipofisio-adrenal durante el curso de la esclerosis multiple. Rev Neurol Argen 1: 3-10.
- De Andrés C (2003) Interes de los brotes en la esclerosis multiple: FisiopatologÃa y tratamiento. Rev Neurol 36: 1058-1064.
- Gómez B, Escobar A. Estrés Y (2006) sintema inmune. Rev Mex Neurosci 7: 30-38.
- Sánchez-López MP, Olivarez-Perez T, Nieto-Barco A, Hernandez-Perez MA, Barroso-Ribal J (2004) Esclerosis multiple y depresión. Rev Neurol X 38: 524-529.
- Smith SM (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialog Clin Neurosci 8: 383-395.
- Kowarik MC, Soltys S, Bennett JL (2014) The Treatment of neuromyelitis optica. J Neuroopthalmol 34:70-82.
- Rafael H, Mego R, Moromizato P, Garcia W (2002) Omental transplantation for temporal lobe epilpesy. Report pf two cases. Neurol India 50: 71-74.
- Rafel H (2015) Omental transplantation for neuroendocrinological disorders. Am J Neurodegener Dis 4: 1-12.
- Rafael H (2017) Omental transplantation for neuromyelitis optica. J Neurol Stroke 6: 00194
- Carnero E, Leguizamon F, Colla PE, Alonso R (2013) Neuromyelitis optica:Clinical and therapeutic Update. Neurol Argent 5: 259-269.
- Chiquete E, Navarro-Bonnet J, Ayala-Armas R, Gutierrez-Gutierrez N, Solorzano-Melendez A, et al. (2010)Neuromielitis optica:Actualización clÃnica. Rev Neurol 51: 289-294.
- Bukhari W, Barnett MH, Broadley SA(2012) Molecular pathogenesis of neuromyelitis optica. Int J Mol Sci 13: 12970-12993.
- Hadjigeorglou I, Villar C, Fernandez MA, Mirallave A, Arnau A, et al.( 2013) Esclerosis multiple pediátrica. Rev Esp Escl Mult 25: 16-23.
- Polman ChH, Reingold SC, Banwell B, Clanet M, Cohen JA, et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald Criteria. Ann Neurol 69: 292-302.
- Brownlee NJ, Haydy TA, Fazekas F, Miller DH (2017)Diagnosis of multiple sclerosis:Progress and challanges. Lancet 389: 1336-1346.
- Loma I, Heyman R (2011) Multiplre sclerosis:Pathogenesis and Treatment. Curr Neuropharm 9: 409-416.
- Weiner HL (2008) A shift from adaptive to innate immunity :A potential mechanism of disease progression in multiple sclerosis. J Neurol 255: 3-11.
- Porras-Betancourt M, Nuñez-Orozco L, Plasencia-Alvarez NI, Quiñonez-Aguilar S, Sauri-Suarez S, et al. (2007) Esclerosi multiple. Rev Mex Neurosci 8: 57-66.
- Gouin JP, Hantsoo L, Kiecolt-Glaser JK (2008) Immune dysregulation and chronic stress among older adults:A review. Neuroimmunomodulation 215: 251-259.
- Riise T, Mohr DC, Munger KL, Rich-Edwards JW, Kawachi I, et al. (2011) Stress and the risk of multiple sclerosis. Neurology 76: 1866-1871.
- Lovera J, Reza T (2013) Stress in multiple sclerosis :Review of new developments and future directions. Curr Neurol Neurosci Rep 13: 398.
- Chiquete E, Valle-Rojas D, Rodriguez-Saldaña J, Rodriguez-Flores M, Aguirre-Garcia J, et al. (2012) Aterosclerosis carotidea e intracraneal en una población pediátrica:Un estudio de autopsia-Rev Mex Neurosci 13: 93-97.
- Rafael H (2016) Aspirin against atherosclerosis intracranial arterial stenosis. J Neurol Neurophysiol 7: 1000403.
- Rafael H (2017) Etiopathogenesis of central nervous system gliomas. J Oncol Transl Res 3: 1000122.
- Rafael H, Valadez MT (2014) Disfunción cerebral mÃnima.II:Etiologia y fisiopatologÃa. Salud Pública Méx 28: 495-503.
- Rafael H, Valadez MT (1987) Disfunción cerebral mÃnima.III:Tratamiento (reporte preliminar ) Salud Pública Méx 29: 55-60.
- Mitsonis CI, Patagas C, Zervas I, Stagos K (2009) The effects of stressful life events on the course of multiple sclerosis.Int J Neurosci 119: 315-335.
- Huitinga I, De Groot CJ, van der Valk P, Kamphorst W, Tilders FJ (2001) Hypothalamic lesions in multiple sclerosis. J Neuropathol Exp Neurol 260: 1208-1218.
- Rapaport B, Karceski S (2012)Multiple sclerosis and stress. Neurology79: e47-e49.
- Qiu W, Raven S, Wu JS, Bunde Ch, Hollingsworth P, et al. (2011) Hypothalamic lesions in multiple sclerosis. J Neurol Neurosurg Psychiat 82: 819-822.
- Geurts JJG, Bo L, Roosendaal SD, Hazes T, Daniels R, et al. (2007)Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 66: 819-827.
- Rose JW, Houtchens M, Lynch SG (2000) Multiple sclerosis. Available at:http://library.med.utah.edu/kw/ms/epidemiology.htnl.
- Tardieu M, Deiva K (2013) Rare inflammatory disease of the white matter and mimics of multiple sclerosis and related disorders. Neuropediatrics 44: 302-308.
- Sofroniew MV (2015) Astrocyte barrier to neurotoxic inflammmation. Nature Rev Neurosci 16: 249-263.
- Ballabh P, Braun A, Nedergoard M (2004) The blood-brain barrier:An overview, structure, regulation, and clinical implications. Neurobiol Dis 16: 1-13
- Escobar A, Gómez GB (2008) Barriera hematoencefalica:Neurobiologia, implicaciones clÃnicas y efectos del estrés sobre el desarrollo. Rev Mex Neurosci 9: 395-405.
- de Vries HE, Kuiper J, de Boer AG, van Berkel TJ, Breiner DD (1997) The blood-brain barrier in neuroinflammation diseases. Pharmacol Res 99: 143-155.
- Rafael H (2014) Omental transplantation for neurodegenerative diseases. Am J Neurodegener Dis 3: 50-63.
- Rafael H (1995) Tejidos donadores de catecolaminas:Una revisión.Diagnostico(Peru) 34: 42-49.
- Wingerchuk DM (2007) Lennon VA, Lucchinetti CF, Pittock SJ, Weinschenker BG. The spectrum of neuromyelitis optica-Lancet Neurol 6: 805-815.
- Minagar A, Jy W, Jimenez JJ, Alexander JS (2006) Multiple sclerosis as a vascular disease. Neurol Res 28: 230-235.
- Peschl P, Bradl M, Höftberger R, Berger T, Reindl M (2017)Myelin oligodendrocyte glycoprotein: Deciphering a target in inflammatory demyelination disease.Front Immunol 8: 529.
- Papadopoulos MC, Bennett JL, Verkman AS (2014) Treatment of neuromyelitis optica:Stat of the art and emerging therapies. Nat Res Neurol 10: 493-506.
- Rafael H, Ayulo V, Lucar A (2003) Patogenesis de la aterosclerosis:Base hemodinámica y factores de riesgo.Rev Climaterio(Mex ) 6: 125-128.
- Sudaram G, Brew BJ, Jones SP, Adams S, Lim CK (2014) Quinolinic acid toxicity on oligodendroglial cells:Relevance for multiple sclerosis and therapeutic strategies. J Neuroinflam 11: 204.
- De Bie, Lim CK, Guillemin GJ (2016) Progesterone alters knurenine pathway activation in IFN-gamma activited macrophages: Relevance for neuroinflammatory diseases. Int J Tryptoph Res 9: 89-93.
- Hou L, Zhang X, Wang D, Baccarelli A (2012) Environmental chemical Exposures and human epigenetic. Int J Epidemiol 41: 79-105.
- Rafel H (2016) Therapeutic methods against insulin resistance. J Endocrinol Metab 6: 1-11.
- Rosenberg GA (2002) Matrix metalloproteinases and neuroinflammation in multiple sclerosis. Neuroscientist 8: 586-595.
- D´Mello F, Baidy N, Marcal H, Guillemin G, Rossi F, et al. (2016) Cytotoxic effects of environmental toxins on human glial cells. Neurotox Res 31: 245-258.
- Petkovic F, Castellano B (2016) The role of interleukin-6 in central nervous system demyelination. Neural Regener Res 11: 1922-1923.
- Guillamon-Vivances T, Gómez-Pinedo U, Matias-Guiu J (2015) Astrocyte in neurodegnerative diseases:. Function and molecular description. Neurologia(Esp) 30: 119-129.
- Tabata H (2015) Diverse subtipe of astrocytes and their development during corticogenesis. Front Neurosci 9: 00114.
- Howe CL, Kaptzan T, Magaña SM, Ayers-Ringles JR, LaFrance-Corey RG (2014) Neuromyelitis optica IgG stimulates an immunological response in rat astrocyte cultures. Glia 62: 692-708.
- Marignier R, Nicolle A, Watrin Ch, Touret M, Cavagna S, et al. (2010) Oligodendrocyte are damaged by meuromyelitis optica immunoglobulin G via astrocyte injury. Brain 13: 2578-2591.
- Preston GM, Carroll TP, Guggine WB, Agre P (1992) Appearance of water channels in xonopus oocytes expressing cell CHIP28 protein. Science 256: 385-387.
- Cohly HHP, Isokpehi R, Rajnarayanan RV (2008) Compartmentalizationof aquaporins in the human intestine. Int J Environ Res Publi Health 5: 115-119.
- Verkman AS (2005) More than just water channels; Unexpected celular roles of aquaporins. J Cell Sci 118: 3225-3232.
- Huber VJ, Tsujita M, Kwaee IL, Nakada T (2009) Inhibition of aquaporin-4 by antiepileptic drugs. Bioorg Med Chem17: 418-424.
- Yu H, Qi GL, Wang L, Chen L, Deng Z, et al. (2016) Aquaporin 4 inhibition decreased systhesis of cytokines by acetozolamide in the hippocampus of rats with pentrazol-induced chronic epilepsy. Genet Mol Res 15: 15039012.
- Barnett MH, Prineas JW, Buckland ME, Parratt JD, Pollard JD (2012) Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy. Mult Scler 18: 108-112.
- Rafael H, David JO, Vilca AS (2015) Omental transplantation for infantile cerebral palsy. J Neurol Sci(Turk) 32: 146-153.
- Russo V, Yu C, Billiveau P, Hamilton A, Flynn LE (2014) Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cell Transl Med 13: 206-217.
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzales-Perez O, Rowitch D (2006) Origen of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 26: 7907-7918.
- Van Tilborg E, de Theije CGM, van Hal M, Wagenaar N, de Vries LS, et al. (2017) Origin and dynamics of oligodendrocytes in the developing brain:Implications for perinatal white matter injury- Glia 66: 221-238.
- Naruse M, Ishizaki Y, Ikenaka K, Hitoshi S (2017) Origin of oligodendrocytes inmammalian forebrains:a revised perspective. J Physiol Sci 67: 63-70.
- Quiñonez-Hinojosa A, Sinai N, Soriano-Navarro M, Gonzales-Perez O, Mirzadeh Z, et al.(2006) Cellular composition and cyto-architecture of the adult human subventricular zone:A niche of neural stem cells. J Comp Neurol 494: 415-434.
- Baumann N, Pham-Dinh D (2001) Biology of oligodendrocytes and myelin in the mammalian central nervous system. Physiol Rev 81: 871-827.
- Hashimoto R, Hori K, Owa T, Miyashita S, Dewa K, et al. (2016) Origin of oligodendrocytes in the cerebellum, whose development is controlled by the transcription factor SOX 9. Mech Dev 140: 25-40.
- Hajihosseini M, Tham TN, Dubois-Dalcq M (1996) Origin of oligodendrocytes within the human spinal cord. J Neurosci 16: 7981-7994.
- Dodson RF, Wai-Fong L (1972) Perivascular oligodendrocytes in the striatum of the squirrel monkey. J Neurol Sci 17: 237-244.
- Morell P, Quarles RH, Norton WT (1994) Myelin formation, structure, and biochemistry. Basic neurochemistry:Molecular, cellular, and medical aspects. New York, Raven Press pp117-143.
- Bateffeld A, Klooster J, Kole MHP (2016) Myelinating satellite oligodendrocytes are integrated in glial syncytium constraining neuronal high-frequency activity. Nat Commun 7: 11298.
- Ludwing SK (1979) The perineuronal satellite oligodendrocyte. Acta Neuropathol 47: 49-53.
- Jackman N, Ishii A, Bansal R (2009) Oligodendrocyte development and myelin biogénesis: Parising out the roles of glycosphingolipids. Physiol 24: 290-297.
- Larocca JN, Rodriguez-Gabin AG (2002) Myelin biogénesis:Vesicle transport in oligodendrocytes. Neurochem Res 27: 1313-1329.
- Horrocks LA (1967) Composition of myelin from peripheral and central nervous system of the monkey. J Lipid Res 8: 569-576.
- Patwardhan RV, Minager A, Kelley RE, Nanda A (2006) Neurosurgical Treatment of multiple sclerosis. Neural Res 28: 320-325.
- Riggs HE, Griffiths JO (1938) Anamalies of the circle of Willis in persons with nervous and mental disorders- Arch Neurol Psychiat 39: 1353-1354.
- Rhoton Jr AL (2002) The supratentorial arteries. Neurosurgery 51: 53-120.
- Flora GC, Baker AB, Loewenson RB, Klassen AC (1968) A comparative study of cerebral atherosclerosis in males and females. Circulation 38: 859-869.
Citation: Rafael H (2018) Omental Transplantation against NMO and MS: A Method to Decrease or Prevent Relapses. Neurol Clin Therapeut J 2: 102.
Copyright: © 2018 Rafael H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4701
- [From(publication date): 0-2018 - Feb 27, 2026]
- Breakdown by view type
- HTML page views: 3683
- PDF downloads: 1018