Peripartum Cardiomyopathy: Four Case Reports with Different Outcomes
Received: 29-Jul-2017 / Accepted Date: 09-Aug-2017 / Published Date: 28-Aug-2017
Abstract
Peripartum cardiomyopathy (PC) represents a rare, life-threatening condition of late pregnancy or early puerperium but, most likely because of its rare incidence, it remains a poorly understood clinical entity. Indeed, its etiology and clinical management, as well as its prognosis, are still debated. The following reports illustrate three consecutive cases referred to our department who were all diagnosed with PC but who followed completely different clinical courses. All cases were represented by young (Case 1: 30 years-old; Case 2: 32 years-old Case 3: 25 years-old; Case 4:28 years-old ), Caucasian, primiparous, female patients without any concomitant comorbidities who were admitted to our department with a severely reduced left ventricular ejection fraction (LVEF). Case 1 was admitted for cardiogenic shock one day after giving birth whereas Case 2, 3 and 4 nearly two months after delivery for decompensated heart failure (HF). Case 1, besides the standard pharmacological and non-pharmacological therapy for acute HF, received a single dose of cabergoline and, within three weeks, she completely recovered. Otherwise, Case 2 received only standard therapy for acute HF and, though not completely, she showed a significant LVEF improvement at the discharge 1 month later. Finally, Case 3, whose clinical course was complicated by LV thrombosis and arrhythmic storm, underwent to urgent heart transplantation procedure after nearly two months of hospitalization. Finally Case 4 received standard heart failure therapy, restored completely her cardiac function and recently completed her second pregnancy without any complications. Above described cases support the great heterogeneity in clinical presentation and course of PC and seems to confirm that a delayed diagnosis, as well as LV thrombosis, are among factors strongly associated with a poor outcome.
Keywords: Cardiomyopathy; Sympathetic neurotransmission; Hypertension
78892Introduction
Peripartum cardiomyopathy (PC) represents a rare, life-threatening condition of late pregnancy or early puerperium, being its diagnosis usually suspected when a left ventricular systolic dysfunction and symptoms of heart failure occur between the last month of pregnancy and the first five months postpartum (Table 1) [1,2]. Notwithstanding age over 30 years, multiparity, twin pregnancies, history of hypertension as well as black race are all factors thought to increase the risk for developing this condition, for none of them a certain role has been yet demonstrated [3]. The etiopathogenesis of PC was described in the mice as the increase of oxidative stress causing the augmentation of oxydative LDL and deletion of gene STAT 3 ( the protective gene against oxydative distress [4,5]. This STAT 3 arrangement put at high risk to PC by increasing of Cathepsin D and expression of activated prolactin (aPRO). The aPRO provoked the pro-apoptotic and antiangiogenesis of prolactin that got involved also cardiac cells [5].
| Diagnostic Criteria of Peripartum cardiomyopathy | |
|---|---|
| Development of Heart Failure | Last month of pregnancy →5 month postpartum |
| Clinical History | Absence pre-existing heart disease |
| Risk Factors | Older maternal Age Multiparity Multifetal pregnancy African Descent High blood pressure Prior toxin exposure Use of certain medication to prevent premature labor |
| Cause | Undefined Prior viral illness Abnormal immune response Nutritional deficiencies Coronary artery spasm Small-vessel disease Defective antioxidant defenses Genetic |
| Arrhythmia | Risk of abnormal heart rhythm |
| Echocardiographic details | LVED dimension >2.7 cm/m2 M-mode fractional shortening < 30% LVEF <0.45 |
| Note: LVED: left ventricular end diastolic; LVEF: left ventricular ejection fraction | |
Table 1: Diagnostic criteria of peripartum cardiomyopathy.
From a clinical viewpoint, PC is known to be characterized by a rapid course and a relatively high probability for spontaneous recovery [6], mortality rates ranging between 6 to 10% in developed countries and nearly half of patients returning to normal left ventricular function [4]. However, delayed diagnosis, advanced New York Heart Association (NYHA) functional class, and left ventricular thrombosis, as well as coexisting comorbidities are all factors associated with a poor outcome or, at least, with a delayed recovery [7,8]. Finally, concerning the therapeutic management in the early phase of PC, although it mostly overlaps with that of other types of acute decompensated heart failure, including conventional pharmacologic (inotropes, diuretics, vasodilators, etc.) and nonpharmacologic therapies (i.e., ultrafiltration, mechanical support, heart transplantation, etc.) [9-12], it should be mentioned how the association with specific targeted therapies (i.e., bromocriptine or cabergoline) might be particularly advantageous in selected cases [13-18]. Unfortunately, mainly because of its rare incidence and heterogeneous presentation, PC still remains a weakly characterized and poorly understood clinical entity.
The following reports illustrate three consecutive cases of patients referred to our center (Heart Transplant Unit, S. Camillo Hospital, Rome – Italy) who were all diagnosed with PC but who followed completely different clinical courses. Notably, general characteristics as well as the main echocardiographic and lab data are all reported in Table 2.
| Variable | Case 1 | Case 2 | Case 3Q | Case 4 |
|---|---|---|---|---|
| Age, years | 30 | 32 | 25 | 28 |
| Race | Caucasian | Caucasian | Caucasian | Caucasian |
| Parity, number | 1 | 1 | 1 | 1 |
| Hypertension | No | No | No | No |
| Comorbidities | No | Marfan Sdr | No | |
| Diagnosis, days PP | 1 | 66 | 60 | 58 |
| Clinical Picture | Cardiogenic shock | Heart failure | Acute heart failure | Heart failure |
| NYHA class | IV | IIIb | IV | IV |
| LVEDV/BSA, ml/m2 | 30 | 25 | 28 | 29 |
| LVEF, % | 20 | 22 | 15 | 20 |
| C.I.,L/min/m2 | 1 | 1 | 1 | 1 |
| PASP, mmHg | 35 | 33 | 45 | 53 |
| Sierology | No | No | No | No |
| WBC, *103/uL | 31.19 | 6.05 | 14.98 | 7.31 |
| C-RP, mg/mL | 12.09 | 0.38 | 33 | 2.67 |
| Procalcitonin, ng/mL | 1.1 | 0.3 | 33.61 | - |
| BNP, pg/mL | 1107 | 1031 | 7713 | 1050 |
| TpnI (ng/mL) | 6.02 | 0.9 | 1.37 | 0.2 |
| Cultural exam | Negative | Negative | Negative | Positive |
Note: Urine culture: E. coli; PP: Post Partum; NYHA: New York Heart Association; LVEDV: Left Ventricular End Diastolic Volume; BSA: Body Surface Area; LVEF: Left Ventricular Ejection Fraction; C.I.: Cardiac Output Indexed for BSA (Echocardiographic Measure); PASP: Pulmonary Artery Systolic Pressure; WBC: White Blood Cells; C-RP: C Reactive Protein; BNP: Brain Natriuretic Peptide; TpnI: Troponin I
Table 2: General and clinical characteristics of the patient at the admission in our department.
Case Presenatation I
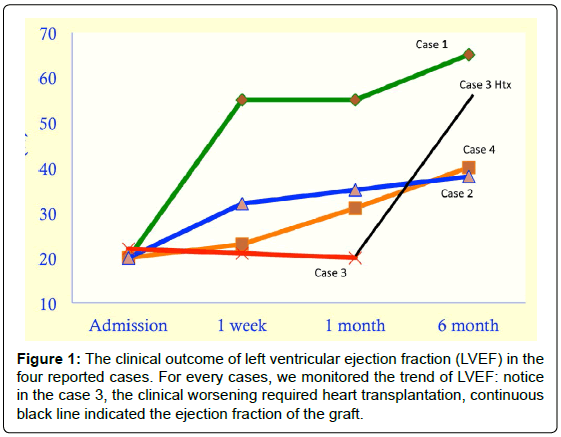
A 30-years-old, primiparous, Caucasian female admitted for cardiogenic shock to the Emergency Department (ED) one day after giving birth (caesarean section). The patient was in relatively good health until the day of delivery, when she developed rapidly progressive dyspnea (NYHA functional class IV), sopraventricular tachycardia (heart rate 130 bpm), and marked hypotension. Upon arrival, the patient was immediately intubated and transferred to our Intensive Care Unit (ICU) for treatment prosecution. At the admission, since that the patient remained in shock with severe lactate acidosis despite administration of noradrenaline, dobutamine, and levosimendan, an intra-aortic balloon pump (IABP) was percutaneously inserted. Moreover, on the basis of a high circulating plasma levels of prolactin, and also by considering the early timing postpartum (just one day), a dopamine D2 receptor agonist was promptly administrated (cabergoline 1 mg, single dose). Finally, the patient underwent also an endomyocardial biopsy in order to exclude a myocarditis etiology. At the third day from the admission in ICU, a respiratory and metabolic improvement was observed and the invasive ventilation was then interrupted. The fourth day it was possible to remove the IABP. During the first week of hospitalization, seriate echocardiographic examination showed a significant and progressive improvement in left ventricular systolic function (left ventricular ejection fraction, LVEF, from 20% to 55%) and a similar behavior was also observed for the main laboratoristic data (Prolactin from 192 ng/ mL to 9.7 ng/mL; Brain Natriuretic Peptide, BNP, from 1107 pg/mL to 442 pg/mL; C-reactive protein, CRP, from 12.09 mg/mL to 6.42 mg/ mL). Once completely stabilized, the patient was transferred to our department and, after initial titration of conventional heart failure therapy with ACE-inhibitors, diuretics and low dose beta-blockers, she was discharged home three weeks after admission. She is still in followup, she is doing well under treatment and, after 6 months, there was a complete LVEF recovery.
Case Presentation II
A 32-years-old, primiparous, Caucasian female referred to our attention from another hospital where she was hospitalized for acute heart failure 66 days after giving birth (spontaneous full-term delivery). The patient had Marfan Syndrome and she was in relatively good health until the 50 days of delivery, when she developed progressive dyspnea (NYHA functional class IIIb), sopraventricular tachycardia, and fatigue. At the admission, a therapeutic approach with dobutamine, diuretics, ACE-inhibitors, and low beta-blockers dose was started. Conversely, given the low circulating plasma level of prolactin (0.4 ng/ mL) and the delayed appearance of clinical picture, cabergoline was not considered useful and, accordingly, not administrated. Six days after, notwithstanding a slight but significant clinical improvement, due to the evidence of repeated episodes of non-sustained ventricular tachycardia, the patient was transferred to our ICU as a precautionary measure. Once in ICU, the patient continued abovementioned therapy with a further progressive improvement in her signs and symptoms of heart failure and, after two days, she was again transferred in our department. During the first two weeks of hospitalization, seriate echocardiographic examination showed a significant and progressive improvement in left ventricular systolic function (LVEF from 20% to 32%) and a similar behavior was also observed for the main lab data (BNP from 955 pg/mL to 522 pg/mL; CRP from 0.4 mg/mL to less than 0.01 mg/mL). Once completely stabilized, and after initial titration of conventional heart failure therapy with ACE-inhibitors, diuretics and low dose beta-blockers, she was discharged home 17 days after admission. The patient is still in follow-up, she is doing well under treatment and is quite asymptomatic (NYHA I-II). However, after 6 months, her LVEF resulted still partially impaired albeit slightly improved (from 32 to 40%).
Case Presentation III
A 25-years-old, primiparous, Caucasian female admitted for acute decompensate heart failure to the Emergency Department (ED) 60 days after giving birth (spontaneous full-term delivery). The patient was in relatively good health until the 50 days of delivery, when she developed progressive dyspnea (NYHA functional class IIIb), and fatigue. Upon arrival, the patient was promptly transferred to the Intensive Coronary Unit where she received standard therapy for acute heart failure, including inotrope, vasodilators and diuretics and, due to the echocardiographic evidence of left ventricular thrombosis (also confirmed by cardiac resonance imaging), anticoagulation with continuous heparin infusion was started. During the first month of hospitalization, seriate echocardiographic assessments did not show any significant improvement in left ventricular systolic function (LVEF from 15% to 20%). Moreover, on the basis of repeated sustained ventricular arrhythmias and one ventricular fibrillation treated with transthoracic DC shock at 360 Joule, the patient received an implantable cardioverter defibrillator as secondary prophylaxis of sudden arrhythmic death. Thereafter, she was referred to our department for treatment prosecution and, mainly, to evaluate her eligibility for heart transplantation. At the admission, the patient was haemodinamically unstable with sopraventricular tachycardia (heart rate equal to 110 bpm), marked hypotension, and severe symptoms of heart failure (NYHA IV). During the first days of hospitalization the standard therapy for acute heart failure did not lead to a significant echocardiographic or lab data improvement (LVEF 20%; BNP 7713 pg). Moreover, in consideration of persistent biventricular thrombosis extended to both jugular veins despite a concomitant warfarin therapy, a full screening for thrombophilic patter was done. The latter showed a severe deficit in Anti-Thrombin (AT) III (26% of activity) as well as in Protein C (39% of activity), both accordingly corrected. After the first week, due to a dramatic worsening in her clinical picture, she was transferred to our ICU where, since that the clinical course was further complicated by an arrhythmic storm, an IABP was percutaneously inserted as a bridge to heart transplantation (surgery procedure 11 days after the IABP placement). The patient is still followed by our dedicated ambulatory, she is completely asymptomatic (NYHA I) and her echocardiographic parameters, as well as her lab data, are all into the normal range.
Case Presentataion IV
A 28-years-old, primiparous, Caucasian female admitted for heart failure to the Emergency Department (ED) 58 days after giving birth (spontaneous full-term delivery). The patient had no clinical history of cardiac disease and other comorbidities. She had developed a respiratory distress and then progressive dyspnea (NYHA functional class IV) and fatigue. She started to receive standard therapy for acute heart failure, including inotrope, vasodilators and diuretics. The echocardiogram evidenced left ventricular ipokinesis and akinesis, low ejection fraction (LVEF <20%), and increase dimension and volume of LV. The anticoagulation oral therapy combined with continuous heparin infusion was started immediately. Meanwhile the seriate echocardiographic assessments documented the functional restore of the heart, she had repeated sustained ventricular arrhythmias (treated with transthoracic DC shock at 360 Joule and anti-arrhythmic drugs), hence the patient received an implantable cardioverter defibrillator as secondary prophylaxis of sudden arrhythmic death. She was discharged 4 weeks after and she patient is still followed by dedicated ambulatory, Nowadays she is completely asymptomatic (NYHA I) and her echocardiographic parameters, as well as her laboratoratoristic data, are all into the normal range.
Discussion
The current brief report described cases all pertaining to young, Caucasian, primiparous, female patients without any concomitant comorbidities with an overlapping clinical picture of decompensated acute heart failure.
According to our experience, the PC was developed less than 10% before partum and the most of them after delivery (Days after delivery: 55 ± 32). The diagnostic suspicion of PC needed immediately clinical and therapeutic managements because of the rapid and uncontrolled hemodynamic worsening. Also according to Donfrancesco et al. [13], we combined Levosimendan to Carbergoline to functional cardiac recovery monitoring by BNP and PRL daily dosages. Case 2 was admitted and treated rapidly but she had not a complete healing.
Case 3 was more complex because she had coagulative disorders causing multiple peripherical and cardiac intracavitary thrombosis. She was treated with specific anticoagulation and mechanical/medical support but without results. Therefore, she was transplanted and she was followed by our dedicated ambulatory.
Case 4 responded optimally and immediately to the medical support and the seriate echocardiogram documented a complete restore of the cardiac functionality. Recently, she completed her second pregnancy without any major and cardiac complications (Figure 1).
Analyzing extensively the four cases, the only differential parameter is leukocytosis: the three cases had neutrophilia instead the case 4 had eosinophilia. At the moment we have not had enough informations and data to explain this unusual type of leukocytosis in our patient but the etiopathogenesis of PC could play a central role (Table 2). This could be a future step of our research.
Conclusion
The peripartum cardiomyopathy is a cause of heart failure after partum requiring rapid and complex medical and mechanical supports. In the most of the clinical cases, the appropriate therapy could restore cardiac functionality completely. However the misunderstood of PM could cause life-threatening complications in patient and its rapid and uncontrolled hemodynamic worsening could require heart transplantation also. A rapid diagnosis and advanced heart failure therapy could reduce PC complications and could restore the cardiac function in order to a good quality of life and in few case (like Case 4) a possibility to another secure pregnancy.
Conflict of Interest
All of the author disclosure any conflict of interest.
Human rights statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. Informed consent was obtained from the patient for being included in the study.
References
- Arany Z, Elkayam U. (2016) Peripartum cardiomyopathy. Circulation 133: 1397-1409.
- Hilfiker Kleiner D, Haghikia A, Nonhoff J, Bauersachs J. (2015) Peripartum cardiomyopathy: Current management and future perspectives. Eur Heart J 36: 1090-1097.
- Lampert MB, Lang RM. (1995) Peripartum cardiomyopathy. Am Heart J 130: 860-870.
- Sliwa K, Hilfiker-Kleiner D, Mebazaa A, Petrie MC, Maggioni AP, et al. (2014) EURObservational research programme: A worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the Heart Failure Association of the European Society of Cardiology Working Group on PPCM. Eur J Heart Fail 16: 589-591.
- Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, et al. (2007) A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128: 589-600.
- Felker GM, Jaeger CJ, Klodas E, Thiemann DR, Hare JM, et al. (2000) Myocarditis and long-term survival in peripartum cardiomyopathy. Am Heart J 140: 785-791.
- Thompson L, Hartsilver E (2016) Peripartum cardiomyopathy. Updat Anaesth.
- Johnson Coyle L, Jensen L, Sobey A (2012) Peripartum cardiomyopathy: REVIEW and practice guidelines. Am J Crit Care 21: 89-98.
- Loyaga-Rendon RY, Pamboukian SV, Tallaj JA, Acharya D, Cantor R, et al. (2014) Outcomes of patients with peripartum cardiomyopathy who received mechanical circulatory support data from the interagency registry for mechanically assisted circulatory support. Circ Hear Fail 7: 300-309.
- ÅasiÅ„ska-Kowara M, Lango R, Kowalik M, Jarmoszewicz K (2014) Accelerated heart function recovery after therapeutic plasma exchange in patient treated with biventricular mechanical circulatory support for severe peripartum cardiomyopathy. Eur J Cardio-thoracic Surg 46: 1035-1036.
- Loyaga-Rendon RY, Pamboukian SV, Tallaj J, Acharya R, Cantor RS, et al. (2014) Outcomes of patients with peripartum cardiomyopathy who received mechanical circulatory support: Data from the INTERMACS registry. Circ Heart Fail 32: S12.
- Lueck S, Sindermann J, Martens S, Scherer M. (2016) Mechanical circulatory support for patients with peripartum cardiomyopathy. J Artif Organs 19: 305-309.
- Donfrancesco S, Cottini M, Lappa A, Nencini C, Picozzi P (2016) Cabergoline, levosimendan and Iabp : Treatment of peripartum cardiomyopathy. ARC Journal of Anesthesiology 1: 1-5.
- De Jong JSSG, Rietveld K, Van Lochem LT, Bouma BJ (2009) Rapid left ventricular recovery after cabergoline treatment in a patient with peripartum cardiomyopathy. Eur J Heart Fail 11: 220-222.
- Chng E, Dalan R (2013) Pituitary apoplexy associated with cabergoline therapy. J Clin Neurosci 20: 1637-1643.
- Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, et al. (2010) Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: A proof-of-concept pilot study. Circulation 121: 1465-1473.
- Habedank D, Kühnle Y, Elgeti T, Dudenhausen JW, Haverkamp W, et al. (2008) Recovery from peripartum cardiomyopathy after treatment with bromocriptine. Eur J Heart Fail 10: 1149- 1151.
- Ichida M, Katsurada K, Komori T, Matsumoto J, Ohkuchi A, et al. (2010) Effectiveness of bromocriptine treatment in a patient with peripartum cardiomyopathy. J Cardiol Cases 2: e28-e31.
Citation: Ricotta A, Cottini M, Della Monica PL, Sbaraglia F, Polizzi V, et al. (2017) Peripartum Cardiomyopathy: Four Case Reports with Different Outcomes. Cardiovasc Ther 2: 119.
Copyright: © 2017 Ricotta A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 16965
- [From(publication date): 0-2017 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 15872
- PDF downloads: 1093