Paediatric Auditory Brainstem Implantation (ABI): A Journey through Surgery and Outcomes
Received: 19-Apr-2018 / Accepted Date: 31-May-2018 / Published Date: 07-Jun-2018 DOI: 10.4172/2161-119X.1000350
Abstract
Introduction: Auditory brain stem implantation (ABI) is an implantable electrode device to treat neural hearing loss, where cochlear implantation (CI) is not possible. ABI system consists of a receiver-stimulator, electrode array and electrode plate internally and a speech processor, microphone and transmitter coil externally. The device is kept on the cochlear nucleus and it stimulates the nucleus by which the patient perceives sound. Knowledge of the anatomical landmarks and variants in anatomy of the brainstem is vital for auditory brainstem implant surgery. Pediatric Auditory Brainstem Implantation is indicated for children with congenital cochlear aplasia, absent/hypoplastic vestibulocochlear nerve (VCN), for whom cochlear implantation is not possible.
Methodology: Retro prospective study (From 2006 till 2017) at Auditory implant centre in Madras ENT Research Foundation, which includes 24 children who had undergone ABI surgery and are being followed up for 1 year, post operatively. Aims were to study the anatomical variants and the outcomes of auditory brainstem implant implantation. To determine if different anatomical variants effect placement of ABI electrode. To assess the outcomes by Categories of auditory Performance (CAP) scores and Speech Intelligibility Ratings (SIR) scores.
Results: All the candidates had significant audiological and verbal outcomes after the auditory brainstem implantation. Though, there was difficulty in insertion of the electrode in subjects with anatomical variants, the outcomes were comparable with other subjects.
Keywords: Sensorineural hearing loss; ABI and electrodes; Anatomical variants; Cerebellar flocculus; Lateral recess; CAP and SIR scores; Outcomes
Introduction
Hearing loss is the most common congenital and acquired sensory deficit among children. Sensorineural hearing loss (SNHL) occurs when sound is not efficiently transduced into electric potentials or when transmission of these signals to higher-order auditory centres is disrupted. Small subset of paediatric patients with severe to profound SNHL loss that will not benefit from the Cochlear Implant (CI) due to a small or absent cochlea or auditory nerve or scarring of the inner ear due to infection or trauma, will benefit from Auditory Brainstem Implant surgery (ABI) [1]. The ABI differs from the CI as it bypasses the cochlea and cochlear nerve to directly stimulate second order neurons of the auditory pathways in the brainstem called the cochlear nucleus [2]. Similar to CI, ABI consists of an external sound processor and an internal part with an electrode, with only variation in the length of the attachment wire and arrangement of electrodes in a titanium plate and covered by Dacron mesh.
The concept of auditory prosthesis has undergone a revolutionary evolution which began with Djourno and Eyries on 25 February 1957 [3]. First successful ABI surgery was performed in 1979 for a woman with Neurofibromatosis type 2 (NF2), by William E. Hitselberger and William F. House at House Ear Institute [4]. In 2001, Colletti performed first paediatric ABI surgery for a case of auditory nerve aplasia [5]. Since then Colletti group from Italy and Sennaroglu group from Turkey have has performed nearly 200 non NF2 paediatric ABI surgeries, including surgery on an 8th month old infant by Colletti [5-7]. Today ABI surgery is recognized as a safe and effective option for stimulating the auditory cortex in children with profound retro-cochlear deafness.
Patients and Methods
This is a retrospective and prospective observational study of 24 paediatric patients who underwent auditory brain stem implant surgeries, at our tertiary referral centre from March 2006 till March 2017. Aims of our study were to study the normal surgical landmarks & anatomical variants encountered, to determine if anatomical variants affect placement of ABI electrode and to study outcomes after ABI and compare the outcomes between implantees with normal surgical anatomy and anatomical variant.
12 electrode channel, Medel (MED-EL LTD, Innsbruck, Austria) ABI was used in all our cases. Institutional research ethics board approval was obtained. Informed consent for participation in the study was taken from all the parents. Inclusion criteria included all children with bilateral profound SNHL loss due to cochlear and cochlear nerve aplasia, labyrinthitis ossificans, cystic/common cavity. Children with bilateral severe to profound hearing loss due to cerebello pontine (CP) angle tumors were excluded from the study as that would distort the CP angle anatomy and also children more than 6 years of age, those with subnormal IQ and children with syndromic multiple disability were excluded from the study in order to reduce bias while comparing outcomes in ABI children.
Etiology of deafness, radiology findings, age at implantation, reviewed from previous medical records and prospectively. Children with complaints of severe hearing deficit since birth associated with delay in speech and language development, were assessed for etiology of deafness and underwent electrophysiological evaluation for hearing and also radiological evaluation of inner ear (high resolution CT and 3T MRI). Patients with profound retro cochlear SNHL were diagnosed and the parents of the children with retro cochlear pathology, were counselled for ABI surgery, duration, likely complications, post-operative switch on, audio visual therapy for 2 years, expected outcomes, overall cost and available government schemes for surgery. An informed written consent from parents for surgery was taken. All required investigations were done and the child was prepared for surgery after comprehensive evaluation and opinions from neurosurgeon, paediatrician, ophthalmologist, cardiologist, anaesthesiologist, speech pathologist, clinical psychologist and occupational therapist. Surgical team comprised of ENT surgeon, Neurosurgeon, Neuro-anesthesiologist, Audiologist. Intra-operative anatomical findings and variants from normal anatomy were noted. Electromyography monitoring of 7 and 9 nerves and intra OP monitoring of evoked auditory brainstem response was done. Subjective grading of cerebellar flocculus and electrode insertion difficulty for different grades of flocculus was assessed intra-operatively. The same data was retrieved from previous medical records, of who had undergone ABI surgery, before the study has started.
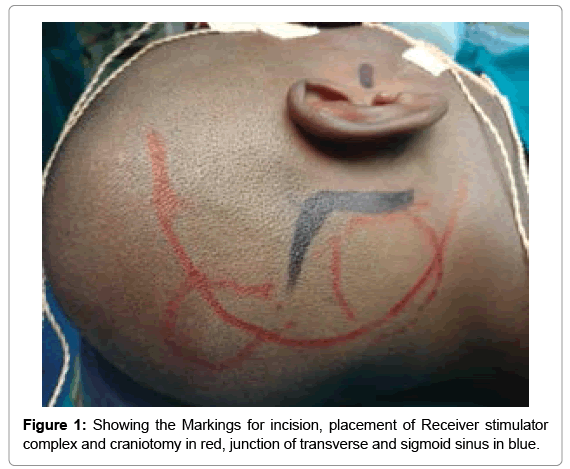
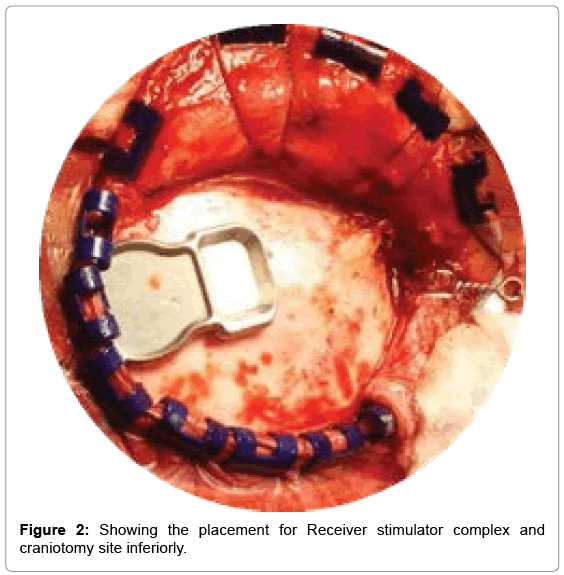
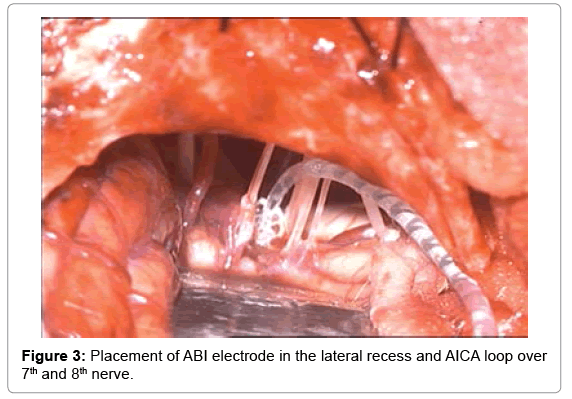
Surgery is done by retrosigmoid approach (Figure 1). After, sub periosteal dissection, 3×3 cm craniotomy is done and extended to visualize the transverse sigmoid sinus junction. ABI receiver stimulator coil bed was drilled with tie-down holes (Figure 2). Dura was inccised and reflected to visualize cerebellum. The later was retracted inferiomedially and cerebral spinal fluid (CSF) was released. Flocculus was identified and graded depending on visibility of choroid plexus and root entry zone of 7th,8th nerve and lower cranial nerves. After arachnoid dissection, foramen of Luschka was identified. When in doubt, location of lateral recess was further confirmed by noting for the egress of CSF during valsalva manoeuvre. Dummy electrode was negotiated through the foramen of Luschka to floor of the 4th ventricle to check optimal positioning of electrodes, followed by placing the permanent electrode through the same route, after fixing receiver stimulator coil (Figure 3).
Prior to placement of electrode, its dacron mesh was trimmed in all our pediatric ABI candidates. This was done because of smaller dimensions of lateral recess in children and to prevent adhesions. The anatomical variations defining foramen of Luschka were also noted.
Intra-operative EABR was measured. EABR in ABI lacks waves I and II waves. Positive auditory responses are multi-peak waveforms generated in first 2-4 min of onset of stimulus, while larger waves with longer amplitude are generally non-auditory responses. After optimal placement, electrodes were secured with surgicel. Dura was closed in water tight manner and craniotomy defect covered with gel foam and bone sandwich. Receiver stimulator area was closed with palva flap and skin wound was closed in layers followed by mastoid dressing.
During the surgery, the following anatomical landmarks were very helpful in guiding the electrode towards the cochlear nucleus, which is not under direct vision.
Surgery-anatomical landmarks
1. Choroid plexus marks the entrance of the lateral recess and the taenia traverses the roof of the lateral recess [8].
2. Flocculus of cerebellum which forms an operculum covering the entry to lateral recess of fourth ventricle [9].
3. Exits of cranial nerves 7, 8 and 9 form a triangle which help in identification of the entrance of the lateral recess of fourth ventricle (~ 5×6 mm) [10].
4. Straight vein, running from 9th/10th/11th cranial nerve complex to 7th/8th cranial nerve complex forming floor of lateral recess and heading to the entrance of the foramen of Luschka [10,11].
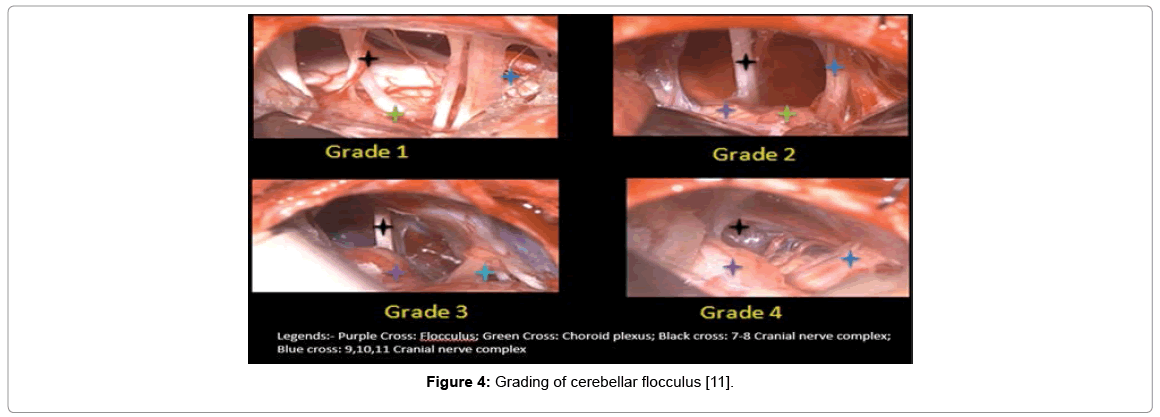
After retracting the cerebellum, flocculus was identified and graded based on grading mentioned in Sunil et al. article [11], Grade 1 is nonvisualization of cerebellar flocculus with a prominent choroid plexus. Grade 2 flocculus is hypoplastic and lying rostrally with a prominent choroid plexus. Grade 3 flocculus is small and more central with a small choroid plexus. Grade 4 cerebellar flocculus is large and central with a small choroid plexus (Figure 4). Grade 1 and Grade 2 flocculus covers the root entry zone of 7th, 8th and lower cranial nerves (9th, 10th and 11th).
Figure 4: Grading of cerebellar flocculus [11].
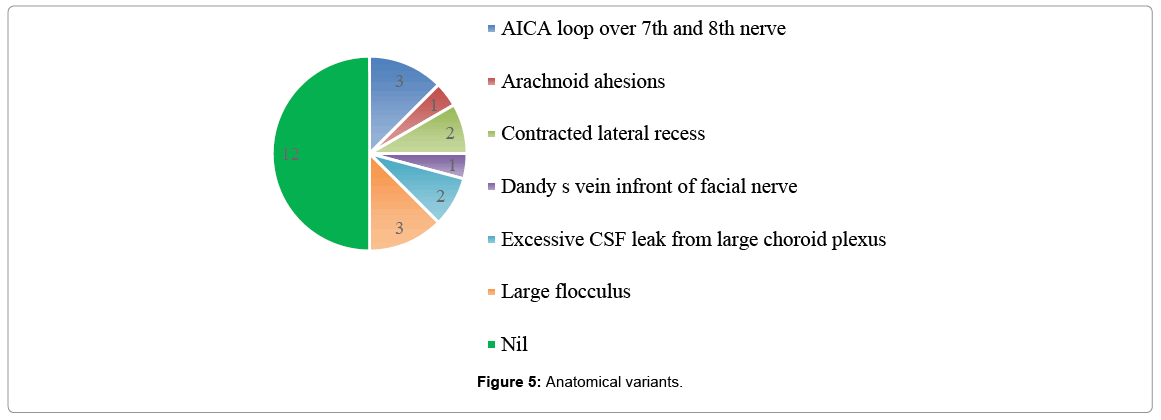
It was noticed that hypoplasia of flocculus was associated with hypertrophy of choroid plexus. Choroid plexus was partially bipolarized to visualize lateral recess. In cases of contracted lateral recess, the opening was widened using dummy electrode. Electrode placement was done superficial to straight vein. Anatomical variations were encountered during ABI surgery (Figure 5), which has made the proper positioning of the ABI challenging in some of the cases. Arachanoid adhesions were released with micro scissors. Glossopharyngeal nerve was taken as guide for proper positioning of implant over the cochlear nucleus, as cochlear nerve was either hypoplastic or absent in most of the cases. In, cases of Anterior inferior cerebellar artery (AICA), the vascular loop was separated from 7th and 8th nerve complex. Electrode insertion was easy in cases of AICA variant, when compared with cases of other anatomical variants. In one of the cases, Dandy’s vein, which are group of superior petrosal vein in posterior fossa, caused difficulty in visualizing the cerebello pontine angle structures [12].
Post operatively children were kept in neuro-ICU care for first 24 to 48 h and later transferred to ward. Suture removal was done on 10th post op day, except for cases with complications, like wound dehiscence or pseudo meningocele. CT scan was done post operatively to confirm position of device. Activation of device was done 6 weeks later with monitoring of vital signs and non-auditory effects. This was followed by intensive auditory verbal habilitation therapy (AVHT) at our centre. Evoked Auditory Brainstem Response (EABR) was done regularly during the follow up period for confirming the device integrity and assessment of the optimal performance. The auditory outcomes were measured with the help of Categories of auditory Performance (CAP) scores and Speech Intelligibility Ratings (SIR) scores at 3 months, 6 months, 9 months and 12 months of implant age. CAP measures supraliminal performance, which reflects everyday auditory performance in a more realistic way. The SIR scores are used to measure the speech intelligibility of the implanted children by quantifying their everyday spontaneous speech [8].
Results
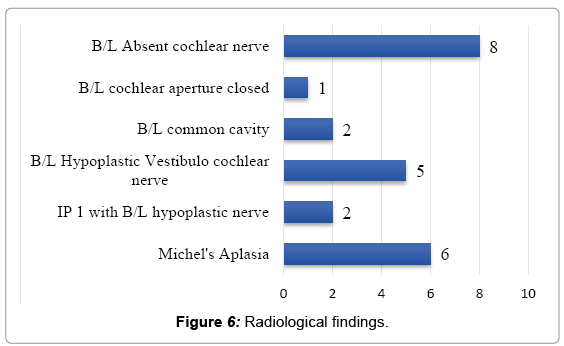
Total of 24 children underwent ABI from 2006 till March 2017. 12 implantees were male and rest were female. The mean age at implantation was 4.16 years. Out of the 24 implantees, 33% of the cases had bilateral absent cochlear nerve, 25% cases had Michle’s aplasia and remaining cases had etiology as shown in Figure 6.
Excessive CSF leak, with no anatomical variant also made positioning of electrode difficult. 2×2 table shows, normal surgical anatomy and variant anatomy cases which had difficulty in electrode insertion. Chi square test was applied to Table 1 and the p value was significant, stating that anatomical variants encountered during ABI surgery made the positioning of the electrode difficult.
| Easy Insertion | Difficult Insertion | |
|---|---|---|
| Anatomical Variants Present | 3 | 9 |
| No anatomical variants | 10 | 2 |
Table 1: Insertion difficulty of ABI in relation to the anatomical variant, ‘p’=0.004 (by chi square test), result is significant, at p<0.05.
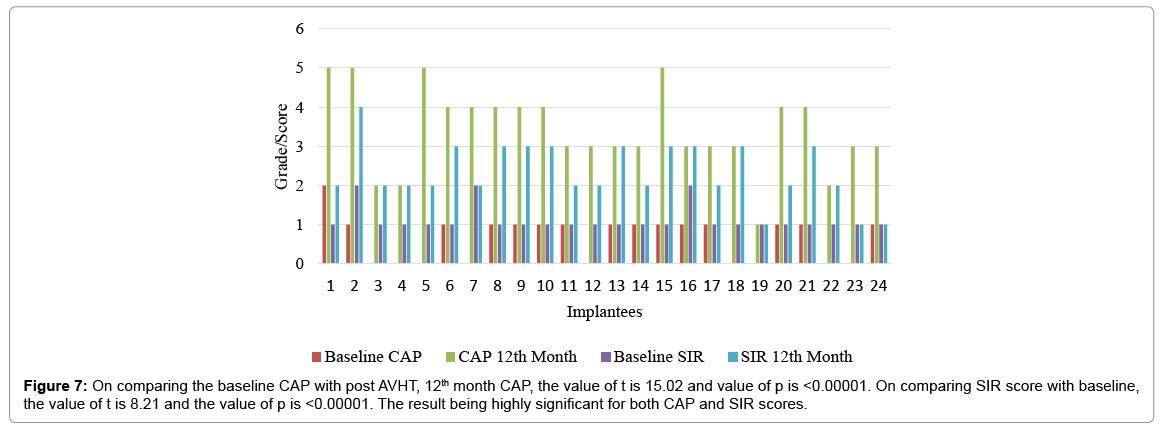
In our cohort, the most common post op complication was psuedomeningocele (4 cases) which was managed with pressure dressing and aspiration was done along with intra venous antibiotics. Other complications were transient clinical facial paresis on the side of surgery, surgical site discharge and wound healing. Later two were treated with course of anti-biotics. Two patients required post op ventilatory support for 2 days in view of transient cerebellar edema. One implantee had a revision surgery due to bio-film formation and was implanted on the other side, year and a half later. All the implantees were followed every 3rd month. Baseline CAP and SIR score of each patient was compared with 12th month CAP and SIR score, respectively. Since the data was normal distributed, paired T test was applied and resultant p value for both CAP and SIR scores were significant, stating that all the implantees had significant improvement with respect to auditory and speech performance, post 1 year of AVHT (Figure 7).
The mean CAP and SIR scores after 1 year of AVHT were 3.42 and 2.33 respectively. CAP and SIR scores of implantees with anatomical variants and no anatomical variants were compared. Chi square test was applied to both the 2×2 tables (Tables 2 and 3). p value for both the tables was not significant, drawing to a conclusion that regardless of the insertion difficulty in challenging cases, CAP scores and SIR scores were similar in all the children after 1 year of AVHT.
| CAP score ≤ 3 | CAP Score>3 | |
|---|---|---|
| Anatomical Variants Present | 8 | 4 |
| No anatomical variants | 5 | 7 |
Table 2: In view of mean CAP score being 3.42, CAP score 3 was used for differentiation in comparison table, ‘p’=0.22 (by chi-square test). This is result is not significant at p ≤ 0.05.
| SIR score ≤ 2 | SIR Score>2 | |
|---|---|---|
| Anatomical Variants Present | 9 | 3 |
| No anatomical variants | 5 | 7 |
Table 3: In view of mean SIR score being 2.33, SIR score 2 was used for differentiation in comparison table, ‘p’=0.09 (by chi-square test). This is result is not significant at p ≤ 0.05.
Discussion
Normal surgical approaches include translabyrinthine approach, retrosigmoid or lateral suboccipital approach [13]. Friedland DR and Wackym PA, in their study concluded that retrosigmoid approach provides excellent visualization of lateral recess [14] and same was used in all our cases. Translabyrinthine approach has a much more limited surgical exposure than retrosigmoid approach and surgical time is prolonged, in view of temporal bone drilling to expose brain stem. In addition, Retrosigmoid approach makes it possible to bypass the mastoid air cells so that intracranial contamination by the middle ear flora can be prevented [11,13]. Sub occipital approach is involved with risk of complications of excessive cerebellar retraction. Middle cranial fossa has the risk of post op facial nerve weakness and surgery is possible with angled endoscopes. Therefore, for the placement of ABI in a child, retrosigmoid approach appears to be advantageous [13].
Children with hypoplastic nerves and cystic cochlea fall into the grey zone for CI vs ABI. Promontory stimulation testing in those cases, will help in deciding the role of CI surgery [15,16].
Knowledge of the anatomical landmarks and variants in anatomy of the brainstem is vital for the operating surgeon for identifying site for placement of ABI electrode and position is confirmed by EABR2. Imaging is indispensable for the preoperative evaluation of patients with brain stem implants. Contraindications to ABI include previous stereotactic radiotherapy which has the risk of radiation necrosis of the cochlear nucleus region and anatomic distortion and fibrosis [17].
Colletti et al. article describes the other anatomic variants encountered, pre-operatively and surgical challneges [9]. The most common surgical complications reported in the pediatric ABI studies included CSF leak and mild/transitory cerebellar edema, which occurred in up to 8.5% and 9.2% of patients, respectively [18,19]. Cases have been reported with some major complications like secondary hydrocephalus, facial palsy, device failure requiring revision surgery and minor complications like wound infections, seroma, balance problems [20]. Surgery at younger age affects outcomes positively, enabling higher neuronal plasticity. The first programming of the ABI electrode is done 6 weeks after the surgery. After switch-on, EABR thresholds can be used as a guide to program the mapping levels in the ABI [21]. To start with, the channels in the center of the electrode are activated. If there are no side effects, then switch on is carried onto other elcetrodes. Switch on is done under neurological monitoring of 7th, 8th and lower cranial nerve monitoring, in an ICU set up. If there is a side effect, the current level is lowered until hearing sensation without any side effects is achieved. If this is not possible, the channel leading to the side effect is closed [13]. Common side effects include stimulation of facial nerve, nystagmus, vagal stimulation, dizziness, change in gait. In our study, 3 patients had facial twitching, 2 patients had bradycardia requiring treatment and 2 patients had imbalance, which resolved over time. Most important factors affecting the outcome depends on the age of implantation, continuation of AVHT, attention deficit hyperactivity disorder, visual problems, associated syndromes and subnormal IQ [22-23]. All the children who were operated at our center had increased communication skills and improvement in lip reading and environmental sound perception. Gratifying results were achievable with intense AVHT, although not comparable to CI children. ABI children, require prolonged monitoring with intense therapy and optimal fine tuning of the maps, to excel in communication.
Conclusion
Use of ABI for children opened a new era in the rehabilitation of patients, in whom CI surgery is contraindicated due to cochlear and retro-cochlear anomalies. Frequent anatomical variations surrounding lateral recess are encountered. Flocculus of the cerebellum is of different grades and higher grades can make the placement of ABI electrodes difficult. Early intervention is required to take advantage of neural plasticity along with a good team approach. Intense audio verbal therapy and habilitation, which is most important following ABI surgery, gave good post op results in most of the cases, despite the surgical difficulties. Research is being done to study clinico- radiological outcomes by comparing MRI of the flocculus with its surgical findings. Neuronavigation and endoscopy may facilitate appreciate the surgical anatomy and proper placement of electrodes. Optogenetic stimulation of cochlear nucleus and the use of light-based neuronal stimulation also offers the promise to increase spatial resolution of current ABI arrays [24]. The journey through paediatric auditory brainstem implantation is an exciting but challenging one. Very few centres across the world have shown promising results, to establish this sophisticated technology as a standard of care for hearing restoration in children with inner ear anomalies.
References
- Parth SV, Elliott KD, Alyson KB, Daniel LJ (2016) Pediatric auditory brainstem implant surgery: A new option for auditory habilitation in congenital deafness. J Am Board Fam Med 29: 286-288.
- Britoneto RV, Bento RF, Yasuda A, Ribas GC, Rodrigues AJ Jr. (2005) Anatomical references in auditory brainstem implant surgery. Braz J Otorhinolaryngol 71: 282-286.
- Eisen MD (2003) The first implanted electrical neural stimulator to restore hearing. Otol Neurotol 24: 500-506.
- Hitselberger WE, House WF, Edgerton BJ, Whitaker S (1984) Cochlear nucleus implants. Otolaryngol Head Neck Surg 92: 52-54.
- Colletti V, Fiorino F, Sacchetto L, Miorelli V, Carner M (2001) Hearing habilitation with auditory brainstem implantation in two children with cochlear nerve aplasia. Int J Pediatr Otorhinolaryngol 60: 99-111.
- Sennaroglu L, Colletti V, Manrique M, Laszig R, Offeciers E, et al. (2011) Auditory brainstem implantation in children and non-neurofibromatosis type 2 patients: A consensus statement. Otol Neurotol 32: 187-191.
- Sennaroglu L, Ziyal I, Atas A, Sennaroglu G, Yucel E, et al. (2009) Preliminary results of auditory brainstem implantation in prelingually deaf children with inner ear malformations including severe stenosis of the cochlear aperture and aplasia of the cochlear nerve. Otol Neurotol 30: 708-715.
- Â Zhou H, Chen Z, Shi H, Wu Y, Yin S (2013) Categories of auditory performance and speech intelligibility ratings of early-implanted children without speech training. PLoS One 8: e53852.
- Colletti G, Mandala M, Colletti L, Colletti V (2015) Surgical visual reference for auditory brainstem implantation in children with cochlear nerve deficiency. Otolaryngol Head Neck Surg 153: 1071–1073.
- Kanowitz SJ1, Shapiro WH, Golfinos JG, Cohen NL, Roland JT Jr (2004) Auditory brainstem implantation in patients with neurofibromatosis type 2. Laryngoscope 114: 2135-2146.
- Sunil G, Shyam SK, Kameswaran M, Vasudevan MC, Ranjith R, et al. (2017) Does cerebellar flocculus size affect subjective outcomes in pediatric auditory brainstem implantation. Int J Pediatr Otorhinolaryngol 97: 30-34.
- Matsushima K, Ribas ES, Kiyosue H, Komune N, Miki K, et al. (2015) Absence of the superior petrosal veins and sinus: Surgical considerations. Surg Neurol Int 6: 34.
- Komune N, Yagmurlu K, Matsuo S, Miki K, Abe H, et al. (2015) Auditory brainstem implantation: Anatomy and approaches. Neurosurgery 11: 306-321.
- Friedland DR, Wackym PA (1999) Evaluation of surgical approaches to endoscopic brainstem implantation. Laryngoscope 109: 175-180.
- Sennaroglu L, Sennaroglu G, Atay G (2013) Auditory brainstem implantation in children. Curr Otorhinolaryngol Rep 1: 80-91.
- Warren FM 3rd, Wiggins RH 3rd, Pitt C, Harnsberger HR, Shelton C (2010) Apparent cochlear nerve aplasia: To implant or not to implant? Otol Neurotol 31: 1088-1094.
- Kameswaran M, Vasudevan MC, Anandkumar RS, Jawahar N, Kiran N, et al. (2005) Auditory brainstem implantation: The first Indian experience. Indian J Otolaryngol Head Neck Surg 57: 58-63.
- Raghunandhan S, Kameswaran M (2010) Auditory brain stem implants. Otorhinolaryngol clinics 2: 151-155.
- Kimberley SN, Elliott DK, Sethi R, Parth VS (2015) Auditory brainstem implant outcomes. Otolaryngol Head Neck Surg 153: 739-750.
- Colletti V, Shannon RV, Carner M, Veronese S, Colletti L (2010) Complications in auditory brainstem implant surgery in adults and children. Otol Neurotol 31: 558-564.
- Raghunandhan S, Kameswaran M, Prashant R, Ranjith R, Chandrashekar R (2013) Clinical study of aided cortical auditory evoked potentials in pediatric auditory brainstem implantees. J Hear Sci 3: 22-29
- Colletti L (2007) Beneficial auditory and cognitive effects of auditory brainstem implantation in children. Acta Otolaryngol 127: 943-946.
- Rafaeldacosta M, Bittencourt AG, Jose N, Carolina AB (2014) Auditory brainstem implants in children: Results based on a review of the literature. J Int Adv Otol 10: 284-90.
- Sidharth VP, Lee DJ (2015) Pediatric auditory brainstem implant surgery. Otolaryngol Clin North Am 48: 1117-1148.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7892
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 6848
- PDF downloads: 1044